Abstract
1. The pattern of breathing during the approach to the steady state following step changes of end-tidal PCO2 and PO2 has been determined in normal conscious human subjects. Three types of step were studied: (a) steps of PA, CO2 against a constant background of hyperoxia (PA, O2 ∼ 200), an almost pure intracranial chemoreceptor stimulus, (b) steps of PA, O2 between ∼ 50 and 80 torr against a background of constant mild hypercapnia, an arterial chemoreceptor stimulus, and (c) steps of PA, CO2 against a background of constant hypoxia (PA, O2 ∼ 50), a mixed stimulus. Steps were small and the responses barely detectable by the subjects.
2. Steps of CO2 in hyperoxia produced the slowest approach to the steady state. A single exponential fitted the ventilation response up to about 4 min (mean half time 83 sec for the `up' and 69 sec for the `down' transients). During the transient the pattern of change of tidal volume (VT) and expiratory time (TE) was the same as in the steady state. Inspiratory time (TI), however, in the early part of the transient, changed in the opposite direction to TE, returning to its steady value only after 1½-3 min. This effect occurred in both `up' and `down' transients and resulted in a smaller change of respiratory frequency than would have been predicted from the steady-state response.
3. Hypoxic steps produced the fastest approach to the steady state with mean half-times for ventilation of 10·9 sec for the `up' transients and 6·6 sec for the `down'. TI followed the same pattern during the transient as in the steady state, whereas TE, following the step out of hypoxia, lengthened to far beyond its final steady value within five breaths of the step, only returning to its steady-state value 3-4 min after the step. This resulted in an exaggerated change of frequency during the early part of the transient.
4. Steps of CO2 in hypoxia, a mixed peripheral and central chemoreceptor stimulus, showed a ventilation response which was best fitted by two exponentials, the half-times of which were consistent with those obtained for the separate responses. The patterning was also consistent with a mixed response, more so for TI than for TE.
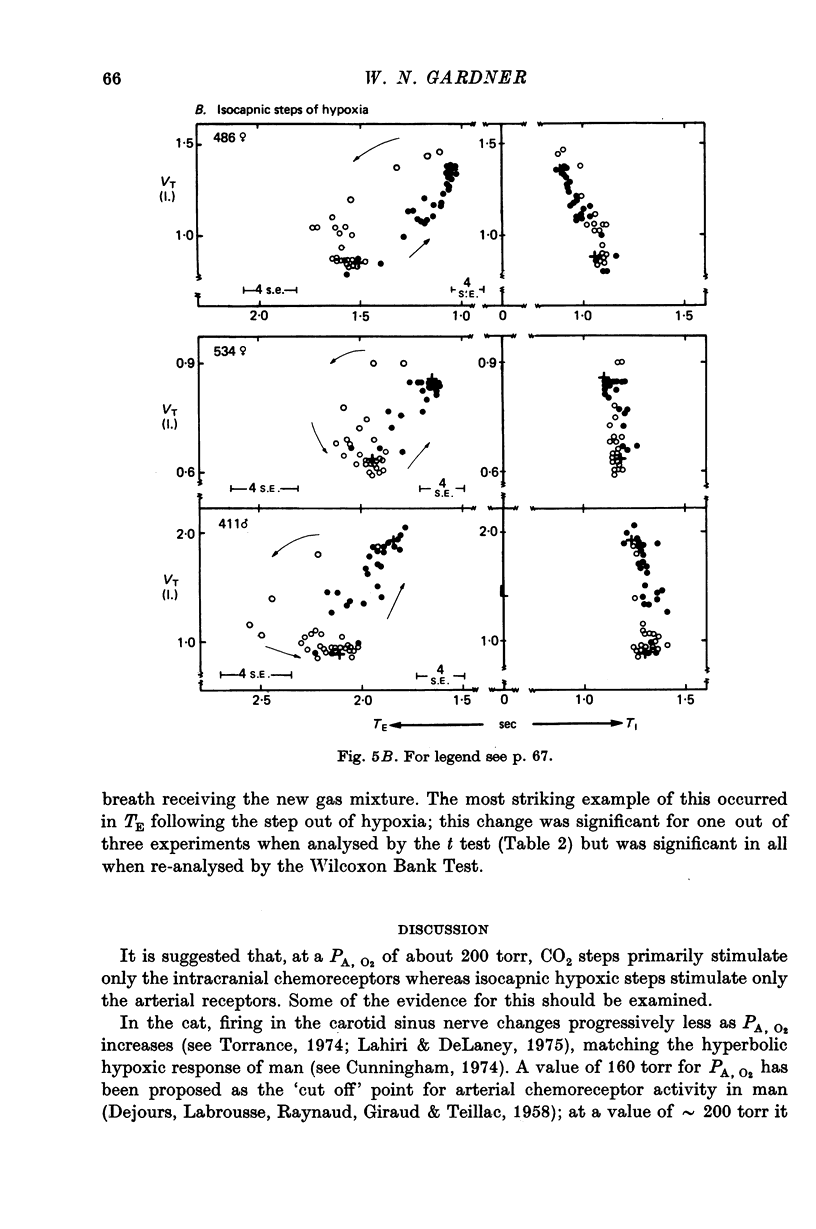
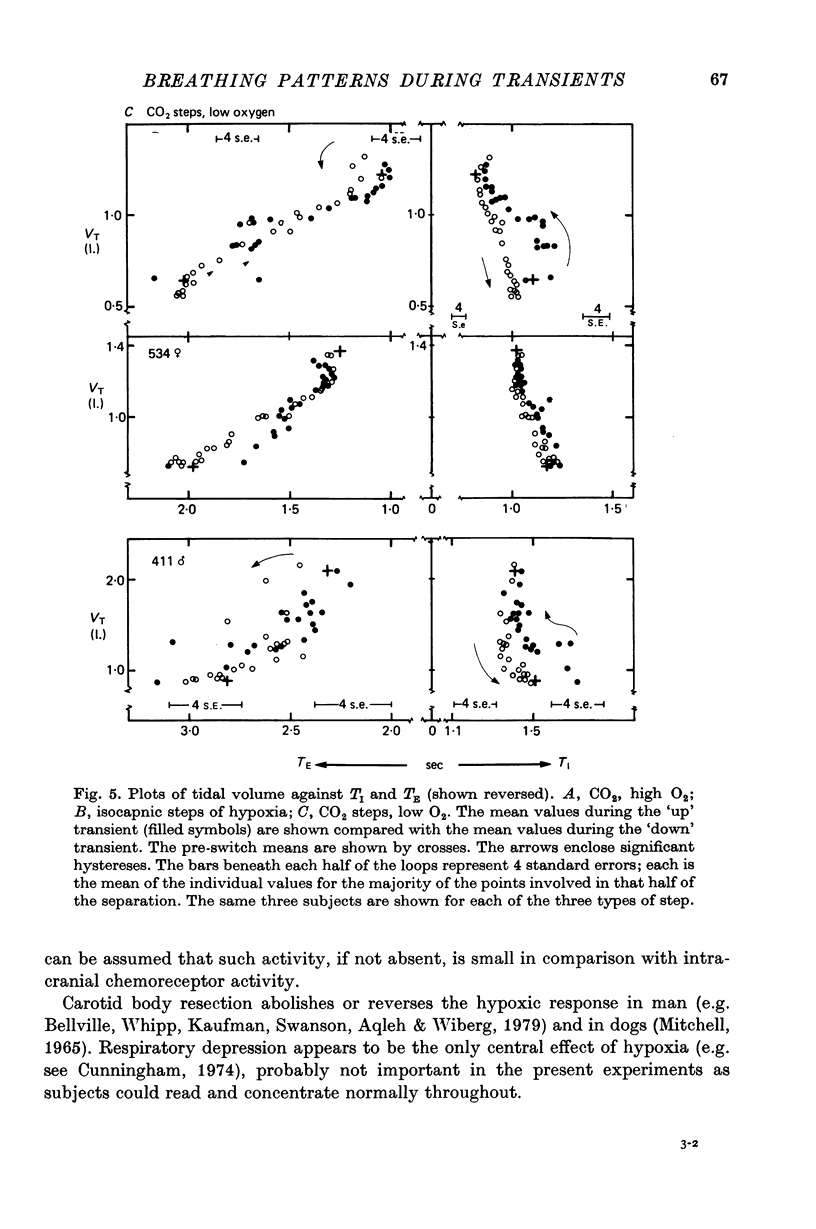
5. The steady-state pattern derived from the pre-switch means was consistent with the pattern previously described.
6. Possible mechanisms are discussed. It is suggested that these results could explain the different patterns seen in the past by those using re-breathing and steady-state techniques.
7. The validity of using one or two breath oxygen or nitrogen tests (or other similar tests) as a quantitative measure of the hypoxic response in man is questioned.
Full text
PDF
















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellville J. W., Whipp B. J., Kaufman R. D., Swanson G. D., Aqleh K. A., Wiberg D. M. Central and peripheral chemoreflex loop gain in normal and carotid body-resected subjects. J Appl Physiol Respir Environ Exerc Physiol. 1979 Apr;46(4):843–853. doi: 10.1152/jappl.1979.46.4.843. [DOI] [PubMed] [Google Scholar]
- Bernards J. A., Dejours P., Lacaisse A. Ventilatory effects in man of breathing successively CO-2-free, CO-2-enriched and CO-2-free gas mixtures with low, normal or high oxygen concentration. Respir Physiol. 1966;1(4):390–397. doi: 10.1016/0034-5687(66)90006-5. [DOI] [PubMed] [Google Scholar]
- Black A. M., McCloskey D. I., Torrance R. W. The responses of carotid body chemoreceptors in the cat to sudden changes of hypercapnic and hypoxic stimuli. Respir Physiol. 1971 Oct;13(1):36–49. doi: 10.1016/0034-5687(71)90063-6. [DOI] [PubMed] [Google Scholar]
- Bradley G. W., von Euler C., Marttila I., Roos B. Transient and steady state effects of CO-2 on mechanisms determining rate and depth of breathing. Acta Physiol Scand. 1974 Nov;92(3):341–350. doi: 10.1111/j.1748-1716.1974.tb05752.x. [DOI] [PubMed] [Google Scholar]
- Clark F. J., von Euler C. On the regulation of depth and rate of breathing. J Physiol. 1972 Apr;222(2):267–295. doi: 10.1113/jphysiol.1972.sp009797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham D. J., Drysdale D. B., Gardner W. N., Jensen J. I., Petersen E. S., Whipp B. J. Very small, very short-latency changes in human breathing induced by step changes of alveolar gas composition. J Physiol. 1977 Apr;266(2):411–421. doi: 10.1113/jphysiol.1977.sp011774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham D. J., Gardner W. N. A quantitative description of the pattern of breathing during steady-state CO2 inhalation in man, with special emphasis on expiration. J Physiol. 1977 Nov;272(3):613–632. doi: 10.1113/jphysiol.1977.sp012063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEJOURS P. Chemoreflexes in breathing. Physiol Rev. 1962 Jul;42:335–358. doi: 10.1152/physrev.1962.42.3.335. [DOI] [PubMed] [Google Scholar]
- DEJOURS P., LABROUSSE Y., RAYNAUD J., GIRARD F., TEILLAC A. Stimulus oxygène de la ventilation au repos et au cours de l'exercice musculaire, à basse altitude (50 m), chez l'homme. Rev Fr Etud Clin Biol. 1958 Feb;3(2):105–123. [PubMed] [Google Scholar]
- Eldridge F. L. Central neural respiratory stimulatory effect of active respiration. J Appl Physiol. 1974 Nov;37(5):723–735. doi: 10.1152/jappl.1974.37.5.723. [DOI] [PubMed] [Google Scholar]
- Flenley D. C., Brash H., Clancy L., Cooke N. J., Leitch A. G., Middleton W., Wraith P. K. Ventilatory response to steady-state exercise in hypoxia in humans. J Appl Physiol Respir Environ Exerc Physiol. 1979 Mar;46(3):438–446. doi: 10.1152/jappl.1979.46.3.438. [DOI] [PubMed] [Google Scholar]
- GRODINS F. S., GRAY J. S., SCHROEDER K. R., NORINS A. L., JONES R. W. Respiratory responses to CO2 inhalation; a theoretical study of a nonlinear biological regulator. J Appl Physiol. 1954 Nov;7(3):283–308. doi: 10.1152/jappl.1954.7.3.283. [DOI] [PubMed] [Google Scholar]
- Gardner W. N. Proceedings: The pattern of breathing following step changes of alveolar PCO2 in man. J Physiol. 1974 Oct;242(2):75P–76P. [PubMed] [Google Scholar]
- Gardner W. N. The pattern of the human respiratory response to step change of PA,O2 [proceedings]. J Physiol. 1978 Feb;275:33P–34P. [PubMed] [Google Scholar]
- Gardner W. N. The relation between tidal volume and inspiratory and expiratory times during steady-state carbon dioxide inhalation in man. J Physiol. 1977 Nov;272(3):591–611. doi: 10.1113/jphysiol.1977.sp012062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand R., Lambertsen C. J. Dynamic respiratory response to abrupt change of inspired CO2 at normal and high PO2. J Appl Physiol. 1973 Dec;35(6):903–913. doi: 10.1152/jappl.1973.35.6.903. [DOI] [PubMed] [Google Scholar]
- Haldane J. S., Meakins J. C., Priestley J. G. The respiratory response to anoxaemia. J Physiol. 1919 May 20;52(6):420–432. doi: 10.1113/jphysiol.1919.sp001841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson M. A., Rao P. S., Torrance R. W. Carbon dioxide sensitivity of aortic chemoreceptors in the cat. Respir Physiol. 1979 Apr;36(3):301–309. doi: 10.1016/0034-5687(79)90043-4. [DOI] [PubMed] [Google Scholar]
- Hey E. N., Lloyd B. B., Cunningham D. J., Jukes M. G., Bolton D. P. Effects of various respiratory stimuli on the depth and frequency of breathing in man. Respir Physiol. 1966;1(2):193–205. doi: 10.1016/0034-5687(66)90016-8. [DOI] [PubMed] [Google Scholar]
- LOESCHCKE H. H., KATSAROS B., ALBERS C., MICHEL C. C. UBER DEN ZEITLICHEN VERLAUF VON ATEMZUGVOLUMEN, ATEM-PERIODENDAUER, ATEMINUTENVOLUMEN UND ENDEXSPIRATORISCHEM CO2-DRUCK BEI EINATRMUNG VON GASGEMISCHEN MIT ERHOETEM CO2-DRUCK. Pflugers Arch Gesamte Physiol Menschen Tiere. 1963;277:671–683. [PubMed] [Google Scholar]
- Lahiri S., DeLaney R. G. Stimulus interaction in the responses of carotid body chemoreceptor single afferent fibers. Respir Physiol. 1975 Sep;24(3):249–266. doi: 10.1016/0034-5687(75)90017-1. [DOI] [PubMed] [Google Scholar]
- Miller J. P., Cunningham D. J., Lloyd B. B., Young J. M. The transient respiratory effects in man of sudden changes in alveolar CO2 in hypoxia and in high oxygen. Respir Physiol. 1974 Feb;20(1):17–31. doi: 10.1016/0034-5687(74)90015-2. [DOI] [PubMed] [Google Scholar]
- Pearson S. B., Cunningham D. J. Some observations on the relation between ventilation, tidal volume and frequency in man in various steady and transient states. Acta Neurobiol Exp (Wars) 1973;33(1):177–188. [PubMed] [Google Scholar]
- Rebuck A. S., Rigg J. R., Saunders N. A. Respiratory frequency response to progressive isocapnic hypoxia. J Physiol. 1976 Jun;258(1):19–31. doi: 10.1113/jphysiol.1976.sp011404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds W. J., Milhorn H. T., Jr, Holloman G. H., Jr Transient ventilatory response to graded hypercapnia in man. J Appl Physiol. 1972 Jul;33(1):47–54. doi: 10.1152/jappl.1972.33.1.47. [DOI] [PubMed] [Google Scholar]
- Reynolds W. J., Milhorn H. T., Jr Transient ventilatory response to hypoxia with and without controlled alveolar PCO2. J Appl Physiol. 1973 Aug;35(2):187–196. doi: 10.1152/jappl.1973.35.2.187. [DOI] [PubMed] [Google Scholar]
- Swanson G. D., Bellville J. W. Step changes in end-tidal CO2: methods and implications. J Appl Physiol. 1975 Sep;39(3):377–385. doi: 10.1152/jappl.1975.39.3.377. [DOI] [PubMed] [Google Scholar]
- Widdicombe J. G., Winning A. Effects of hypoxia, hypercapnia and changes in body temperature on the pattern of breathing in cats. Respir Physiol. 1974 Aug;21(2):203–221. doi: 10.1016/0034-5687(74)90095-4. [DOI] [PubMed] [Google Scholar]


