Abstract
1. The acetylcholine (ACh) responses of cardiac muscle cells from three species of bivalves were studied by intracellular recording and ACh ionophoresis. Heart muscle contraction was abolished by bathing in artificial sea water in which Mn2+ had been substituted for Ca2+.
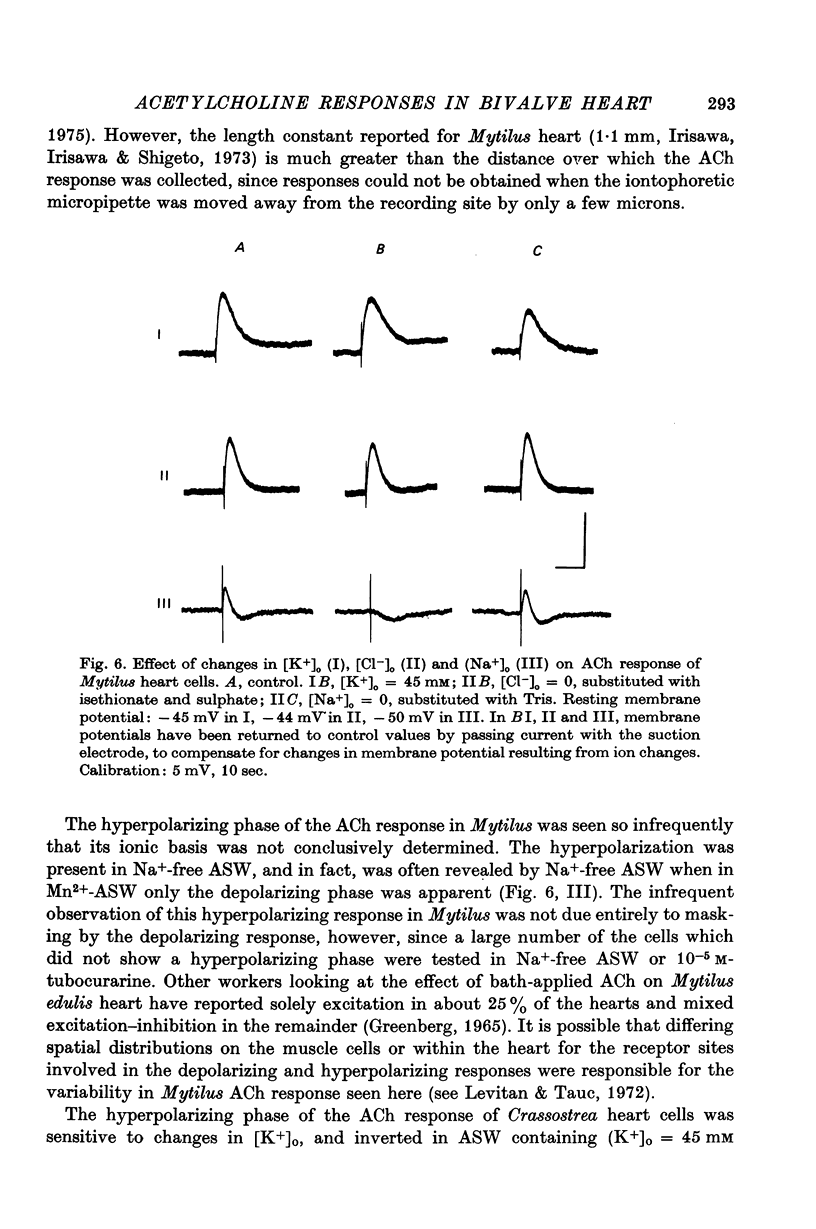
2. Three different types of membrane potential changes were observed in response to ACh pulses: a slow hyperpolarization in the clam Mercenaria mercenaria, a rapid depolarization which was sometimes followed by a slower hyperpolarization in the muscle Mytilus edulis, and a biphasic response consisting of both a rapid depolarization and slower hyperpolarization in the oyster Crassostrea virginica. All responses were accompanied by an increase in membrane conductance, as measured by passing constant current pulses with an extracellular suction electrode.
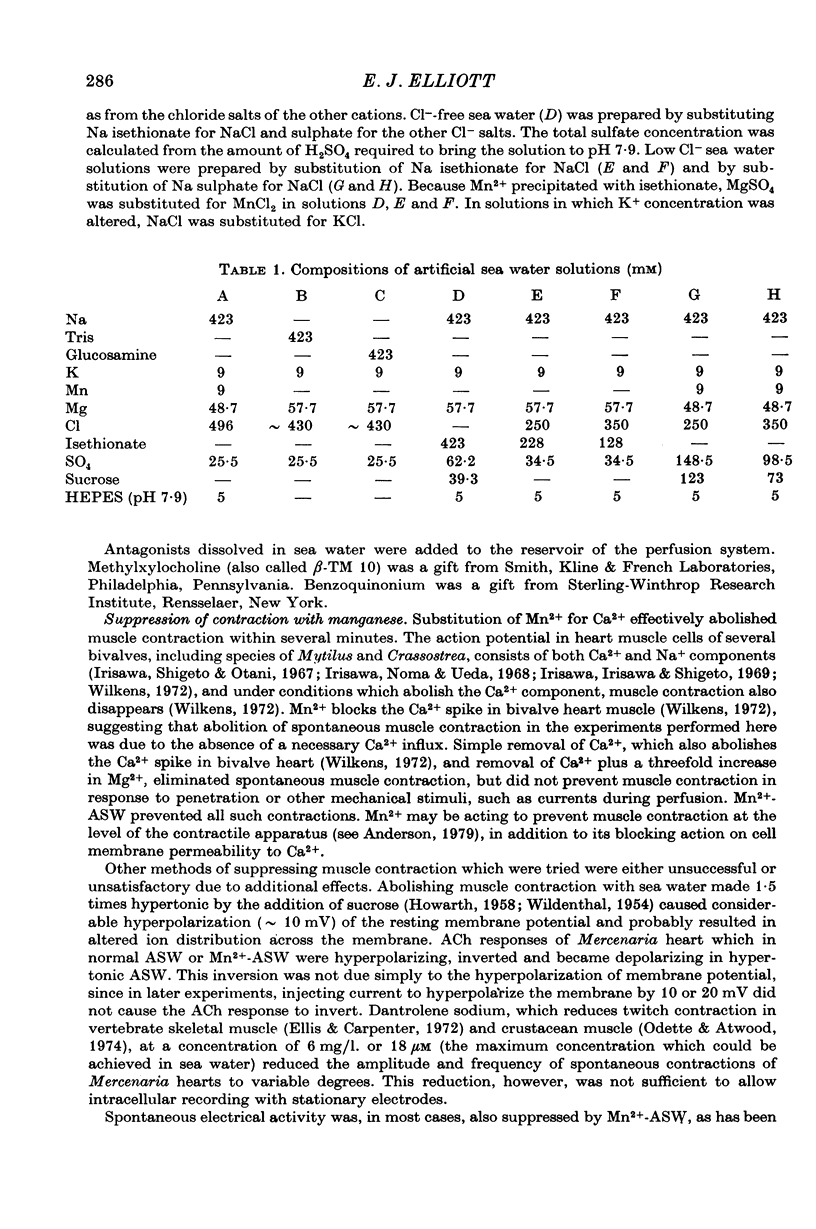
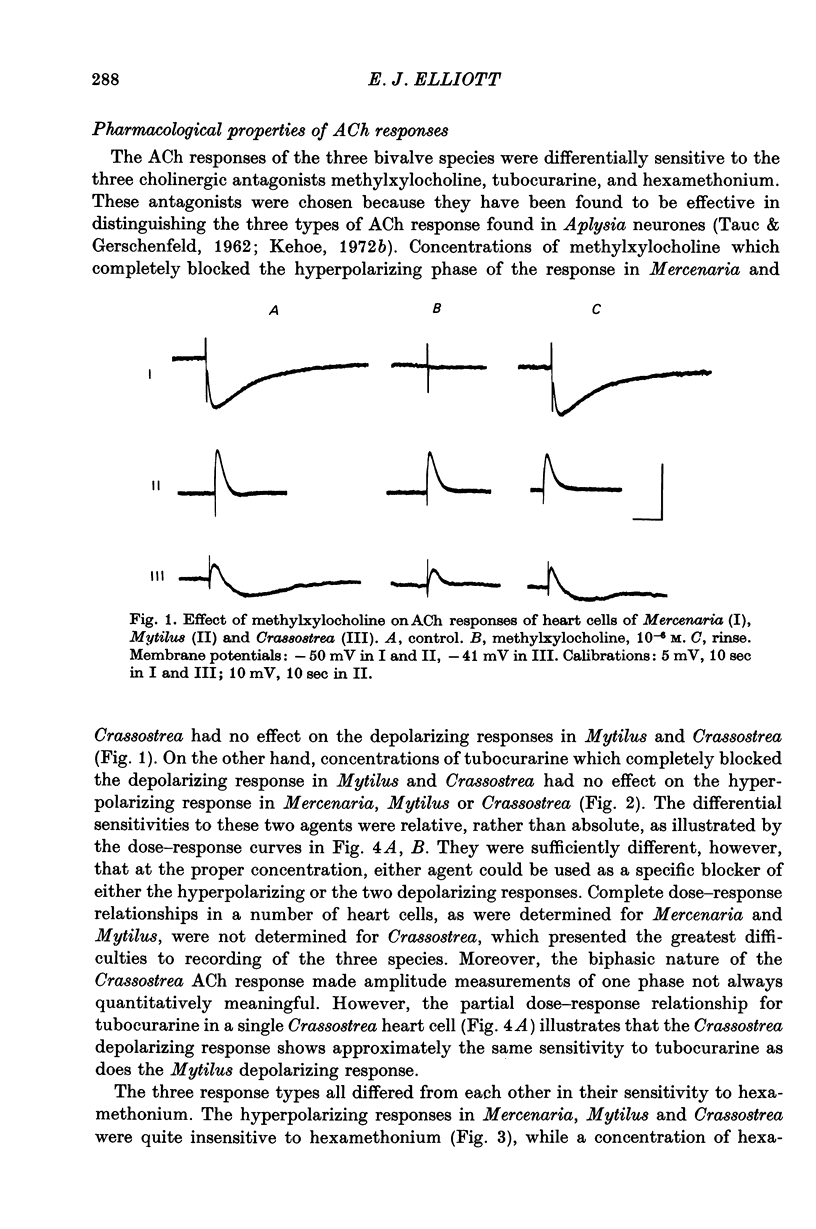
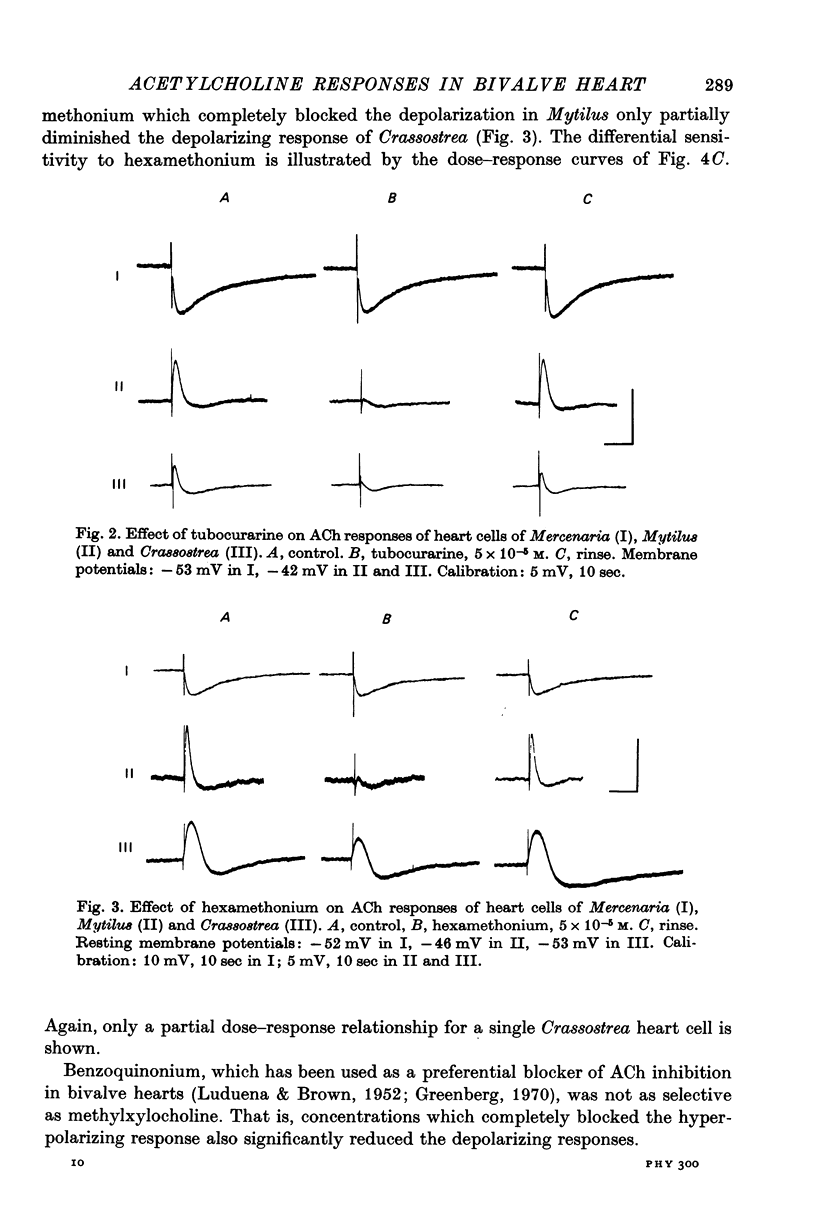
3. The hyperpolarizing response in all three species was blocked most effectively by methylxylocholine and not very effectively by tubocurarine or hexamethonium. The depolarizing response in Mytilus was blocked preferentially by tubocurarine and hexamethonium, while the depolarizing response in Crassostrea was blocked effectively by tubocurarine only.
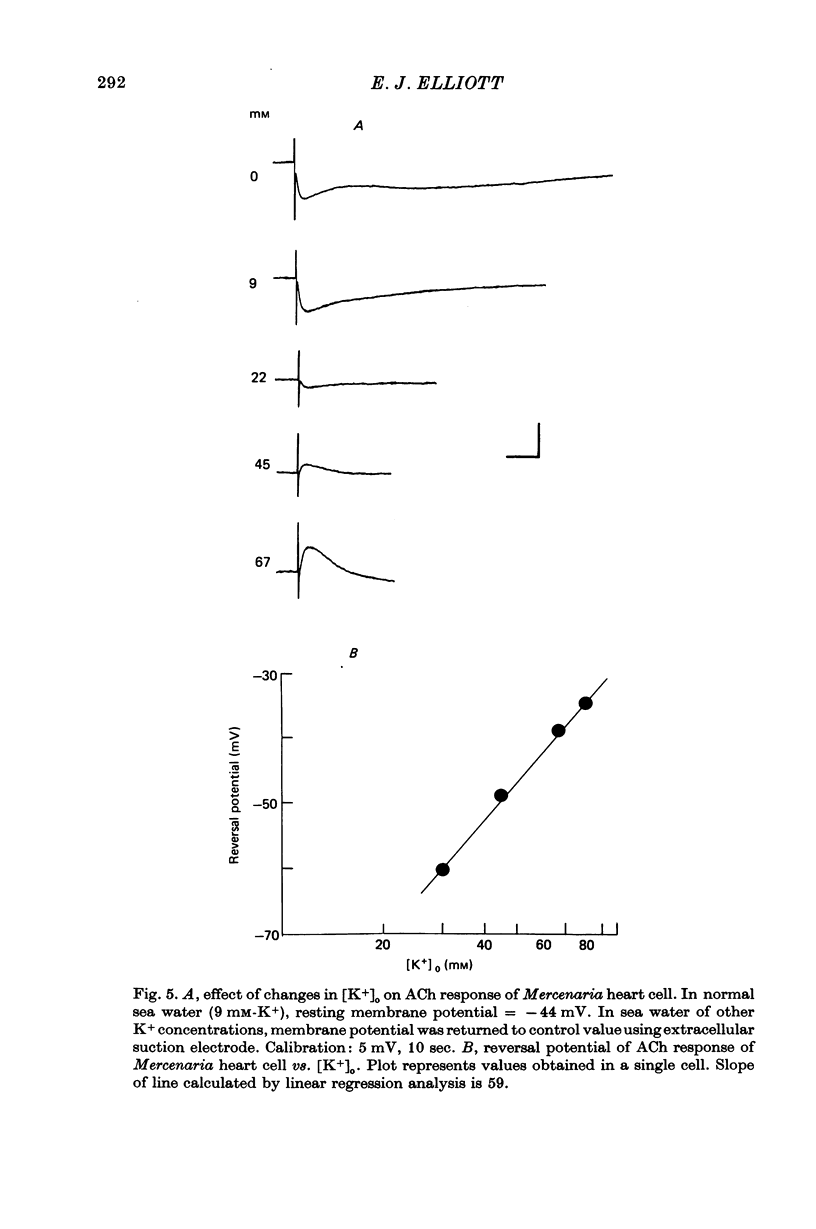
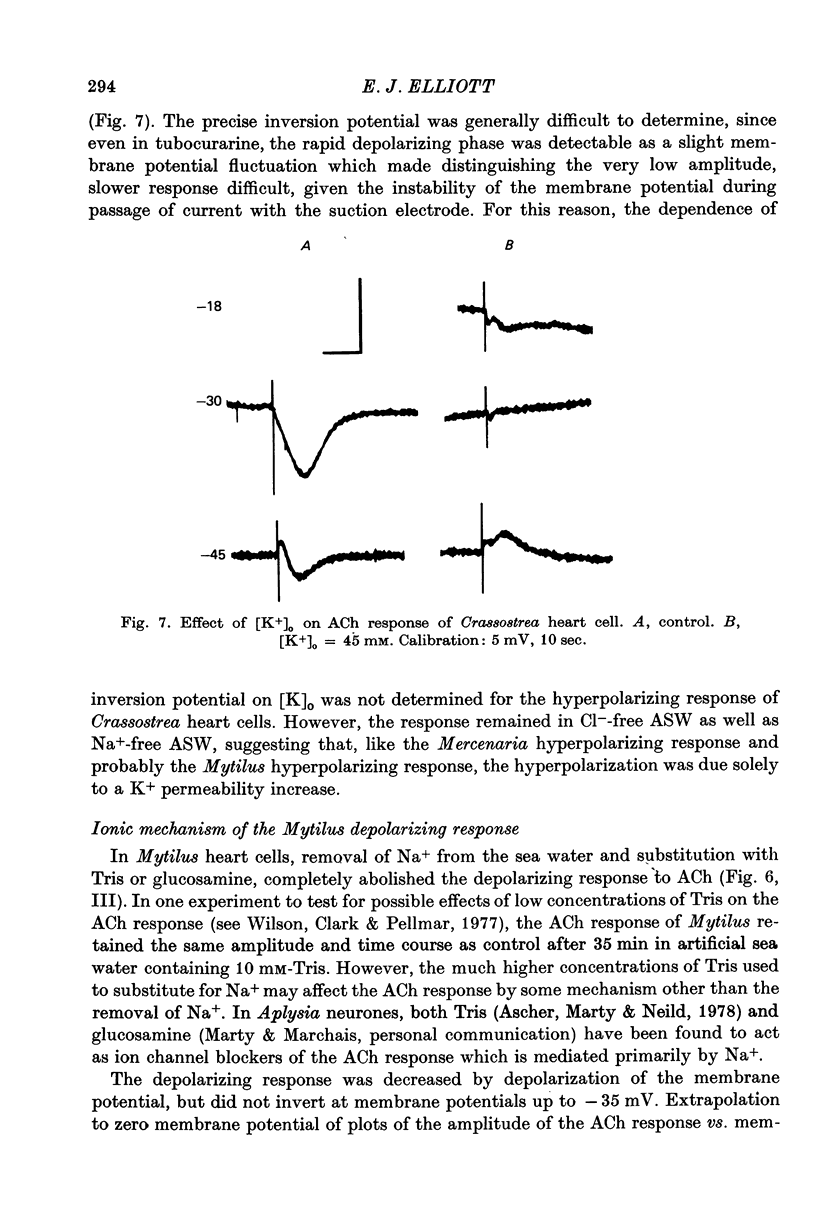
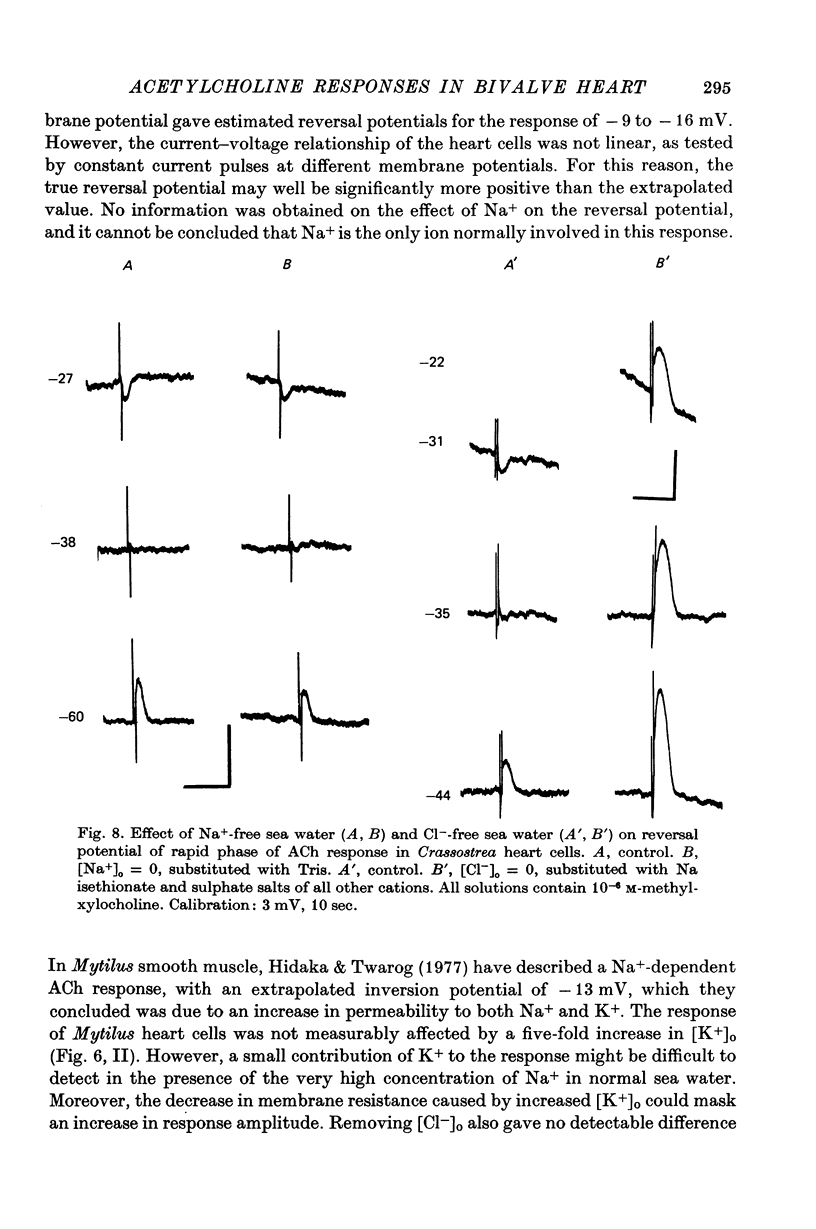
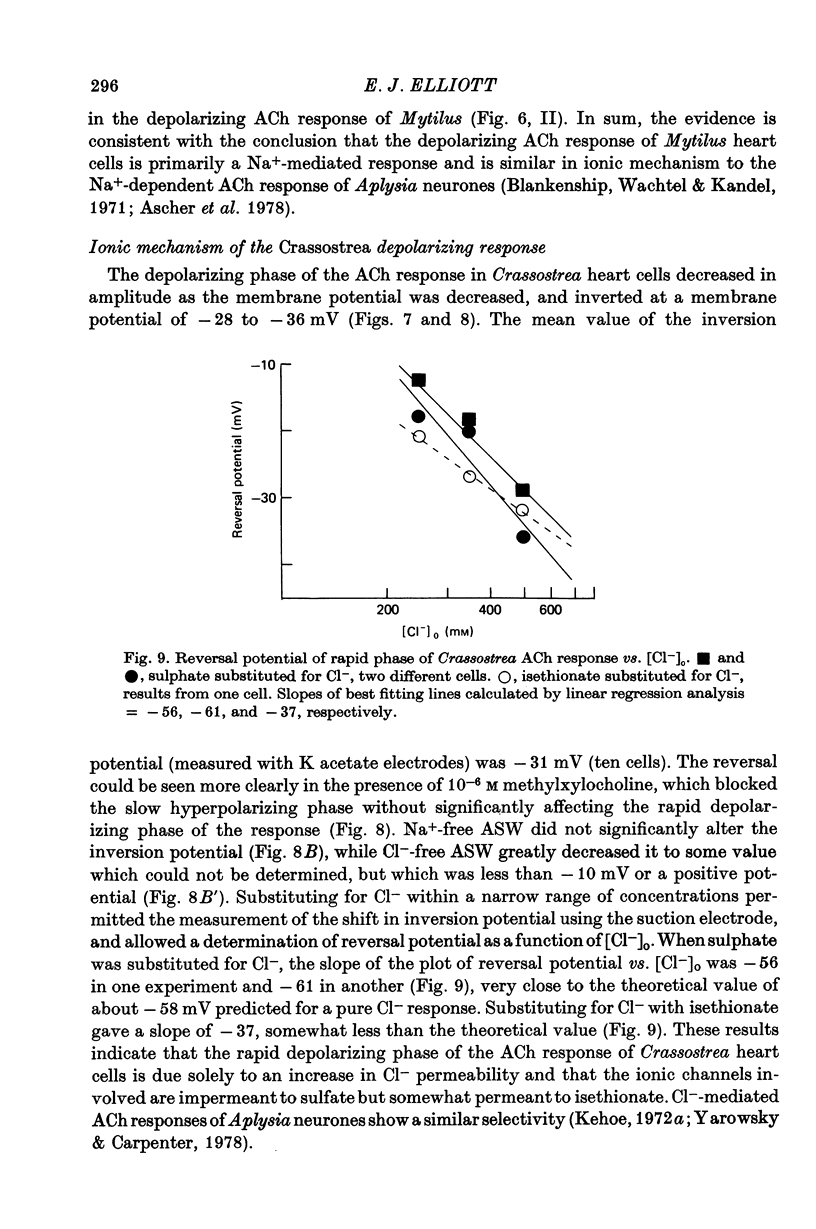
4. The hyperpolarizing response in all three species was not altered by Cl--free or Na+-free sea water, but was affected by changes in external K+. The rate of change of the inversion potential of this response with change in [K+]o was 59 mV per tenfold concentration change. The depolarizing response of Mytilus was not altered by changes in external Cl- or K+, but was abolished in Na+-free sea water. The depolarizing response of Crassostrea was insensitive to external Na+ or K+, inverted at -31 mV in normal sea-water, and was altered by changes in [Cl-]o. Substituting for Cl- with sulphate shifted the inversion potential at a rate of 56-61 mV per tenfold Cl- concentration change.
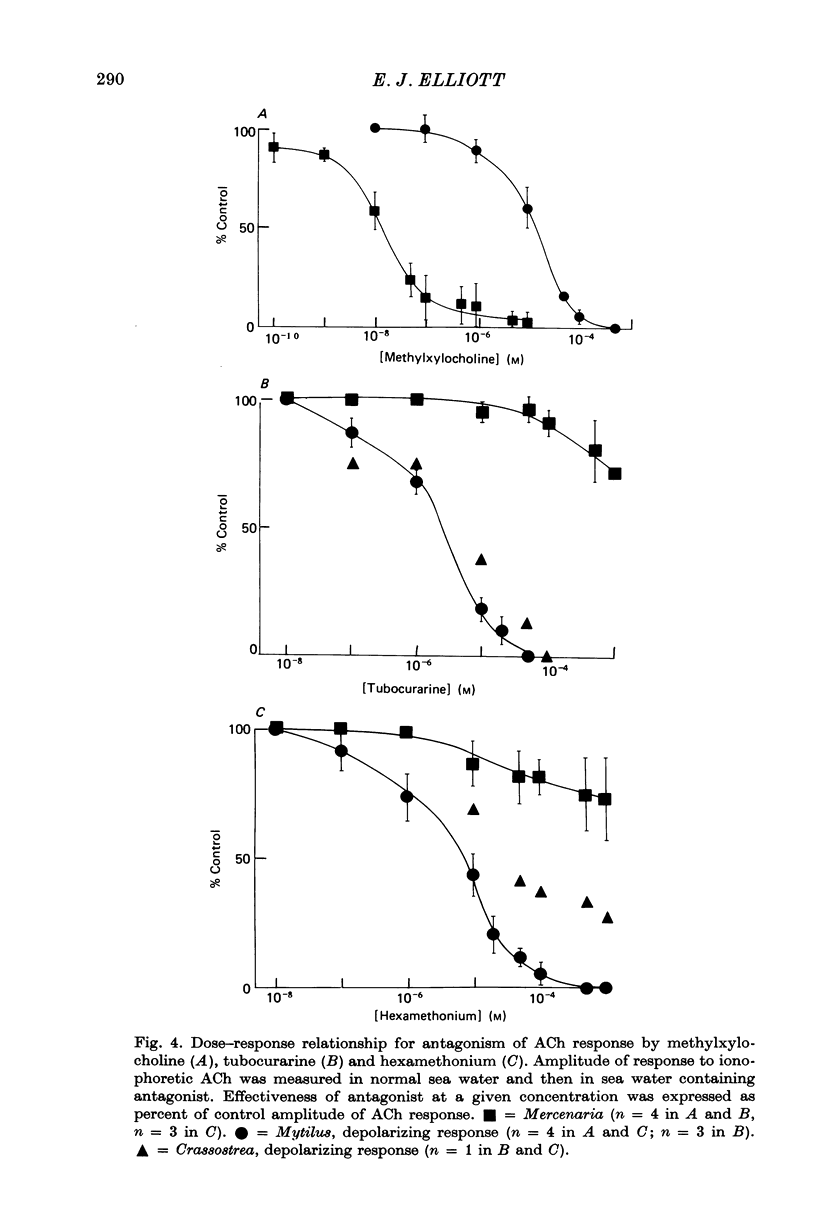
5. The three types of ACh response seen in bivalve heart muscle are similar with respect to time course, pharmacological sensitivity and ionic mechanism to the three types of ACh response described in Aplysia central neurones. Analogies can also be drawn with vertebrate ACh responses.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ascher P., Marty A., Neild T. O. Life time and elementary conductance of the channels mediating the excitatory effects of acetylcholine in Aplysia neurones. J Physiol. 1978 May;278:177–206. doi: 10.1113/jphysiol.1978.sp012299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship J. E., Wachtel H., Kandel E. R. Ionic mechanisms of excitatory, inhibitory, and dual synaptic actions mediated by an identified interneuron in abdominal ganglion of Aplysia. J Neurophysiol. 1971 Jan;34(1):76–92. doi: 10.1152/jn.1971.34.1.76. [DOI] [PubMed] [Google Scholar]
- Bolton T. B. On the latency and form of the membrane responses of smooth muscle to the iontophoretic application of acetylcholine or carbachol. Proc R Soc Lond B Biol Sci. 1976 Aug 27;194(1114):99–119. doi: 10.1098/rspb.1976.0068. [DOI] [PubMed] [Google Scholar]
- Elliott E. J., Kehoe J. Cholinergic receptor in Aplysia neurons: activation by a venom component from the marine snail Conus californicus. Brain Res. 1978 Nov 10;156(2):387–390. doi: 10.1016/0006-8993(78)90525-5. [DOI] [PubMed] [Google Scholar]
- Ellis K. O., Carpenter J. F. Studies on the mechanism of action of dantrolene sodium. A skeletal muscle relaxant. Naunyn Schmiedebergs Arch Pharmacol. 1972;275(1):83–94. doi: 10.1007/BF00505069. [DOI] [PubMed] [Google Scholar]
- GREENBERG M. J. A COMPENDIUM OF RESPONSES OF BIVALVE HEARTS TO ACETYLCHOLINE. Comp Biochem Physiol. 1965 Mar;14:513–539. doi: 10.1016/0010-406x(65)90224-0. [DOI] [PubMed] [Google Scholar]
- Gallagher J. P., Higashi H., Nishi S. Characterization and ionic basis of GABA-induced depolarizations recorded in vitro from cat primary afferent neurones. J Physiol. 1978 Feb;275:263–282. doi: 10.1113/jphysiol.1978.sp012189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ger B. A., Katchman A. N., Zeimal E. V. The slow potassium-dependent acetylcholine current in isolated molluscan neurone: its time course and temperature dependence. Brain Res. 1979 Aug 3;171(2):355–359. doi: 10.1016/0006-8993(79)90342-1. [DOI] [PubMed] [Google Scholar]
- Ger B. A., Zeimal E. V. Pharmacological study of two kinds of cholinoreceptors on the membrane of identified completely isolated neurones of Planorbarius corneus. Brain Res. 1977 Jan 31;121(1):131–139. doi: 10.1016/0006-8993(77)90443-7. [DOI] [PubMed] [Google Scholar]
- Gerschenfeld H. M. Chemical transmission in invertebrate central nervous systems and neuromuscular junctions. Physiol Rev. 1973 Jan;53(1):1–119. doi: 10.1152/physrev.1973.53.1.1. [DOI] [PubMed] [Google Scholar]
- Greenberg M. J. A comparison of acetylcholine structure-activity relations on the hearts of bivalve molluscs. Comp Biochem Physiol. 1970 Mar 15;33(2):259–294. doi: 10.1016/0010-406x(70)90349-x. [DOI] [PubMed] [Google Scholar]
- HOWARTH J. V. The behaviour of frog muscle in hypertonic solutions. J Physiol. 1958 Nov 10;144(1):167–175. doi: 10.1113/jphysiol.1958.sp006093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell H. C., Kuffler S. W., Stickgold R., Yoshikami D. Synaptic excitation and inhibition resulting from direct action of acetylcholine on two types of chemoreceptors on individual amphibian parasympathetic neurones. J Physiol. 1977 Oct;271(3):817–846. doi: 10.1113/jphysiol.1977.sp012027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidaka T., Twarog B. M. Neurotransmitter action on the membrane of Mytilus smooth muscle--I. Acetylcholine. Gen Pharmacol. 1977;8(2):83–86. doi: 10.1016/0306-3623(77)90032-5. [DOI] [PubMed] [Google Scholar]
- Irisawa H., Noma A., Ueda R. Effect of calcium on the spontaneous activities of the oyster myocardium in sodium free solution. Jpn J Physiol. 1968 Apr 15;18(2):157–168. doi: 10.2170/jjphysiol.18.157. [DOI] [PubMed] [Google Scholar]
- Irisawa H., Shigeto N., Otani M. Effect of Na+ and Ca2+ on the excitation of the Mytilus (bivalve) heart muscle. Comp Biochem Physiol. 1967 Oct;23(1):199–212. doi: 10.1016/0010-406x(67)90488-4. [DOI] [PubMed] [Google Scholar]
- Kehoe J. Ionic mechanisms of a two-component cholinergic inhibition in Aplysia neurones. J Physiol. 1972 Aug;225(1):85–114. doi: 10.1113/jphysiol.1972.sp009930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe J., Sealock R., Bon C. Effects of alpha-toxins from Bungarus multicinctus and Bungarus caeruleus on cholinergic responses in Aplysia neurons. Brain Res. 1976 May 14;107(3):527–540. doi: 10.1016/0006-8993(76)90142-6. [DOI] [PubMed] [Google Scholar]
- Kehoe J. The physiological role of three acetylcholine receptors in synaptic transmission in Aplysia. J Physiol. 1972 Aug;225(1):147–172. doi: 10.1113/jphysiol.1972.sp009932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe J. Three acetylcholine receptors in Aplysia neurones. J Physiol. 1972 Aug;225(1):115–146. doi: 10.1113/jphysiol.1972.sp009931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze D. L., Brown A. M. Internal potassium and chloride activities and the effects of acetylcholine on identifiable Aplysia neurones. Nat New Biol. 1971 Feb 24;229(8):229–231. doi: 10.1038/newbio229229a0. [DOI] [PubMed] [Google Scholar]
- Kuwasawa K., Hill R. B. Interaction of inhibitory and excitatory junctional potentials in the control of a myogenic myocardium: the ventricle of Busycon canaliculatum. Experientia. 1972 Jul 15;28(7):800–801. doi: 10.1007/BF01923139. [DOI] [PubMed] [Google Scholar]
- LUDUENA F. P., BROWN T. G., Jr Mytolon and related compounds as antagonists of acetylcholine on the heart of Venus mercenaria. J Pharmacol Exp Ther. 1952 Jun;105(2):232–239. [PubMed] [Google Scholar]
- Ladle R. O., Walker J. L. Intracellular chloride activity in frog heart. J Physiol. 1975 Oct;251(2):549–559. doi: 10.1113/jphysiol.1975.sp011107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitan H., Tauc L. Acetylcholine receptors: topographic distribution and pharmacological properties of two receptor types on a single molluscan neurone. J Physiol. 1972 May;222(3):537–558. doi: 10.1113/jphysiol.1972.sp009813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebeswar G., Goldman J. E., Koester J., Mayeri E. Neural control of circulation in Aplysia. III. Neurotransmitters. J Neurophysiol. 1975 Jul;38(4):767–779. doi: 10.1152/jn.1975.38.4.767. [DOI] [PubMed] [Google Scholar]
- Marty A., Ascher P. Slow relaxations of acetylcholine-induced potassium currents in Aplysia neurones. Nature. 1978 Aug 3;274(5670):494–497. doi: 10.1038/274494a0. [DOI] [PubMed] [Google Scholar]
- Odette L. L., Atwood H. L. Dantrolene sodium: effects on crustacean muscle. Can J Physiol Pharmacol. 1974 Aug;52(4):887–890. doi: 10.1139/y74-113. [DOI] [PubMed] [Google Scholar]
- PILGRIM R. L. The action of acetylcholine on the hearts of lamellibranch molluscs. J Physiol. 1954 Jul 28;125(1):208–214. doi: 10.1113/jphysiol.1954.sp005150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter S. D., Greenberg M. J. Elapid alpha-toxins have no effect on the cholinergic responses of bivalve myocardia. Experientia. 1978 Dec 15;34(12):1608–1609. doi: 10.1007/BF02034704. [DOI] [PubMed] [Google Scholar]
- Purves R. D. Muscarinic excitation: a microelectrophoretic study on cultured smooth muscle cells. Br J Pharmacol. 1974 Sep;52(1):77–86. doi: 10.1111/j.1476-5381.1974.tb09689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeto N. Excitatory and inhibitory actions of acetylcholine on hearts of oyster and mussel. Am J Physiol. 1970 Jun;218(6):1773–1779. doi: 10.1152/ajplegacy.1970.218.6.1773. [DOI] [PubMed] [Google Scholar]
- TAUC L., GERSCHENFELD H. M. A cholinergic mechanism of inhibitory synaptic transmission in a molluscan nervous system. J Neurophysiol. 1962 Mar;25:236–262. doi: 10.1152/jn.1962.25.2.236. [DOI] [PubMed] [Google Scholar]
- Tomita T. Electrophysiology of mammalian smooth muscle. Prog Biophys Mol Biol. 1975;30(2-3):185–203. doi: 10.1016/0079-6107(76)90009-2. [DOI] [PubMed] [Google Scholar]
- WELSH J. H., TAUB R. Structure-activity relationships of acetylcholine and quaternary ammonium ions. J Pharmacol Exp Ther. 1950 Jul;99(3):334–342. [PubMed] [Google Scholar]
- WELSH J. H., TAUB R. The action of acetylcholine antagonists on the heart of Venus mercenaria. Br J Pharmacol Chemother. 1953 Sep;8(3):327–333. doi: 10.1111/j.1476-5381.1953.tb00802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts J. A., Pierce S. K. Acetylcholinesterase: a useful marker for the isolation of sarcolemma from the bivalve (Modiolus demissus demissus) myocardium. J Cell Sci. 1978 Dec;34:193–208. doi: 10.1242/jcs.34.1.193. [DOI] [PubMed] [Google Scholar]
- Wildenthal K., Adcock R. C., Crie J. S., Templeton G. H., Willerson J. T. Negative inotropic influence of hyperosmotic solutions on cardiac muscle. Am J Physiol. 1975 Dec;229(6):1505–1509. doi: 10.1152/ajplegacy.1975.229.6.1505. [DOI] [PubMed] [Google Scholar]
- Wilkens L. A. Electrophysiological studies on the heart of the bivalve mollusc, Modiolus demissus. II. Ionic basis of the action potential. J Exp Biol. 1972 Apr;56(2):293–310. doi: 10.1242/jeb.56.2.293. [DOI] [PubMed] [Google Scholar]
- Wilson W. A., Clark M. T., Pellmar T. C. Tris buffer attenuates acetylcholine responses in Aplysia neurons. Science. 1977 Apr 22;196(4288):440–441. doi: 10.1126/science.15317. [DOI] [PubMed] [Google Scholar]
- Yarowsky J., Carpenter D. O. A comparison of similar ionic responses to gamma-aminobutyric acid and acetylcholine. J Neurophysiol. 1978 May;41(3):531–541. doi: 10.1152/jn.1978.41.3.531. [DOI] [PubMed] [Google Scholar]



