Abstract
1. A method is described for the isolation and vascular perfusion in vitro of the mandibular gland of the rabbit. The perfusate is a physiological salt solution containing glucose as the only metabolic substrate.
2. During perfusion with solutions containing acetylcholine, the gland secretes vigorously at a rate and in a manner similar to that seen in vivo. Although the gland becomes oedematous during perfusion, the extent of this oedema appears to have no influence on secretory ability: the perfused glands were capable of functioning for at least 4 h, and often for more than 6 h.
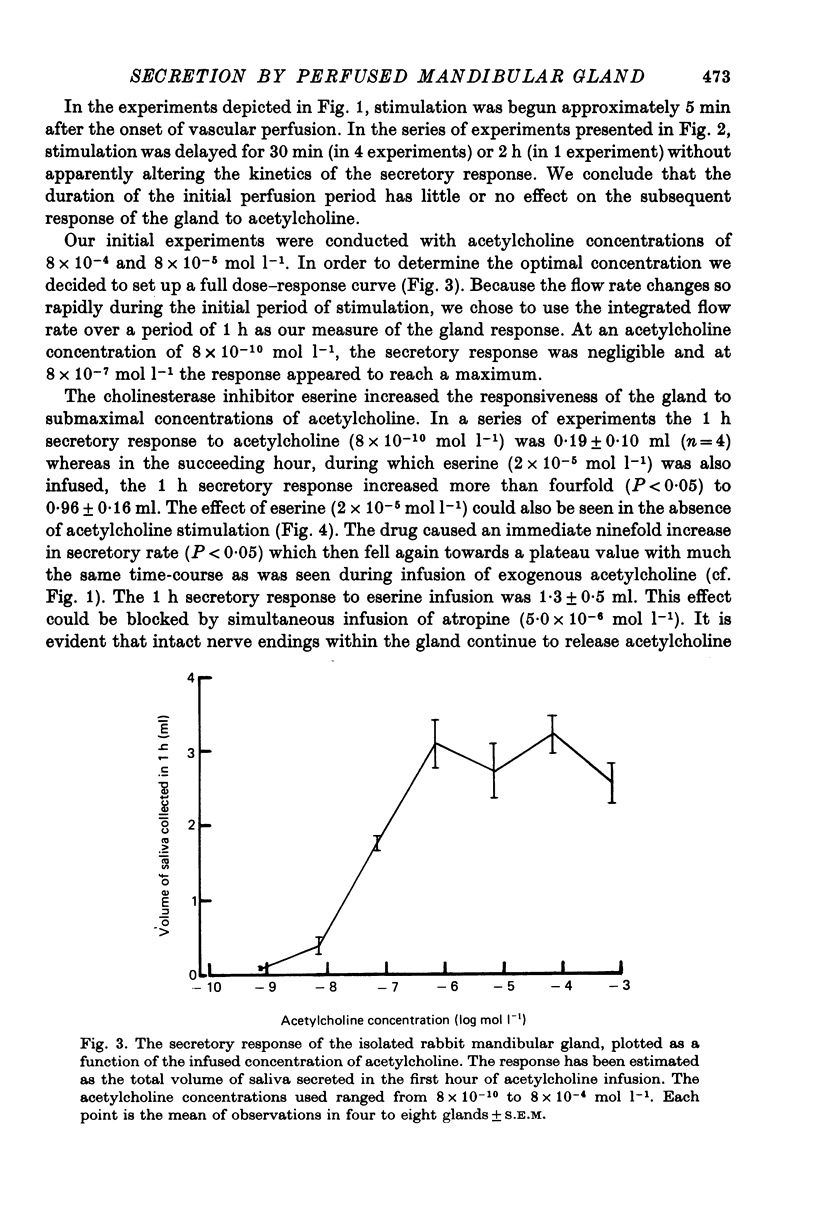
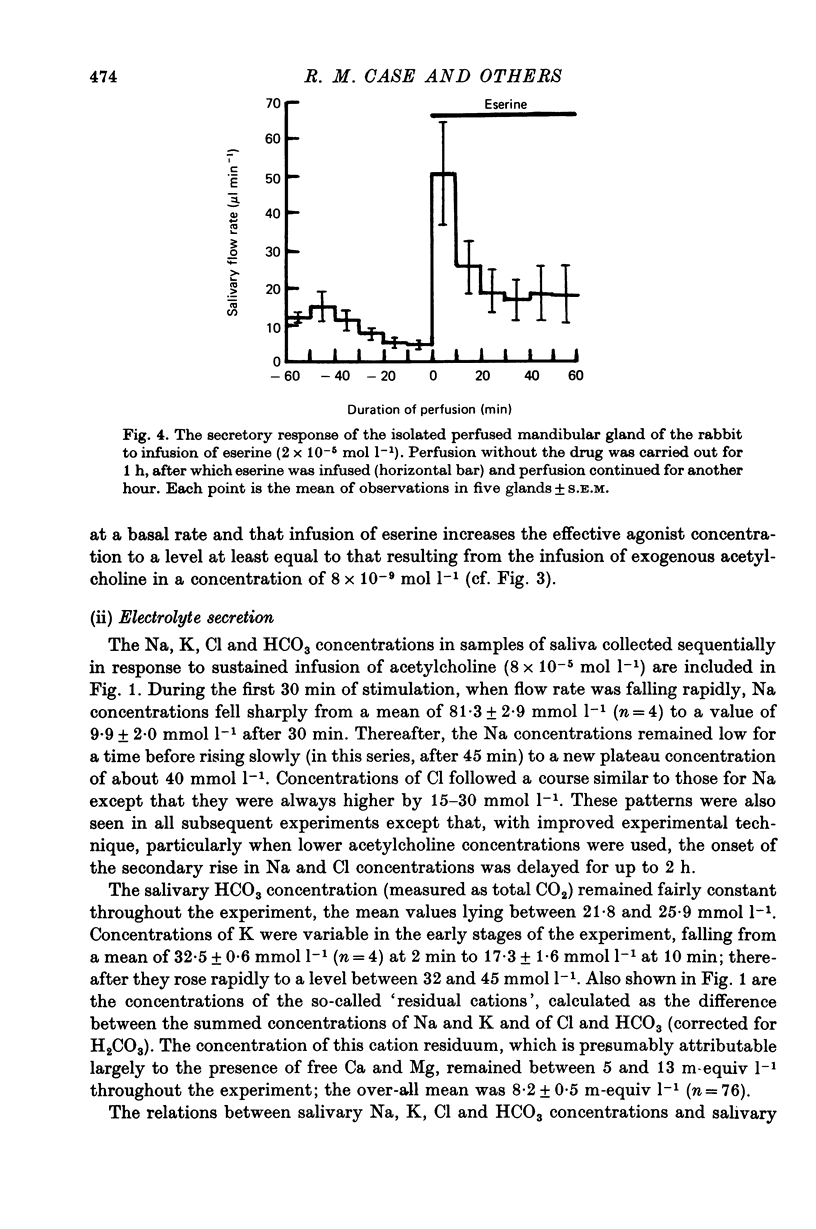
3. Acetylcholine evoked a small secretory response at a concentration of 8 × 10-9 mol l-1 and a maximum response at 8 × 10-7 mol l-1. Eserine (2 × 10-5 mol l-1) evoked secretory responses comparable to those evoked by acetylcholine in a concentration of 8 × 10-9 mol l-1. Secretion, whether unstimulated or evoked by acetylcholine or eserine, could be blocked completely by atropine.
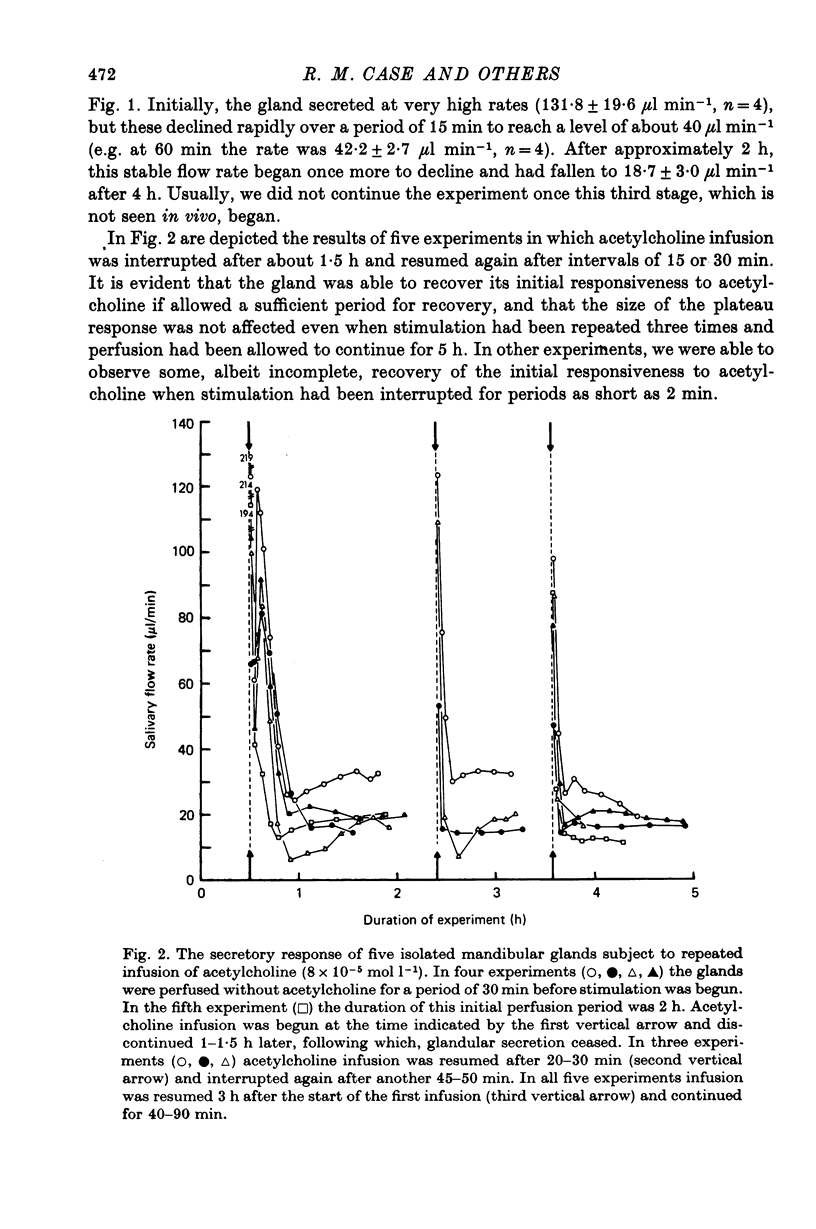
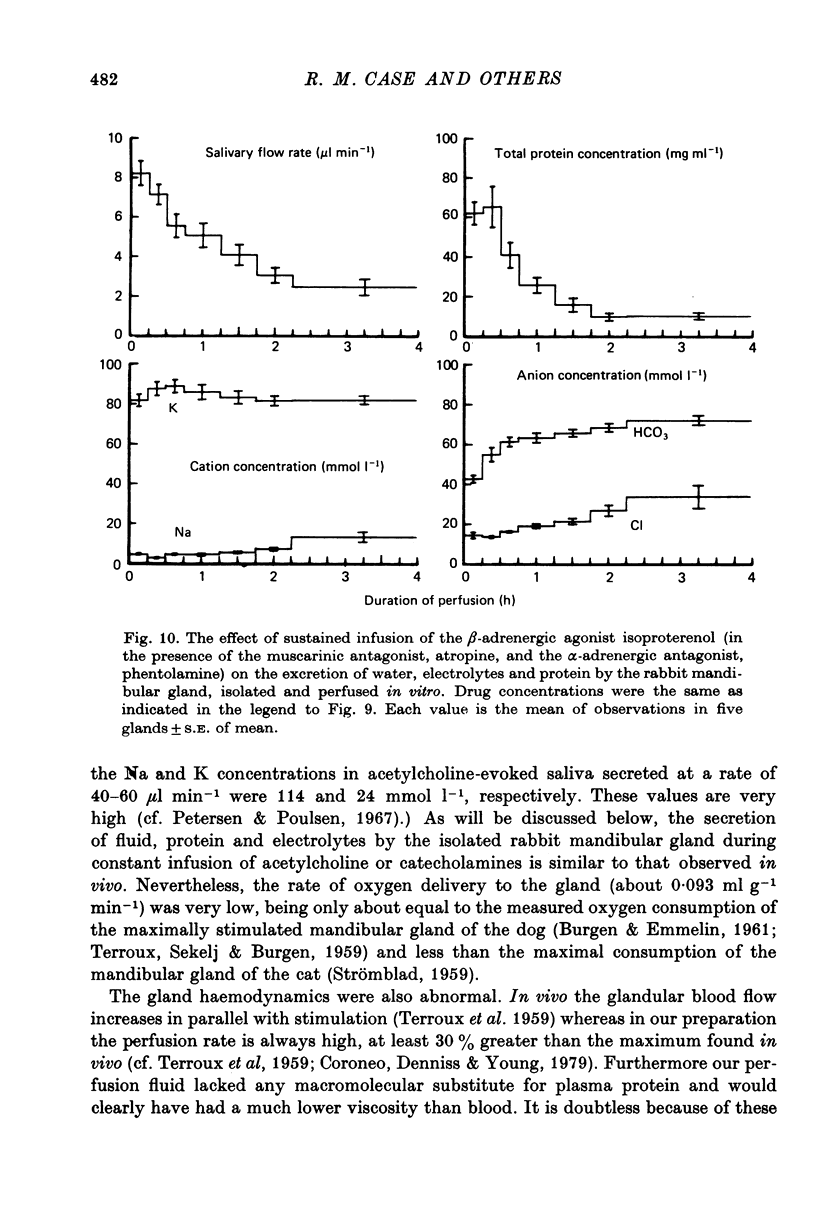
4. During prolonged stimulation with acetylcholine, the fluid secretory response declined rapidly over a period of about 15 min from an initial high value to a much lower plateau value. After 3 or more hours of stimulation, the secretory response began once more to decline, this time towards zero. If, before the second period of decline begins, stimulation is interrupted for about 30 min, the gland recovers its initial responsiveness to further stimulation with acetylcholine.
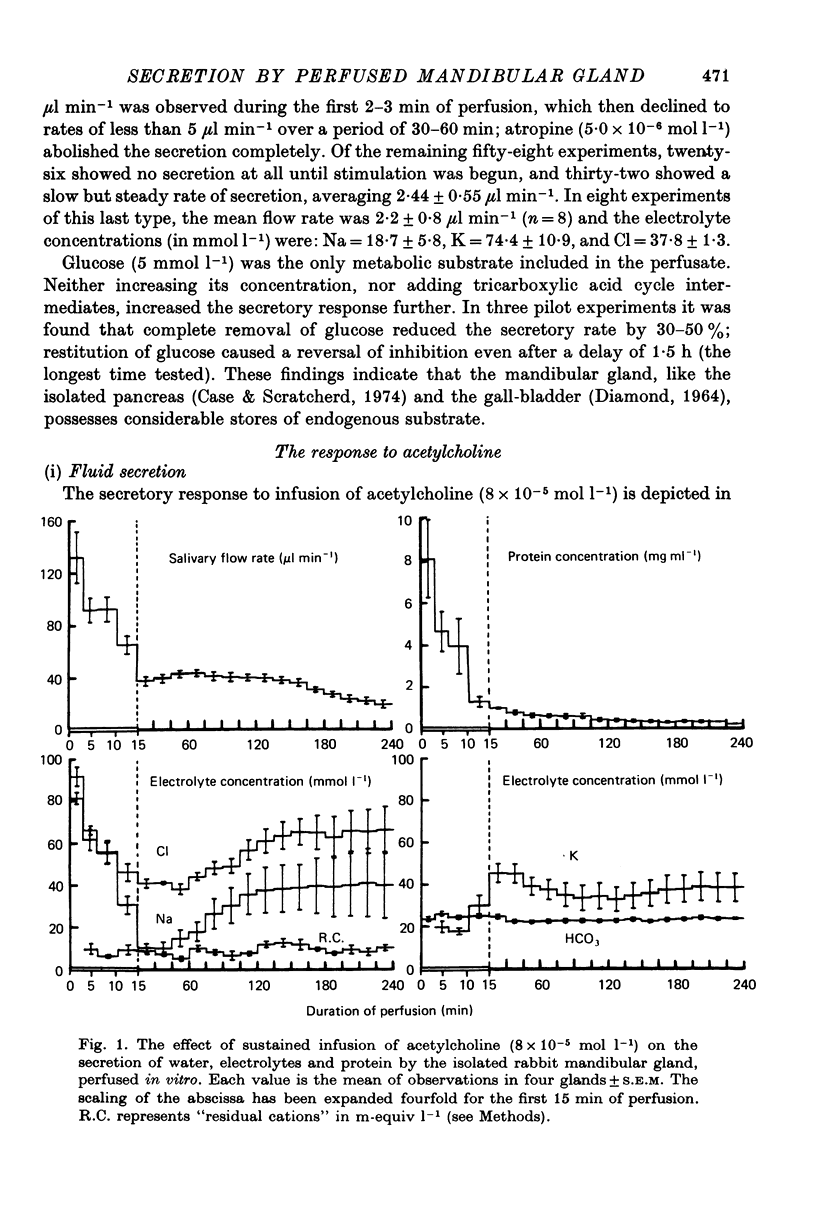
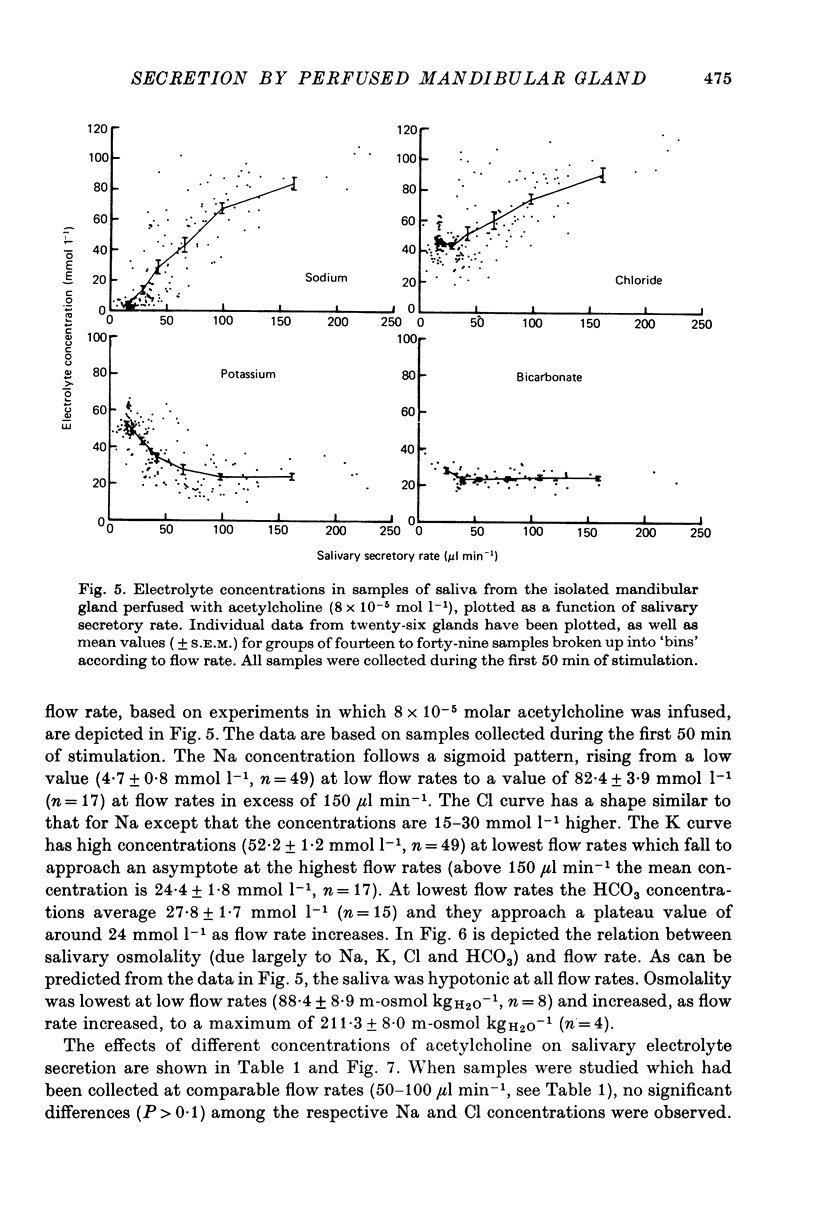
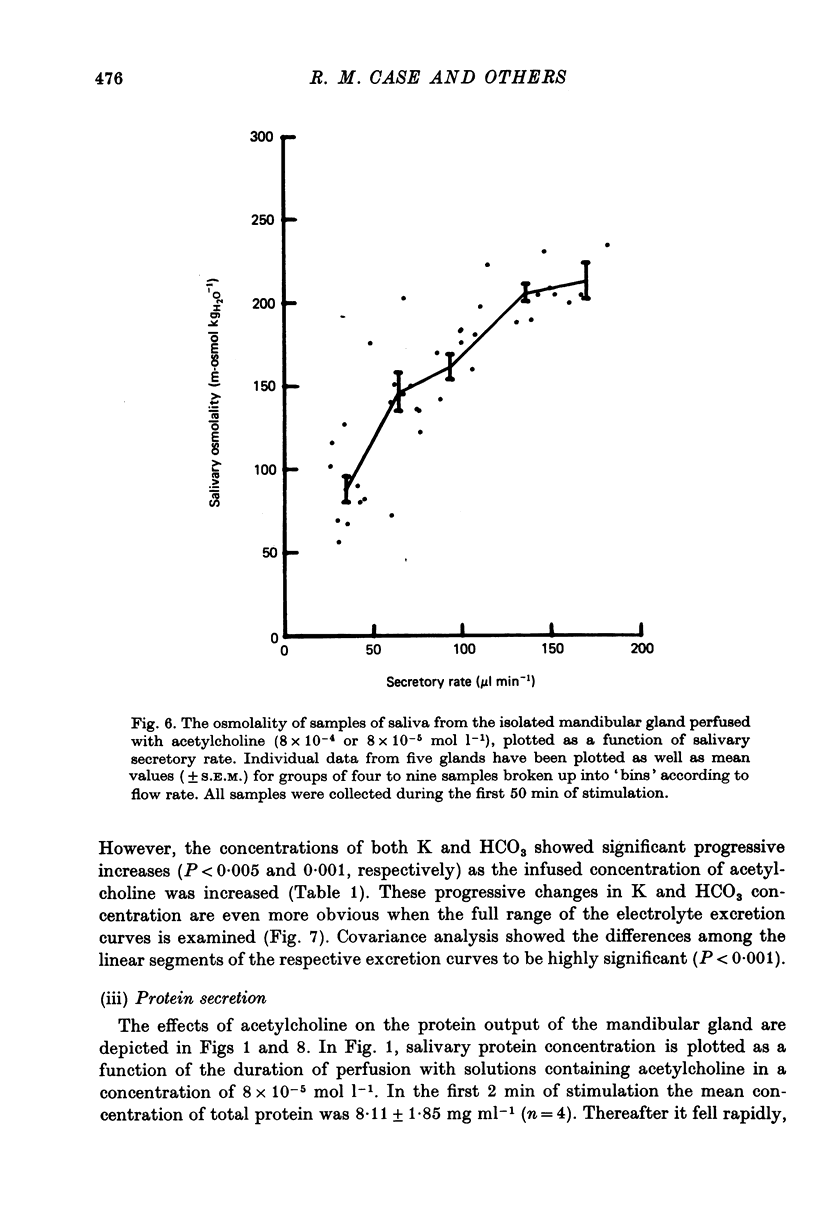
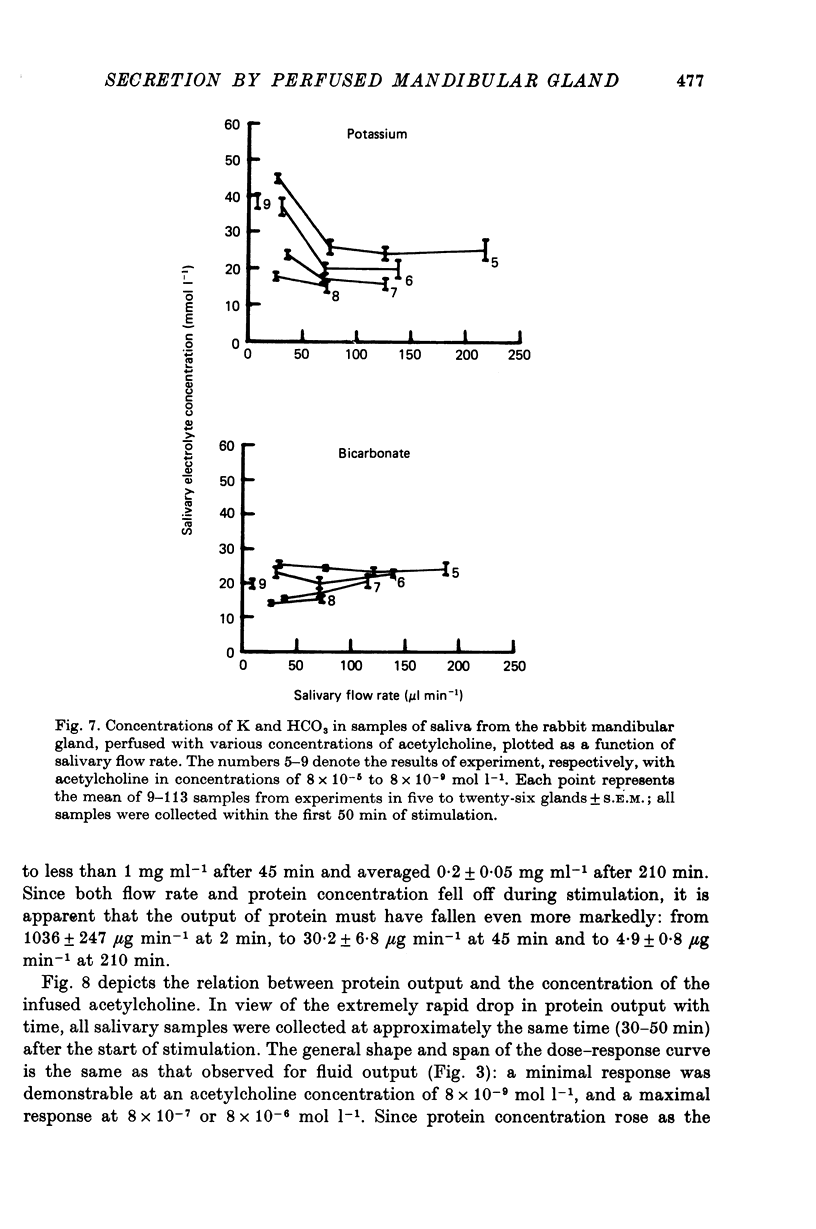
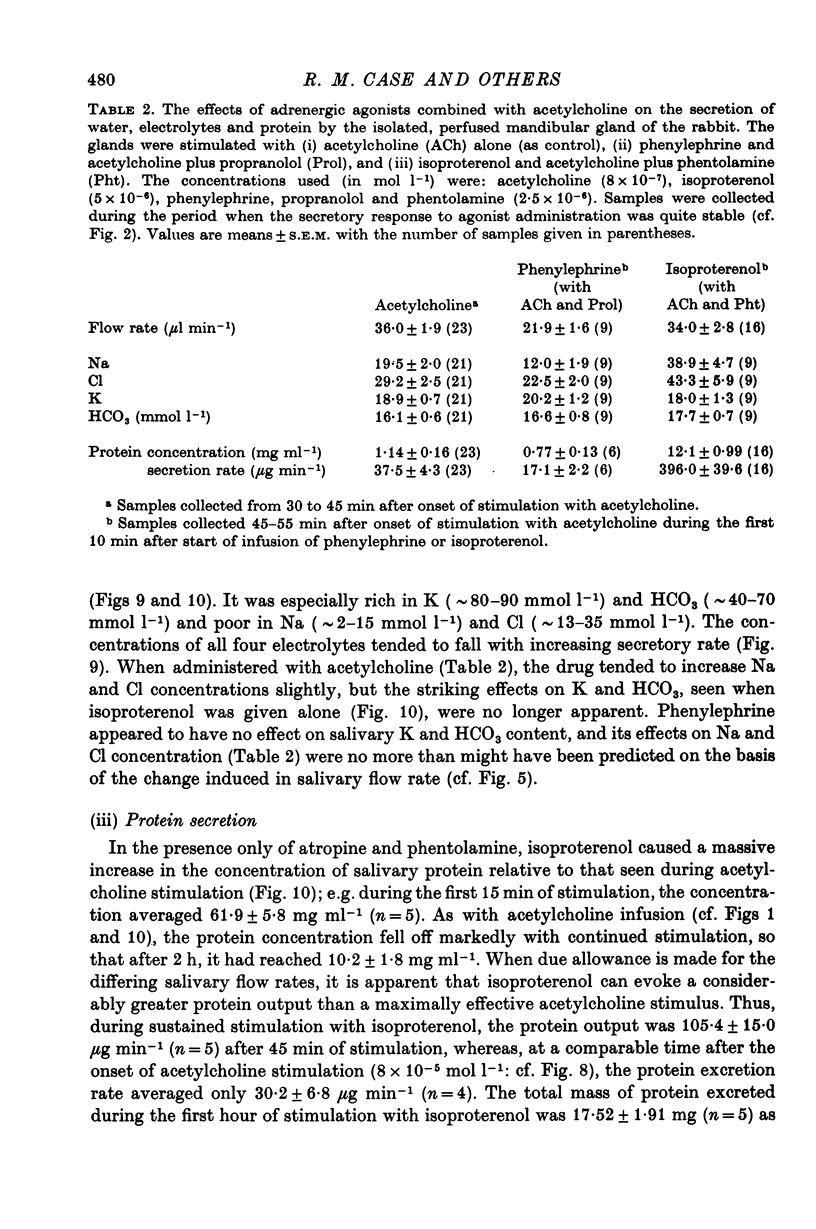
5. The Na, K, Cl and HCO3 concentrations and the osmolality of acetylcholine evoked saliva exhibited flow-dependency similar to that seen in vivo. The concentrations of Na and Cl, but not K and HCO3, increased by about 25 mmol l-1 during periods of prolonged stimulation with acetylcholine even though the salivary secretory rate was constant. The concentrations of K and HCO3, but not Na and Cl, increased progressively as the concentration of infused acetylcholine was increased.
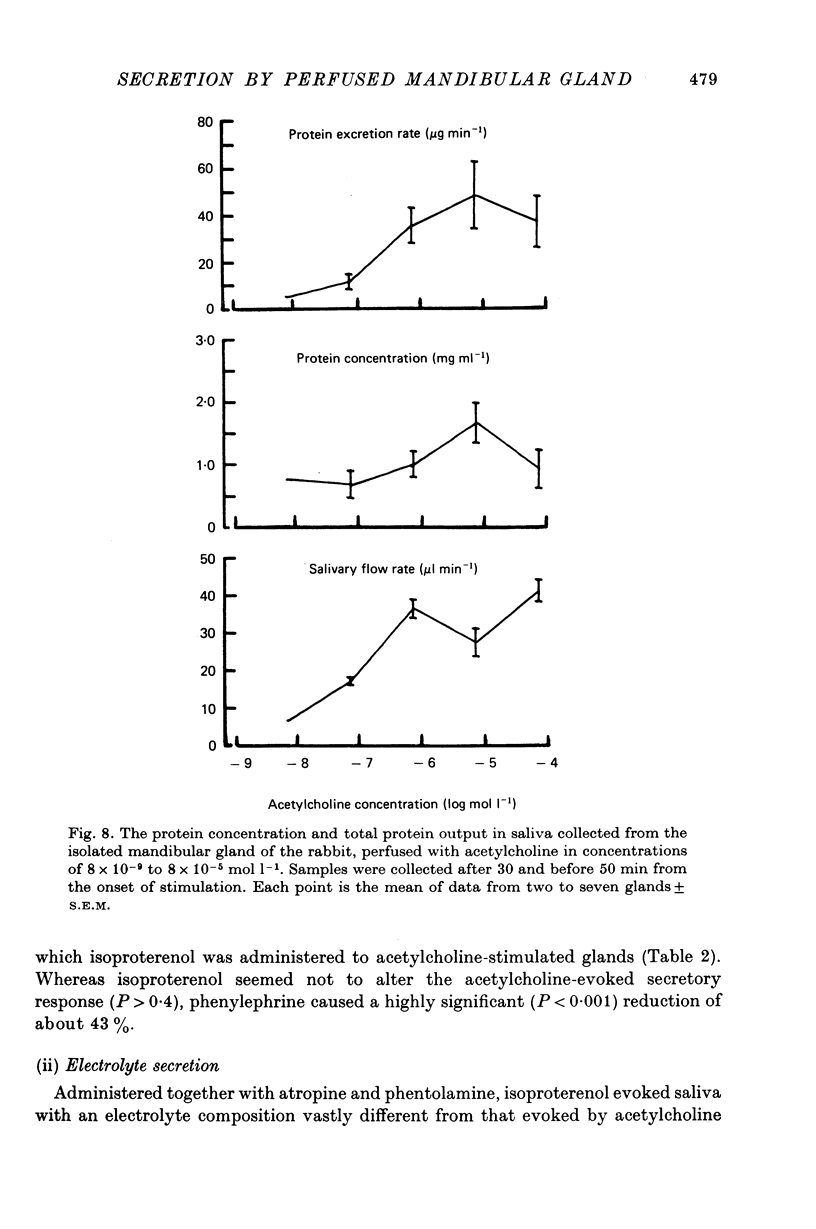
6. Salivary protein secretion increased with increasing concentrations of acetylcholine to a greater extent than did fluid secretion. During continuous stimulation, the rate of protein secretion fell off much faster than the rate of fluid secretion.
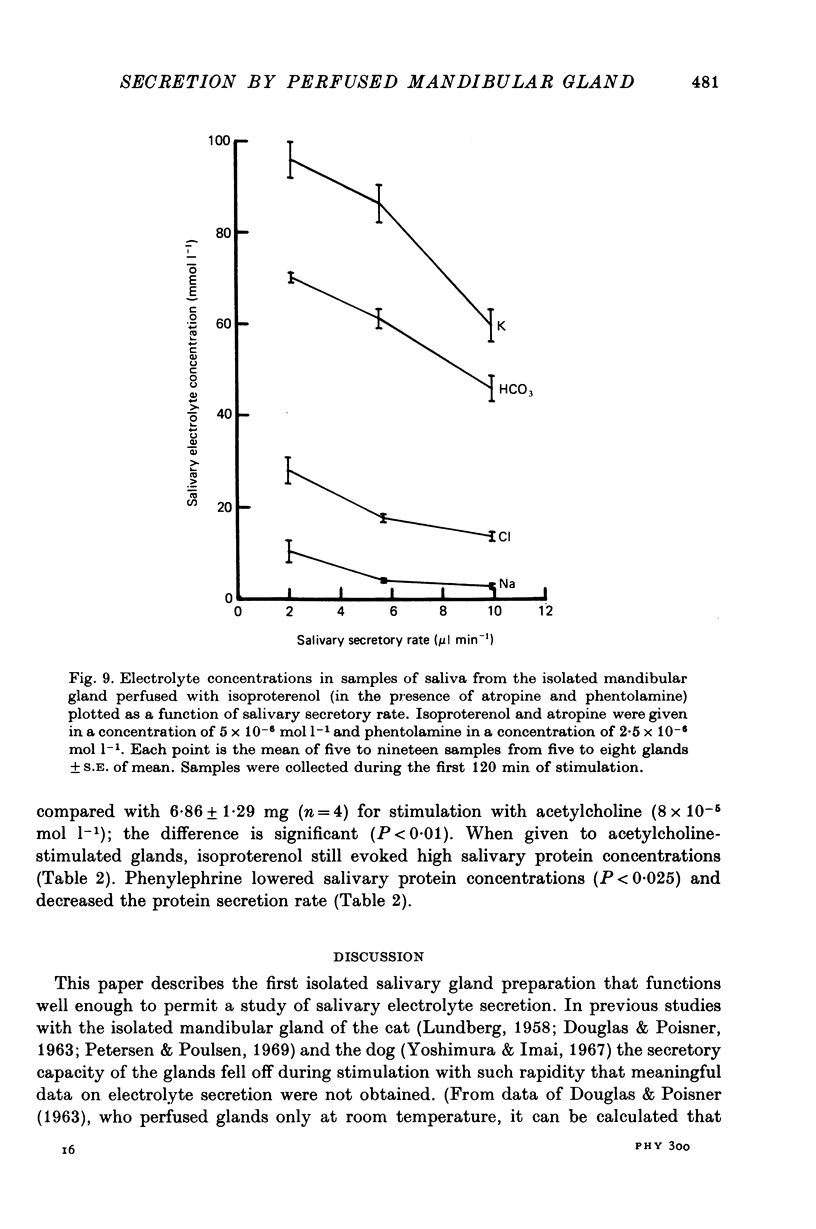
7. The β-adrenergic agonist isoproterenol evoked a fluid secretory response only equal to about 5% of that evoked by acetylcholine, but still the response declined during continued stimulation. The electrolyte composition of isoproterenol-evoked saliva was vastly different from that evoked by acetylcholine, being particularly rich in K and HCO3. The isoproterenol-evoked saliva was also extremely rich in protein so that the total protein secretion evoked by isoproterenol was much greater than that evoked by acetylcholine.
8. The α-adrenergic agonist phenylephrine was without stimulatory effect on salivary fluid secretion and caused a reduction in the secretory response to acetylcholine. The drug had little or no effect on the electrolyte content of acetylcholine-evoked saliva and appeared to reduce its protein content.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anrep G. V. The metabolism of the salivary glands: I. The relation of the chorda tympani to the nitrogen metabolism of the submaxillary gland. J Physiol. 1921 Mar 15;54(5-6):319–331. doi: 10.1113/jphysiol.1921.sp001932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. The interaction of cyclic nucleotides and calcium in the control of cellular activity. Adv Cyclic Nucleotide Res. 1975;6:1–98. [PubMed] [Google Scholar]
- Case R. M., Scratcherd T. The secretion of alkali metal ions by the perfused cat pancreas as influenced by the composition and osmolality of the external environment and by inhibitors of metabolism and Na+, K+-ATPase activity. J Physiol. 1974 Oct;242(2):415–428. doi: 10.1113/jphysiol.1974.sp010715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coroneo M. T., Denniss A. R., Young J. A. The action of physalaemin on electrolyte excretion by the mandibular and sublingual salivary glands of the rat. Pflugers Arch. 1979 Sep;381(3):223–230. doi: 10.1007/BF00583253. [DOI] [PubMed] [Google Scholar]
- DIAMOND J. M. TRANSPORT OF SALT AND WATER IN RABBIT AND GUINEA PIG GALL BLADDER. J Gen Physiol. 1964 Sep;48:1–14. doi: 10.1085/jgp.48.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Poisner A. M. The influence of calcium on the secretory response of the submaxillary gland to acetylcholine or to noradrenaline. J Physiol. 1963 Mar;165(3):528–541. doi: 10.1113/jphysiol.1963.sp007076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inada M., Mello A., De Castro A. L. Influência dos íons cálcio e potássio na resposta secretora da glândula submandibular isolada d perfundida, à estimulaço parassimpática. Rev Bras Pesqui Med Biol. 1976 Dec;9(5-6):265–271. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LUNDBERG A. Electrophysiology of salivary glands. Physiol Rev. 1958 Jan;38(1):21–40. doi: 10.1152/physrev.1958.38.1.21. [DOI] [PubMed] [Google Scholar]
- Mangos J. A., McSherry N. R., Irwin K., Hong R. Handling of water and electrolytes by rabbit parotid and submaxillary glands. Am J Physiol. 1973 Aug;225(2):450–455. doi: 10.1152/ajplegacy.1973.225.2.450. [DOI] [PubMed] [Google Scholar]
- Martin C. J., Frömter E., Gebler B., Knauf H., Young J. A. The effects of carbachol on water and electrolyte fluxes and transepithelial electrical potential differences of the rabbit submaxillary main duct perfused in vitro. Pflugers Arch. 1973 Jun 26;341(2):131–142. doi: 10.1007/BF00587320. [DOI] [PubMed] [Google Scholar]
- Martin C. J., Young J. A. A microperfusion investigation of the effects of a sympathomimetic and a parasympathomimetic drug on water and electrolyte fluxes in the main duct of the rat submaxillary gland. Pflugers Arch. 1971;327(4):303–323. doi: 10.1007/BF00588450. [DOI] [PubMed] [Google Scholar]
- NORDENFELT I., OHLIN P. Supersensitivity of salivary glands of rabbits. Acta Physiol Scand. 1957 Nov 26;41(1):12–17. doi: 10.1111/j.1748-1716.1957.tb01506.x. [DOI] [PubMed] [Google Scholar]
- Petersen O. H., Poulsen J. H. Excretion of sodium and potassium in cat submandibular saliva. Acta Physiol Scand. 1967 Jun;70(2):158–167. doi: 10.1111/j.1748-1716.1967.tb03611.x. [DOI] [PubMed] [Google Scholar]
- Poulsen J. H. Time course of acetylcholine-induced K + release from the perfused cat submandibular gland. Pflugers Arch. 1973 Feb 6;338(3):201–206. doi: 10.1007/BF00587387. [DOI] [PubMed] [Google Scholar]
- STROMBLAD B. C. Gaseous metabolism of the normal and denervated submaxillary gland of the cat. J Physiol. 1959 Mar 12;145(3):551–561. doi: 10.1113/jphysiol.1959.sp006161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneyer C. A., Sucanthapree C., Schneyer L. H. Neural regulation of calcium and amylase of rat parotid saliva (39891). Proc Soc Exp Biol Med. 1977 Oct;156(1):132–135. doi: 10.3181/00379727-156-39891. [DOI] [PubMed] [Google Scholar]
- Schneyer L. H., Young J. A., Schneyer C. A. Salivary secretion of electrolytes. Physiol Rev. 1972 Jul;52(3):720–777. doi: 10.1152/physrev.1972.52.3.720. [DOI] [PubMed] [Google Scholar]
- Smaje L. H. Spontaneous salivation in the rabbit submandibular gland. J Physiol. 1973 May;231(1):179–193. doi: 10.1113/jphysiol.1973.sp010227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprecher R. L., Yoshida Y., Schneyer L. H. Partial isolation and perfusion of rat submaxillary gland. Proc Soc Exp Biol Med. 1967 Aug-Sep;125(4):1227–1229. doi: 10.3181/00379727-125-32320. [DOI] [PubMed] [Google Scholar]
- TERROUX K. G., SEKELJ P., BURGEN A. S. Oxygen consumption and blood flow in the submaxillary gland of the dog. Can J Biochem Physiol. 1959 Jan;37(1):5–15. [PubMed] [Google Scholar]
- Yoshimura H., Imai Y. Studies on the secretory potential of acinal cells of the dog submaxillary gland and its ionic dependency. Jpn J Physiol. 1967 Jun;17(3):280–293. doi: 10.2170/jjphysiol.17.280. [DOI] [PubMed] [Google Scholar]
- Young J. A., Martin C. J. The effect of a sympatho- and a parasympathomimetic drug on the electrolyte concentrations of primary and final saliva of the rat submaxillary gland. Pflugers Arch. 1971;327(4):285–302. doi: 10.1007/BF00588449. [DOI] [PubMed] [Google Scholar]
- Young J. A. Salivary secretion of inorganic electrolytes. Int Rev Physiol. 1979;19:1–58. [PubMed] [Google Scholar]



