Abstract
1. Electrotonic potentials were recorded from the superficial smooth muscle cells of the guinea-pig vas deferens using the method of Abe & Tomita (1968), in response to low-amplitude, long-duration (greater than or equal to 2 sec) pulses. 2. Averaging techniques were used to increase the signal/noise ratio, and the intracellularly recorded electrotonic potentials were corrected for extracellular voltage drop across the bath series resistance. 3. Since the length of tissue in the stimulating and recording compartments affects the time course of electrotonic potentials (see Appendix and Bywater & Redman, 1978) the passive membrane properties were measured with known amounts of tissue in these two compartments. 4. The length constant (lambda) was 0.86 mm and the membrane time constant (tau m) 270 msec. 5. Excitatory junction potentials (e.j.p.s) were recorded and averaged in response to field stimulation of intact branches of the hypogastric nerve. The mean time constant of the exponential decay phase of the e.j.p. (288 msec) was similar to the membrane time constant (tau m = 270 msec). 6. As the e.j.p.s showed little change in amplitude or time constant of decay when recorded up to several millimetres from the stimulating electrode it was assumed that the tissue was isopotential during the e.j.p., and an estimate was made of the time course of the underlying junctional current. 7. The estimated time course of the junctional current during an e.j.p. was similar to the observed time course of a spontaneous junction potential (s.e.j.p.). 8. As the time course of the junctional current during an s.e.j.p.is similar to the time course of the potential change it is likely that the factors which determine the time current underlying the s.e.j.p. also determine the time course of the e.j.p. current.
Full text
PDF



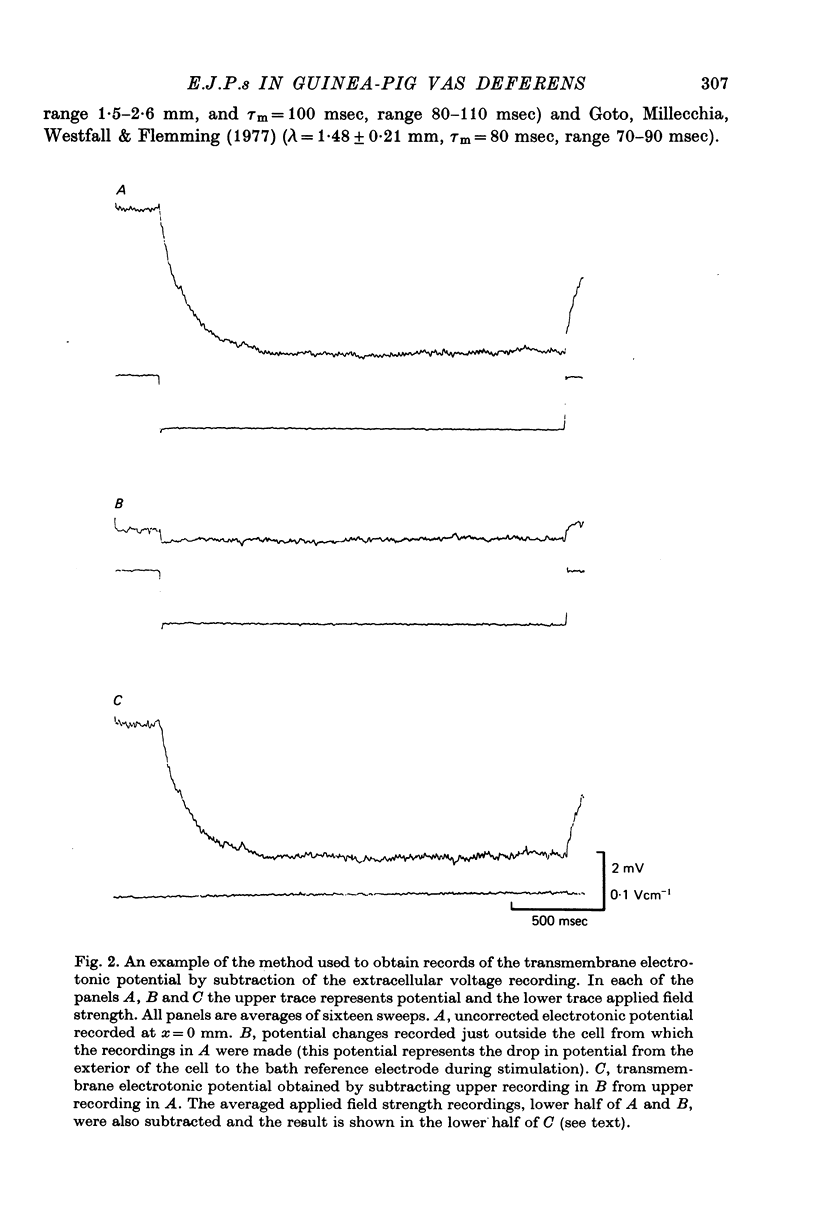


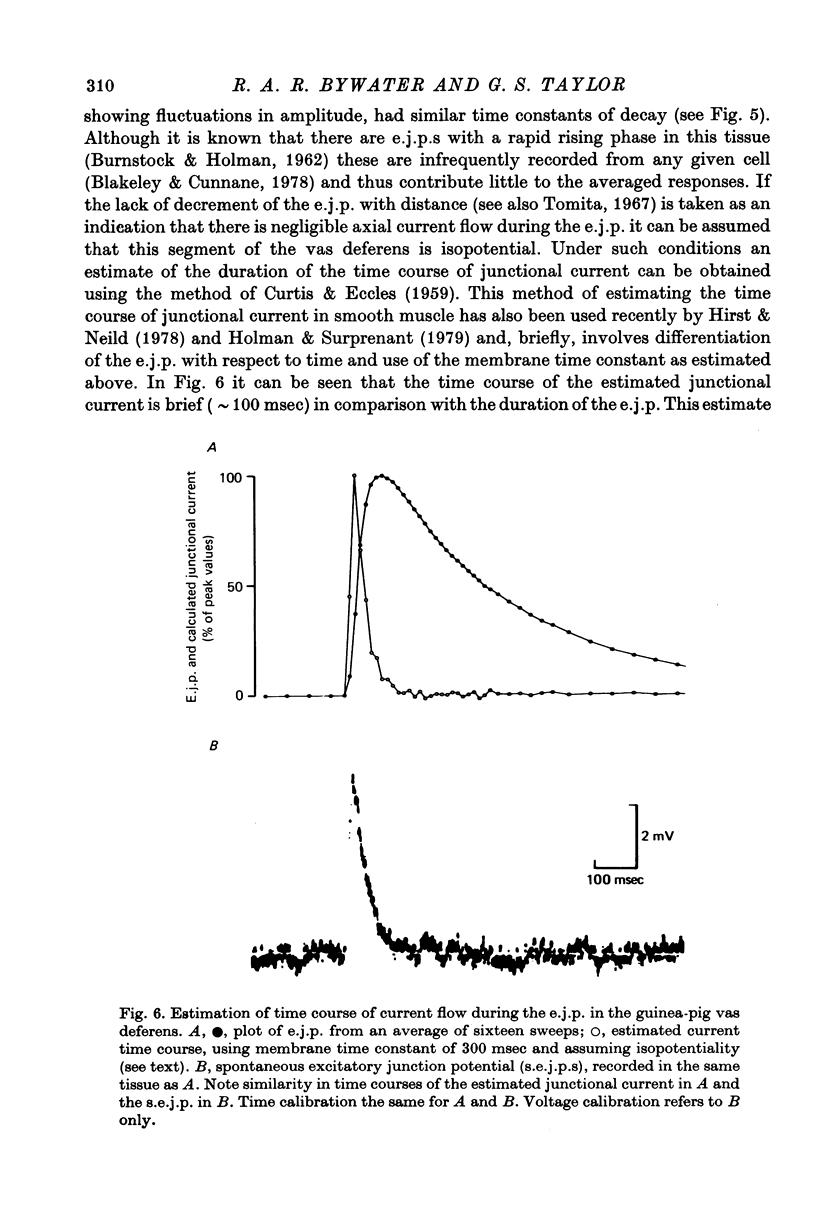



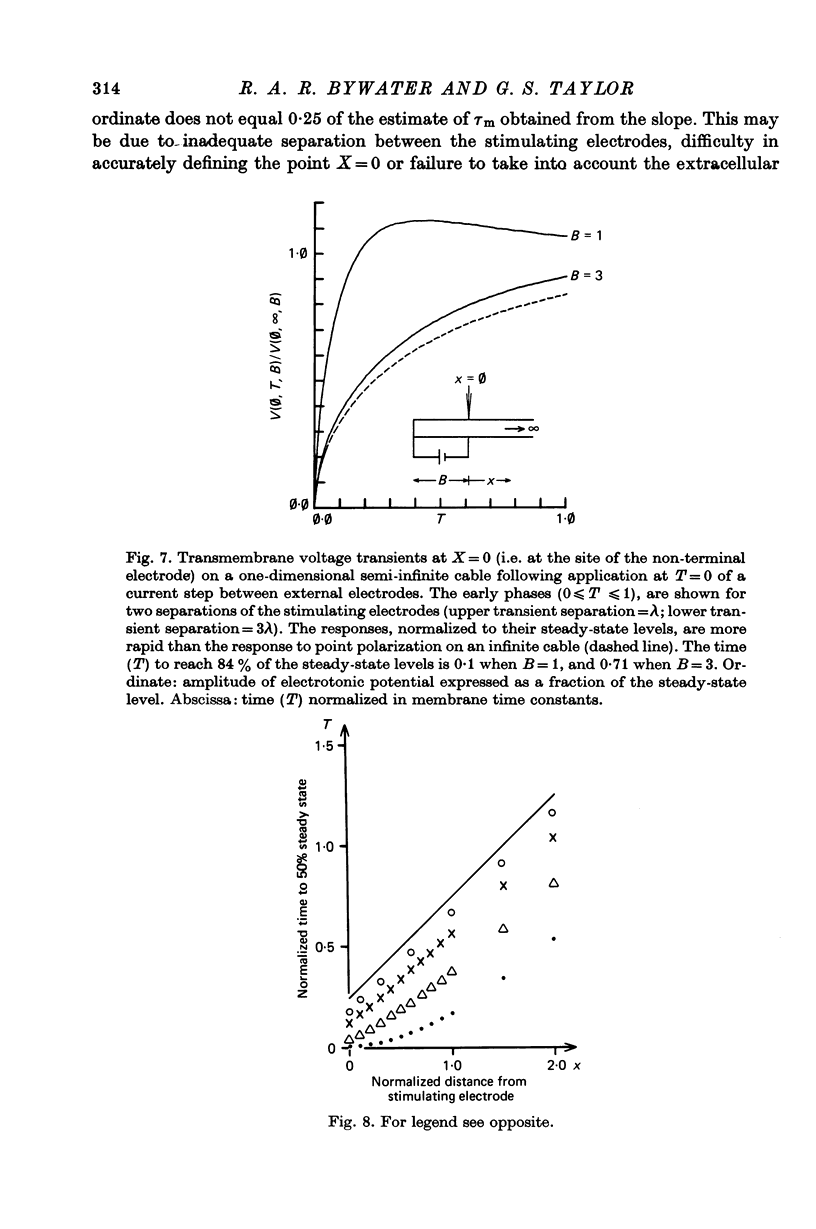


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe Y., Tomita T. Cable properties of smooth muscle. J Physiol. 1968 May;196(1):87–100. doi: 10.1113/jphysiol.1968.sp008496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNSTOCK G., HOLMAN M. E. Spontaneous potential at sympathetic nerve endings in smooth muscle. J Physiol. 1962 Mar;160:446–460. doi: 10.1113/jphysiol.1962.sp006858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Middleton J. An electrophysiological analysis of the effects of amine-uptake blockers and alpha-adrenoceptor blockers on adrenergic neuromuscular transmission. Br J Pharmacol. 1975 Sep;55(1):87–95. doi: 10.1111/j.1476-5381.1975.tb07615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R. Structure and electrical properties of the autonomic neuromuscular junction. Philos Trans R Soc Lond B Biol Sci. 1973 Mar 15;265(867):25–34. doi: 10.1098/rstb.1973.0006. [DOI] [PubMed] [Google Scholar]
- Bennett M. R. The effect of intracellular current pulses in smooth muscle cells of the guinea pig vas deferens at rest and during transmission. J Gen Physiol. 1967 Nov;50(10):2459–2475. doi: 10.1085/jgp.50.10.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURTIS D. R., ECCLES J. C. The time courses of excitatory and inhibitory synaptic actions. J Physiol. 1959 Mar 12;145(3):529–546. doi: 10.1113/jphysiol.1959.sp006159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., JAEGER J. C. The relationship between the mode of operation and the dimensions of the junctional regions at synapses and motor end-organs. Proc R Soc Lond B Biol Sci. 1958 Jan 1;148(930):38–56. doi: 10.1098/rspb.1958.0003. [DOI] [PubMed] [Google Scholar]
- Furness J. B. The effect of external potassium ion concentration on autonomic neuro-muscular transmission. Pflugers Arch. 1970;317(4):310–326. doi: 10.1007/BF00586580. [DOI] [PubMed] [Google Scholar]
- Goto K., Millecchia L. L., Westfall D. P., Fleming W. W. A comparison of the electrical properties and morphological characteristics of the smooth muscle of the rat and guinea-pig vas deferens. Pflugers Arch. 1977 Apr 25;368(3):253–261. doi: 10.1007/BF00585204. [DOI] [PubMed] [Google Scholar]
- Hirst G. D., Neild T. O. An analysis of excitatory junctional potentials recorded from arterioles. J Physiol. 1978 Jul;280:87–104. doi: 10.1113/jphysiol.1978.sp012374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman M. E., Surprenant A. M. Some properties of the excitatory junction potentials recorded from saphenous arteries of rabbits. J Physiol. 1979 Feb;287:337–351. doi: 10.1113/jphysiol.1979.sp012663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljung B. Nervous and myogenic mechanisms in the control of a vascular neuroeffector system. Acta Physiol Scand Suppl. 1970;349:33–68. [PubMed] [Google Scholar]
- OGSTON A. G. Removal of acetylcholine from a limited volume by diffusion. J Physiol. 1955 Apr 28;128(1):222–223. doi: 10.1113/jphysiol.1955.sp005300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita T. Current spread in the smooth muscle of the guinea-pig vas deferens. J Physiol. 1967 Mar;189(1):163–176. doi: 10.1113/jphysiol.1967.sp008161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita T. Electrical responses of smooth muscle to external stimulation in hypertonic solution. J Physiol. 1966 Mar;183(2):450–468. doi: 10.1113/jphysiol.1966.sp007876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita T. Electrophysiology of mammalian smooth muscle. Prog Biophys Mol Biol. 1975;30(2-3):185–203. doi: 10.1016/0079-6107(76)90009-2. [DOI] [PubMed] [Google Scholar]


