Abstract
Heat shock protein 60s (hsp60) are remarkably immunogenic, and both T-cell and antibody responses to hsp60 have been reported in various inflammatory conditions. To clarify the role of hsp60 in T-cell responses in periodontitis, we examined the proliferative response of peripheral blood mononuclear cells (PBMC), as well as the cytokine profile and T-cell clonality, for periodontitis patients and controls following stimulation with recombinant human hsp60 and Porphyromonas gingivalis GroEL. To confirm the infiltration of hsp60-reactive T-cell clones into periodontitis lesions, nucleotide sequences within complementarity-determining region 3 of the T-cell receptor (TCR) β-chain were compared between hsp60-reactive peripheral blood T cells and periodontitis lesion-infiltrating T cells. Periodontitis patients demonstrated significantly higher proliferative responses of PBMC to human hsp60, but not to P. gingivalis GroEL, than control subjects. The response was inhibited by anti-major histocompatibility complex class II antibodies. Analysis of the nucleotide sequences of the TCR demonstrated that human hsp60-reactive T-cell clones and periodontitis lesion-infiltrating T cells have the same receptors, suggesting that hsp60-reactive T cells accumulate in periodontitis lesions. Analysis of the cytokine profile demonstrated that hsp60-reactive PBMC produced significant levels of gamma interferon (IFN-γ) in periodontitis patients, whereas P. gingivalis GroEL did not induce any skewing toward a type1 or type2 cytokine profile. In control subjects no significant expression of IFN-γ or interleukin 4 was induced. These results suggest that periodontitis patients have human hsp60-reactive T cells with a type 1 cytokine profile in their peripheral blood T-cell pools.
Periodontitis is a chronic inflammatory disease characterized by mononuclear cell infiltration into the gingival tissues, leading to connective tissue destruction and alveolar bone resorption. Although periodontal bacteria are the causative agents in periodontitis, subsequent progression and disease severity are thought to be determined by the host immune responses (28). The precise mechanisms of tissue destruction, however, have not been fully elucidated; nevertheless, a number of reports have implicated autoimmune responses in the disease process (2, 5, 12).
Collagen type 1, a major component of the periodontium, has been considered to be one of the target antigens of this autoimmune response due to the fact that high titers of anti-collagen type I antibody are found in the sera (12), and that collagen type I-specific T-cell clones can be identified in the inflamed gingival tissues, of periodontitis patients (38). Heat shock protein 60 (hsp60) has also been suggested as another important candidate antigen. hsp60 belongs to a family of related proteins which have been conserved during evolution. Despite being highly homologous between prokaryotic and eukaryotic cells, hsp60s are strongly immunogenic, and immune responses to microbial hsp60s are speculated to initiate chronic inflammatory diseases in which autoimmune responses to human hsp 60 may be central to pathogenesis (16). Major periodontopathic bacteria such as Porphyromonas gingivalis (13, 19), Actinobacillus actinomycetemcomitans (21), Fusobacterium nucleatum (37), Prevotella intermedia (37), Bacteroides forsythus (37), and Campylobacter rectus (11) are reported to produce hsp's homologous to Escherichia coli GroEL. We have previously demonstrated that the frequency of seropositivity and titers of antibodies to human hsp60 and P. gingivalis GroEL were significantly higher in periodontitis patients than in periodontally healthy control subjects (29). Furthermore, affinity-purified serum antibodies to human hsp60 and P. gingivalis GroEL cross-reacted with P. gingivalis GroEL and human hsp60, respectively. These results suggest that an immune response based on the molecular mimicry between P. gingivalis GroEL and human hsp60 may play a role in periodontitis.
hsp60 has been reported as the dominant microbial antigen for T cells, and in fact, T cells with specificity for mycobacterial hsp60 have been identified in both the human (20) and murine (15) systems. The concept that hsp60 is a dominant antigen in mycobacterial infection has led to the suggestion that hsp's are abundantly produced by the bacteria in order to enhance survival inside the host macrophages and so avoid attack by toxic molecules (42). It has also been reported that self-hsp60 can be recognized by T cells specific for mycobacterial hsp60, suggesting the presence of T cells with specificity for cross-reactive epitopes (20). However, periodontopathic bacteria usually colonize and proliferate extracellularly, and T-cell responses in periodontitis have not been clarified in the context of molecular mimicry between human and periodontopathic bacterial hsp60s.
In order to gain further insight into the role of hsp60 and hsp60-reactive T cells, we investigated the proliferative responses of peripheral blood mononuclear cells (PBMC) from patients with severe periodontitis and from healthy control subjects to bacterial and human hsp60s. Further, the presence of reactive T-cell clones to hsp60 in periodontitis lesions was examined by determining the nucleotide sequences within complementarity-determining region 3 (CDR3) region of the T-cell receptor (TCR) β-chain of hsp60-reactive T-cell clones and comparing them with those of T cells infiltrating the periodontitis lesion. Because the cytokine profile of the reactive T-cell clones is important in determining the type of response, we also analyzed gamma interferon (IFN-γ) and interleukin-4 (IL-4) at both the mRNA and protein levels following stimulation with hsp60s.
MATERIALS AND METHODS
Subjects and specimen collection.
Gingival tissue samples were obtained at the time of periodontal surgery (flap surgery) from 16 patients with moderate to severe periodontitis (mean age, 40.4 ± 9.9 years; range, 26 to 55 years) referred to the periodontal clinic of Niigata University Dental Hospital. All patients were classified as having chronic periodontitis with no systemic disorders. The mean probing depth, probing attachment level, and radiographic bone resorption were 6.5 ± 1.4 mm (range, 4 to 9 mm), 7.5 ± 1.7 mm (range, 4 to 10 mm) and 58.2 ± 27.1% (range, 10 to 100%), respectively. Alveolar bone resorption was measured on the proximal surface of each tooth on a radiograph. The distances from the cemento-enamel junction (CEJ) and alveolar bone ledge (ABL) to the root apex (RA) were measured. Alveolar bone resorption, expressed as percent bone loss, was calculated as (CEJ-to-ABL distance)/(CEJ-to-RA distance) × 100 (26). Informed consent was obtained from all patients before inclusion in the study. Gingival tissue from each specimen was immediately frozen in liquid nitrogen and stored at −80°C until RNA separation. PBMC were separated by Ficoll-Paque (Pharmacia Fine Chemicals, Piscataway, N.J.) density gradient centrifugation from 20 to 30 ml of autologous peripheral blood. PBMC were also separated from 10 periodontally healthy subjects (mean age, 38.0 ± 7.7 years; range, 30 to 49 years) with probing attachment levels of <4 mm and minimal bone resorption at all sites.
Cell culture and proliferation assay.
PBMC were suspended in RPMI 1640 (GIBCO BRL, Grand Island, N.Y.) supplemented with 10% human AB serum (C-Six Diagnostics, Inc., Mequon, Wis.), 20 mM HEPES buffer (GIBCO BRL), 100 U of penicillin/ml, 100 μg of streptomycin (GIBCO BRL)/ml, 2 mM glutamine (GIBCO BRL), and 5 × 10−5 M 2-mercaptoethanol. To determine the optimal concentrations of the recombinant human hsp60 (StressGen Biotechnologies Corp., Victoria, British Columbia, Canada) and P. gingivalis GroEL, the latter was prepared as described previously (29), and cells were cultured at concentrations of 3 × 105/well in a 96-well culture plate (Nunc, Roskilde, Denmark) and stimulated with various doses of hsp60 and P. gingivalis GroEL (0.1 to 10 μg/ml) for 6 days in a humidified atmosphere of 5% CO2 in air at 37°C. For subsequent experiments, a dose of 10 μg/ml was used, because the response at this dose was maximal. Cultures were pulsed for the last 18 h with 0.5 μCi of [3H]thymidine/well. Cells were harvested onto glass fiber filters by a cell harvester (Lab Science, Tokyo, Japan), and [3H]thymidine incorporation was measured by a liquid scintillation counter (Packard Instrument Co., Downers Grove, Ill.)
Determination of major histocompatibility complex (MHC) restriction of T-cell proliferation.
Inhibition of proliferative responses of PBMC stimulated with human hsp60 was examined by using monoclonal antibodies to human HLA-DP (clone BraFB6, IgG2b), HLA-DQ (clone SPV-L3, IgG2a), and HLA-DR (clone BRA-30, IgG2a) (all from Neomarkers, Fremont, Calif.) and isotype-matched control antibodies (Chemicon International, Inc., Temecula, Calif.). No preservatives were contained in any of the monoclonal antibodies. The monoclonal antibodies were added to cultures at a final concentration of 10 μg/ml. Cultures were then incubated in the presence of human hsp60 for 6 days, pulsed, and harvested as described for the proliferation assay.
RNA separation and cDNA synthesis.
Cultures were also set up for single-strand conformation polymorphism (SSCP) analysis of the TCR β-chain and for cytokine mRNA expression. Cells were cultured at a concentration of 106/well in a 24-well culture plate (Corning Inc., Corning, N.Y.) as for the proliferation assay. Total RNAs from gingival tissues and PBMC were separated by using Isogen (Nippon Gene, Tokyo, Japan) according to the manufacturer's instructions. The RNA samples were further purified by successive treatment with DNase I (GIBCO BRL, Gaithersburg, Md.), phenol-chloroform-isoamyl alcohol (GIBCO BRL), and ethanol sedimentation.
The first-strand cDNA was synthesized using Moloney murine leukemia virus reverse transcriptase (GIBCO BRL) and 50 μM random hexanucleotides (Takara Shuzo Co., Ltd., Shiga, Japan) from 2 μg of total RNA in a reaction buffer (GIBCO BRL) containing 50 mM Tris-HCl (pH 8.3), 75 mM KCl, and 3 mM MgCl2, supplemented with 0.5 U of RNase inhibitor, 0.1 M dithiothreitol, and deoxynucleoside triphosphates (dNTP) (each at 0.5 mM). The reaction mixture was incubated at 37°C for 60 min and then heated at 95°C for 5 min.
SSCP analysis.
PCR utilizing the 22 Vβ family-specific 5′ primers coupled with the common Cβ 3′ primer designed by Choi et al. (7) was performed with 2.5 U of Taq DNA polymerase (Takara) in a final volume of 15 μl containing 6 pmol of each primer, 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, 0.01% gelatin, and dNTP (each at 0.2 mM) in an automated DNA thermal cycler (PCR Thermal Cycler MP; Takara). The amplification cycle profile was as follows: denaturation at 94°C for 1 min, annealing at 55°C for 1 min, and extension at 72°C for 1 min. The durations of denaturation in the first cycle and extension in the last cycle were extended for 7 min. After 35 cycles of amplification, the amplified DNA was diluted (1:39) in a denaturing solution (95% formamide, 10 mM EDTA, 0.1% bromophenol blue, 0.1% xylenecyanol) and kept at 90°C for 2 min. The diluted samples (2 μl) were electrophoresed in nondenaturing 4% polyacrylamide gels containing 10% glycerol. Gels were run at a constant power of 35 W for 100 min. After electrophoresis, the DNA was transferred to Immobilon-S membranes (Millipore Intertech, Bedford, Mass.) and visualized after incubations with a biotinylated Cβ probe [5′-A(A/C)AA(G/C)GTGTTCCCACCCGAGGTCG-CTGTGTT-3′], streptavidin, biotinylated alkaline phosphatase, and a chemiluminescent substrate system (Phototope detection kit; New England Biolabs, Inc., Beverly, Mass.).
Sequence analysis.
In order to compare the nucleotide sequences of the CDR3 regions of hsp60-stimulated PBMC, P. gingivalis GroEL-stimulated PBMC, and gingival-tissue derived T cells, DNA was extracted from the small area of the SSCP gel corresponding to the bands demonstrating identical electrophoretic mobility. Extracted DNA was reamplified and purified by agarose gel electrophoresis followed by a DNA purification kit. The recovered DNA fragments were subcloned into pCR 2.1 and transfected into TOP10F′ (Invitrogen Co., San Diego, Calif.). After blue/white screening of recombinant plasmids on a 5-bromo-4-chloro-3-indolyl-β-d-galactoside indicator plate, single white colonies were picked and grown for 12 h at 37°C in Luria-Bertani (LB) broth. After plasmid purification, the correct inserts in positive clones were confirmed by PCR amplification with the respective Vβ and common Cβ primers and were then used for automated sequencing (Pharmacia Biotech, Uppsala, Sweden).
Determination of IFN-γ and IL-4 concentrations in culture supernatant.
After incubation, culture supernatants were removed, aliquoted, and stored at −80°C until analysis for IFN-γ and IL-4 levels with commercially available enzyme-linked immunosorbent assay (ELISA) kits (Immunotech, Marseille, France).
PCR amplification of IFN-γ and IL-4 genes.
cDNA was PCR amplified by using oligonucleotide primers specific for IFN-γ (5′-ATGTAGCGGATAATGGAATCT-3′ and 5′-AACTTGACATTCATGTCTTCC-3′) and IL-4 (5′-ACTGCAAATCGACACCTATTA-3′ and 5′-ATGGTGGCTGTAGAACTGC-3′). PCR amplification was performed in a final volume of 15 μl containing 1.2 μl of cDNA, 0.35 U of Taq DNA polymerase (Takara), 6 pmol of each primer, 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, 0.01% gelatin, and dNTP (each at 0.2 mM) in an automated DNA thermal cycler (Takara). The amplification cycle profile was as follows: denaturation at 94°C for 10 s, annealing at 60°C for 20 s, and extension at 72°C for 30 s. The durations of denaturation in the first cycle and extension in the last cycle were extended for 7 min. After 30 and 35 cycles of amplification for IFN-γ and IL-4, respectively, each PCR product was electrophoresed on a 2% agarose gel, visualized by ethidium bromide staining, and photographed.
Image analysis of cytokine mRNA expression.
The gels were photographed, and their image data were analyzed by using NIH Image (version 1.62) computer software. In order to improve the accuracy of the analysis, the ratio of the gene expression level of each cytokine to that of the β-actin gene was calculated. Briefly, by using Gel Plotting Macros, the total areas of bands of each lane on the gel were calculated. The expression of each cytokine gene relative to that of β-actin was compared between stimulations with human hsp60 and P. gingivalis GroEL.
Statistical analysis.
The proliferative responses and the levels of either cytokine mRNA expression or protein production of PBMC stimulated with human hsp60 and P. gingivalis GroEL were compared. The differences were compared by using an unpaired t test. A probability value of <0.05 was considered statistically significant.
RESULTS
Proliferative responses of PBMC to hsp60 and P. gingivalis GroEL.
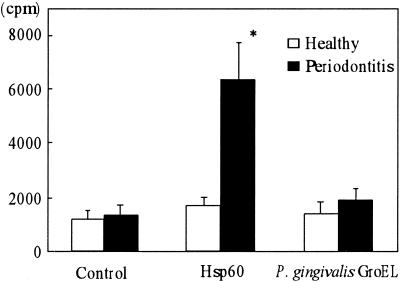
Figure 1 shows the proliferative responses of PBMC from periodontally healthy subjects and periodontitis patients to recombinant human hsp60 and P. gingivalis GroEL. There was no significant difference between control subjects and patients in the baseline response (without stimulation). However, the PBMC from periodontitis patients demonstrated a marked mean proliferative response to human hsp60 4 times greater than that of PBMC from control subjects. This difference was statistically significant (P = 0.015). However, P. gingivalis GroEL did not induce significant proliferative responses in periodontitis patients or in control subjects. We have checked the endotoxin contamination level in the human hsp60 (undetectable) and recombinant P. gingivalis GroEL (12.5 pg/ml) prepared in our laboratory by a Limulus test (Endospecy; Seikagaku Corporation, Tokyo, Japan). These results clearly suggest that T cells proliferated in response to human hsp60 protein but not in response to contaminating endotoxin in the antigen preparation. This is further supported by the fact that P. gingivalis GroEL, which contains a trace amount of endotoxin, did not induce significant proliferation. Thus, it is concluded that the human hsp60 stimulated T cells of periodontitis patients specifically.
FIG. 1.
Proliferative responses to human hsp60 and P. gingivalis GroEL by PBMC from periodontitis patients and control subjects. PBMC at a concentration of 3 × 105/ml were cultured either alone or with 10 μg of human hsp60 or P. gingivalis GroEL/ml for 6 days. Data are expressed as mean counts per minute ± standard errors for either periodontitis patients (n = 16) or control subjects (n = 10). ∗, significantly higher in periodontitis patients than in control subjects.
MHC restriction.
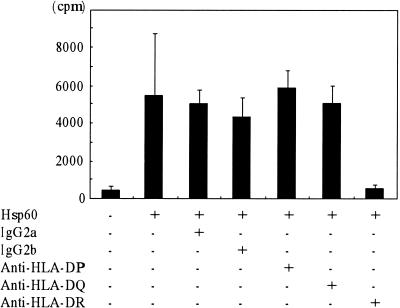
To investigate the MHC restriction of the proliferative response to human hsp60, we tested the effect of anti-HLA-DP, anti-HLA-DQ, and anti-HLA-DR monoclonal antibodies or isotype-matched controls (Fig. 2). The data showed that HLA-DR molecules could function as restricting elements. These results again clearly indicate that the T-cell response to human hsp60 in periodontitis patients is antigen specific.
FIG. 2.
Inhibition of proliferation by class-specific anti-MHC monoclonal antibodies. PBMC from a patient demonstrating a positive proliferative response to human hsp60 were incubated at a concentration of 3 × 105/ml with 10 μg of human hsp60/ml in the presence or absence of monoclonal antibodies recognizing different MHC class II gene products for 6 days. Isotype-matched control antibodies were also included. Data are expressed in mean counts per minute ± standard errors of triplicate cultures.
Cytokine expression in response to hsp60 and P. gingivalis GroEL.
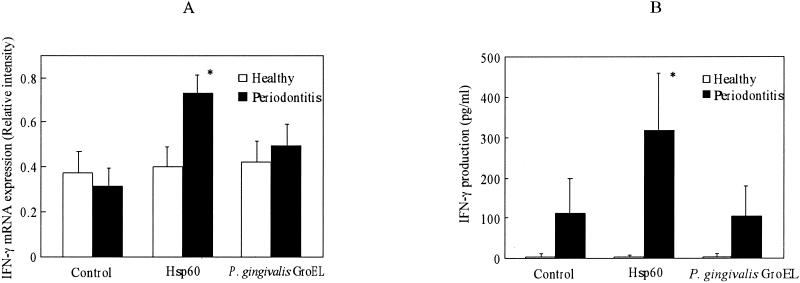
Expression of IFN-γ and IL-4 in response to human hsp60 and P. gingivalis GroEL was examined at both the mRNA and protein levels. As shown in Fig. 3A, there was no significant difference between the control group and periodontitis patients in the spontaneous expression of IFN-γ mRNA. However, there was a significant up-regulation of mRNA expression with hsp60 stimulation in periodontitis patients but not in control subjects (P = 0.02). P. gingivalis GroEL had no or little effect on IFN-γ mRNA expression in PBMC for control subjects or periodontitis patients, respectively. As shown in Fig. 3B, IFN-γ was almost undetectable in the culture supernatants of control subjects irrespective of the presence or absence of stimulants. In periodontitis patients, IFN-γ production tended to be higher than in control subjects in the absence of stimulants. On the other hand, human hsp60 induced significantly higher production of IFN-γ in periodontitis patients but not in control subjects. P. gingivalis GroEL had no effect on IFN-γ production in either control subjects or periodontitis patients.
FIG. 3.
Effects of human hsp60 and P. gingivalis GroEL on the expression of mRNA (A) and protein (B) of IFN-γ by PBMC in periodontitis patients and control subjects. PBMC were stimulated with 10 μg of human hsp60 or P. gingivalis GroEL/ml for 6 days. The intensity of cytokine gene expression was calculated relative to the intensity of β-actin gene expression as a control. Cytokine levels in the culture supernatants were determined by using an ELISA kit according to the manufacturer's instructions. Data are expressed as means ± standard errors for either periodontitis patients (n = 16) or control subjects (n = 10). ∗, significantly higher in periodontitis patients than in control subjects.
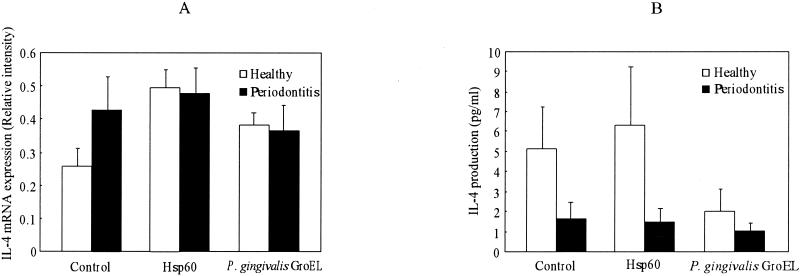
For IL-4, both human hsp60 and P. gingivalis GroEL tended to increase mRNA expression in healthy subjects. However, the effects were not significant. In contrast to IFN-γ, there was no significant difference in IL-4 mRNA expression in periodontitis patients between unstimulated and stimulated cultures (Fig. 4A).
FIG. 4.
Effects of human hsp60 and P. gingivalis GroEL on expression of mRNA (A) and protein (B) of IL-4 by PBMC in periodontitis patients and control subjects. PBMC were stimulated with 10 μg of human hsp60 or P. gingivalis GroEL/ml for 6 days. The intensity of cytokine gene expression was calculated relative to the intensity of β-actin gene expression as a control. Cytokine levels in the culture supernatants were determined by using an ELISA kit according to the manufacturer's instructions. Data are expressed as means ± standard errors for either periodontitis patients (n = 16) or control subjects (n = 10). No significant difference was observed between periodontitis patients and control subjects irrespective of the presence or absence of stimulation.
It was interesting that down-regulation of IL-4 production was observed in periodontitis patients without stimulation. Furthermore, there was no stimulatory effect of human hsp60 or P. gingivalis GroEL on IL-4 production in periodontitis patients. IL-4 production in periodontitis patients remained at low levels irrespective of the culture conditions. Although P. gingivalis GroEL tended to down-regulate IL-4 production in control subjects, this effect was not statistically significant (Fig. 4B).
Effects of human hsp60 and P. gingivalis GroEL on peripheral blood T-cell clonality and T-cell clonality in gingival tissue
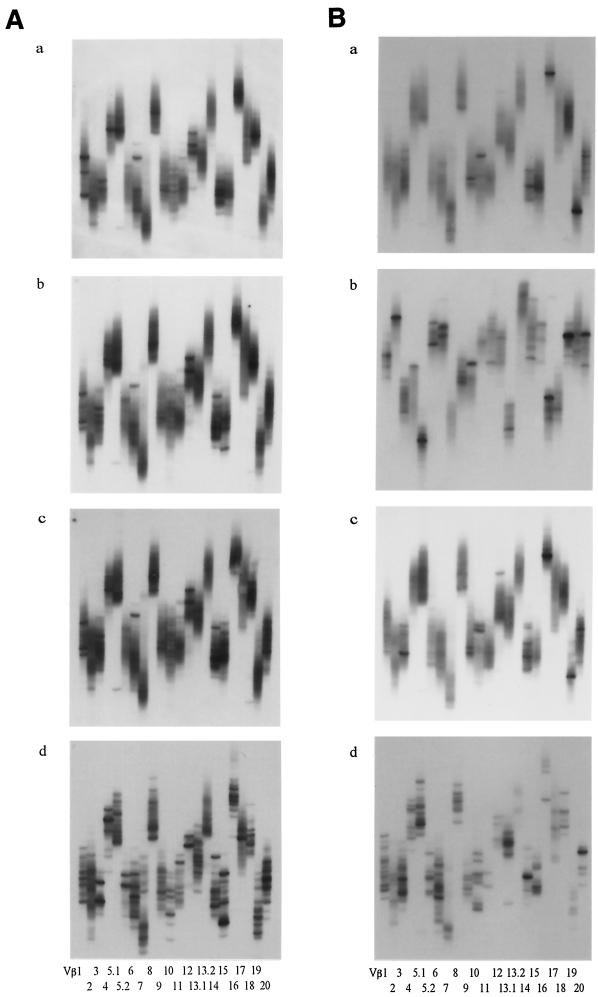
Stimulation with human hsp60 and P. gingivalis GroEL demonstrated distinctive effects on the T-cell clonalities of periodontitis patients and control subjects. Although unstimulated T cells demonstrated smear patterns with a few bands in both periodontitis patients and control subjects (Fig. 5Aa and Ba), a number of distinct bands appeared after stimulation with human hsp60 or P. gingivalis GroEL in periodontitis patients (Fig. 5Ab and Bc). However, in most of the control subjects, clonal accumulation of T cells was hardly observed following stimulation (Fig. 5Ab and Ac). These results indicate that human hsp60 and P. gingivalis GroEL induced clonal expansion in an antigen-specific manner in periodontitis patients preferentially. Furthermore, the emergence of new distinct bands was observed more often in human hsp60-stimulated cultures than in P. gingivalis GroEL-stimulated cultures and was not restricted to particular Vβ families across the patients (Table 1). the other hand, T cells in gingival tissues demonstrated obvious clonal accumulation, as evidenced by a number of distinct bands in both control subjects (Fig. 5Ad) and periodontitis patients (Fig. 5Bd).
FIG. 5.
T-cell clonalities of PBMC with or without stimulation either human hsp60 or P. gingivalis GroEL in a control subject (A) and a periodontitis patient (B). Gingival tissue of periodontitis lesions from the periodontitis patient was analyzed simultaneously. PCR products encoding TCR Vβ genes from PBMC and gingival tissue were analyzed by the SSCP method as described in Materials and Methods. (a) Unstimulated PBMC; (b) Human hsp60-stimulated PBMC; (c) P. gingivalis-stimulated PBMC; (d) Gingival tissue. Each lane represents a particular Vβ family.
TABLE 1.
Numbers of distinct bands emerging in PBMC from periodontitis patients and control subjects after stimulation with hsp60 on P. gingivalis GroEL
| Group and subject | Stimulant | No. of distinct bands for Vβ gene family
|
Total no. of bands | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5.1 | 5.2 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13.1 | 13.2 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | |||
| Periodontitis | ||||||||||||||||||||||||
| P2 | Hsp60 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 4 |
| P. gingivalis GroEL | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| P3 | Hsp60 | 1 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 1 | 9 |
| P. gingivalis GroEL | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| P4 | Hsp60 | 0 | 0 | 1 | 1 | 2 | 2 | 4 | 0 | 3 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 20 |
| P. gingivalis GroEL | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | |
| P5 | Hsp60 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 2 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7 |
| P. gingivalis GroEL | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | |
| P6 | Hsp60 | 0 | 0 | 1 | 0 | 1 | 2 | 1 | 0 | 1 | 0 | 1 | 1 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 12 |
| P. gingivalis GroEL | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | |
| P7 | Hsp60 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 4 |
| P. gingivalis GroEL | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| P8 | Hsp60 | 1 | 1 | 0 | 0 | 2 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 1 | 0 | 1 | 12 |
| P. gingivalis GroEL | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | |
| P9 | Hsp60 | 0 | 2 | 2 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 1 | 2 | 0 | 0 | 4 | 7 | 0 | 0 | 0 | 0 | 0 | 3 | 24 |
| P. gingivalis GroEL | 0 | 1 | 0 | 0 | 2 | 1 | 2 | 0 | 1 | 3 | 0 | 0 | 0 | 2 | 0 | 7 | 1 | 0 | 0 | 0 | 0 | 3 | 23 | |
| P10 | Hsp60 | 0 | 0 | 2 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 12 |
| P. gingivalis GroEL | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 3 | |
| P11 | Hsp60 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 2 | 1 | 5 | 14 |
| P. gingivalis GroEL | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 1 | 0 | 4 | 8 | |
| P12 | Hsp60 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 2 |
| P. gingivalis GroEL | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 1 | 6 | |
| P13 | Hsp60 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 7 |
| P. gingivalis GroEL | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 2 | 3 | 0 | 0 | 1 | 0 | 3 | 15 | |
| P14 | Hsp60 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 3 | 3 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 11 |
| P. gingivalis GroEL | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 3 | |
| P15 | Hsp60 | 0 | 2 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 9 |
| P. gingivalis GroEL | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| P16 | Hsp60 | 0 | 2 | 0 | 1 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 7 |
| P. gingivalis GroEL | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Mean | Hsp60 | 10.3 | ||||||||||||||||||||||
| P. gingivalis GroEL | 4.7 | |||||||||||||||||||||||
| Control | ||||||||||||||||||||||||
| C1 | Hsp60 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| P. gingivalis GroEL | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | |
| C2 | Hsp60 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| P. gingivalis GroEL | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | |
| C3 | Hsp60 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 2 |
| P. gingivalis GroEL | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | |
| C4 | Hsp60 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| P. gingivalis GroEL | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | |
| C5 | Hsp60 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| P. gingivalis GroEL | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 1 | 2 | 3 | 4 | 5.1 | 5.2 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13.1 | 13.2 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | |||
| C6 | Hsp60 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 |
| P. gingivalis GroEL | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| C7 | Hsp60 | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 1 | 1 | 0 | 2 | 0 | 0 | 10 |
| P. gingivalis GroEL | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | |
| C8 | Hsp60 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 2 |
| P. gingivalis GroEL | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| C9 | Hsp60 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| P. gingivalis GroEL | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| C10 | Hsp60 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 2 |
| P. gingivalis GroEL | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 3 | |
| Mean | Hsp60 | 2.3 | ||||||||||||||||||||||
| P. gingivalis GroEL | 1.5 | |||||||||||||||||||||||
Numbers indicate the difference in the number of TCR clonotypes for each Vβ gene between unstimulated and stimulated PBMC. Unstimulated PBMC from patient P1 were not analyzed.
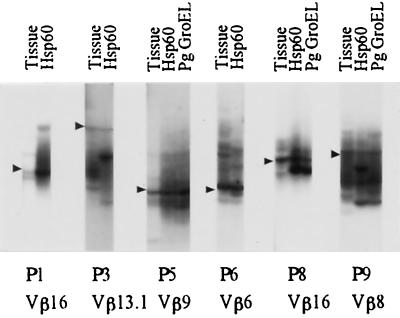
In some cases, identical T-cell clones appeared to be expanded by stimulation with human hsp60 and P. gingivalis GroEL, as the positions of some of the newly emerged bands seemed to be identical with the different stimuli. Moreover, these T-cell clones seemed to exist in the gingival tissues. This assumption is derived from the principle of SSCP analysis that the electrophoretic mobilities of the denatured single-strand DNA fragments can be determined by their nucleotide sequences. Therefore, DNA fragments derived from T-cell clones bearing identical TCR β-chains would show identical electrophoretic mobilities on an SSCP gel. In order to confirm this, PCR products for the expected Vβ family prepared from samples stimulated with human hsp60 and/or P. gingivalis GroEL and from gingival-tissue-derievd samples were applied to adjacent lanes on the same SSCP gel only when there seemed to be distinct bands showing identical electrophoretic mobilities. However, if the position of a band that had newly emerged following stimulation of peripheral blood was apparently different from the position of a distinct band in the autologous gingival samples, this procedure was not carried out. As shown in Fig. 6, SSCP analysis of the expected Vβ family clearly demonstrated that peripheral blood samples stimulated with human hsp60, peripheral blood samples stimulated with P. gingivalis GroEL, and gingival tissue samples showed identical electrophoretic mobilities. For patients P5 and P8, identical T-cell clones were found in human hsp60-stimulated PBMC, P. gingivalis GroEL-stimulated PBMC, and the periodontitis lesion. Identical T-cell clones were found only in hsp60-stimulated PBMC and the periodontitis lesion for patients P1 and P3.
FIG. 6.
Demonstration of common TCRs in PBMC stimulated with hsp's and gingival tissue from the periodontitis patient. PCR products encoding TCR Vβ genes from PBMC and gingival tissue were analyzed by the SSCP method as described in Materials and Methods. Arrowheads indicate identical clones. Tissue, gingival tissue-derived T-cell clones; Hsp60, hsp60-stimulated PBMC; Pg GroEL, P. gingivalis GroEL-stimulated PBMC.
Sequence analysis of the junctional region of expanded T-cell clones following stimulation and of clones accumulated in periodontitis lesions
We further analyzed the nucleotide sequences within the VDJ junctional regions of the clones suspected of having identical TCR β-chains. A small piece of the gel corresponding to the band was cut, and the extracted DNA was subjected to PCR amplification. In order to avoid cross-contamination, each band was cut from a different gel.
As shown in Table 2, clones which had identical electrophoretic mobilities on the SSCP gel demonstrated identical sequences within the junctional regions, confirming that human hsp60 and P. gingivalis GroEL induced expansion of the same T-cell clones in some patients. Moreover, those T-cell clones accumulated in the inflamed gingival lesions of periodontitis patients. Identical clones were also found in human hsp60-stimulated PBMC and the periodontitis lesion but not in P. gingivalis GroEL-stimulated PBMC for some patients. In contrast, the same T-cell clone in P. gingivalis GroEL-stimulated PBMC and the periodontitis lesion was seen for patient P9. These results clearly indicate that human hsp60-reactive T cells that also cross-react with P. gingivalis GroEL account for at least part of the infiltrating T cells in periodontitis lesions.
TABLE 2.
Amino acid sequences of the common TCR Vβ genes from T cells in gingival tissues and hsp60- and P. gingivalis GroEL-stimulated PBMC in periodontitis patients
| Patient (Vβ family) and sample | Amino acid sequencea
|
Jβ | Frequency | |
|---|---|---|---|---|
| Vβ | N-D-N | |||
| P1 (Vβ 16) | ||||
| Gingiva | CASS | RTLQ | 2.5 | 4/17 |
| hsp60-stimulated PBMC | CASS | RTLQ | 2.5 | 3/17 |
| P3 (Vβ 13.1) | ||||
| Gingiva | CASS | YSTGTGS | 1.1 | 4/7 |
| hsp60-stimulated PBMC | CASS | YSTGTGS | 1.1 | 3/11 |
| P5 (Vβ 9) | ||||
| Gingiva | CASS | QERLAGPP | 2.2 | 3/4 |
| hsp60-stimulated PBMC | CASS | QERLAGPP | 2.2 | 2/6 |
| P. gingivalis GroEL-stimulated PBMC | CASS | QERLAGPP | 2.2 | 3/18 |
| P6 (Vβ 6) | ||||
| Gingiva | CASS | SGLNR | 2.7 | 4/5 |
| hsp60-stimulated PBMC | CASS | SGLNR | 2.7 | 3/4 |
| P8 (Vβ 16) | ||||
| Gingiva | CASS | HARD | 2.4 | 7/33 |
| hsp60-stimulated PBMC | CASS | HARD | 2.4 | 2/20 |
| P. gingivalis GroEL-stimulated PBMC | CASS | HARD | 2.4 | 2/24 |
| P9 (Vβ 8) | ||||
| Gingiva | CASS | APGTAF | 1.5 | 3/28 |
| P. gingivalis GroEL-stimulated PBMC | CASS | APGTAF | 1.5 | 3/30 |
| P9 (Vβ 8) | ||||
| Gingiva | CA | IRPS | 2.3 | 2/28 |
| hsp60-stimulated PBMC | CASS | RPSG | 2.7 | 4/24 |
| P. gingivalis GroEL-stimulated PBMC | CASS | RPSG | 2.7 | 2/30 |
Junctional sequences of TCR Vβ genes, which were commonly found in gingival tissues and hsp60-stimulated and P. gingivalis GroEL-stimulated PBMC from patients whose SSCP gels are presented in Fig. 5.
DISCUSSION
Periodontitis is a chronic inflammatory disease caused by a group of gram-negative bacteria. Despite the fact that a number of so-called periodontopathic bacteria are believed to be involved in the initiation and progression of the disease, our recent study has clearly demonstrated that the T cells infiltrating lesions recognize only a restricted number of antigens or epitopes (41). Due to their high conservation among various microbial pathogens and their ability to induce very strong cellular and humoral immune responses, hsp60s have been suggested as possible candidate antigens in periodontitis. Recently, we demonstrated that self-hsp60 might also be a target for autoimmune responses in periodontitis due to its molecular mimicry of the bacterial homologue GroEL (29). This led us to investigate T-cell responses to human hsp60 and P. gingivalis GroEL in periodontitis patients.
In the present study we clearly demonstrated a strong proliferative T-cell response to human hsp60 in periodontitis patients compared with periodontally healthy control subjects. In order to exclude the possibility that contaminating endotoxins in the recombinant protein could be inducing this T-cell response, we determined endotoxin levels in human hsp60 and P. gingivalis GroEL. While P. gingivalis GroEL contained 12.5 pg of endotoxins/ml, no endotoxins were found in human hsp60. If the T-cell response in the present study were caused by contaminating endotoxins, P. gingivalis GroEL would be more stimulatory than human hsp60. Furthermore, the proliferative response to human hsp60 was inhibited by anti-MHC class II antibodies. Thus, we conclude that the elevated T-cell response to human hsp60 in periodontitis patients is antigen specific.
In the present study the proliferative response to P. gingivalis GroEL was much lower than that to human hsp60. In contrast, the proliferative response of PBMC to mycobacterial hsp65 was higher than that to human hsp60 in rheumatoid arthritis patients (18). The reasons for this observation are as yet unknown. It could be speculated that because periodontopathic bacteria almost always reside in periodontal pockets (i.e., outside of the body), the immune system may not be effective in eradicating the infection. Instead, the continuous insult by pathogenic substances, including bacterial hsp60, may overstimulate T cells, which, in turn, may induce anergy. Alternatively, P. gingivalis GroEL may stimulate regulatory T cells (Tr cells), which play a critical role in the generation and maintenance of tolerance. Under normal conditions, potentially self-reactive T cells in the periphery are effectively controlled by different mechanisms leading to peripheral tolerance (8, 14, 27). In some models, down-regulation of the TCR in response to continuous challenge with self-antigen maintains tolerance among peripheral T cells (8, 27). Recent findings suggest that Tr cells are enriched within the CD4+ CD25+ subset (4, 30, 32) and that the function of Tr cells is crucially dependent on signaling via cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), which was found to be constitutively expressed on these cells (24, 25, 31). In this respect, Aoyagi et al. demonstrated that stimulation of PBMC with P. gingivalis antigen induced concomitant elevation of CD25 expression and CTLA-4 expression within the CD4+ subset in periodontitis patients (3).
The immunogenicity of P. gingivalis GroEL may not be as strong as that of human hsp60. However, because of the higher homology of P. gingivalis GroEL with other periodontopathic bacteria-derived GroELs than with human hsp60 (11), levels of antibody to P. gingivalis GroEL in periodontitis patients are elevated and maintained partially by the mechanism of molecular mimicry.
Cross-reactive T-cell responses against hsp's from bacteria and the host have been found in a number of studies (1, 17, 20, 23). These studies usually used synthetic peptides as antigens. In the present study, experiments were performed to evaluate whether physiologic processing generates self-epitopes presented by host cells to cross-reactive T cells specific for microbial hsp60. To this end, we employed reverse transcriptase PCR-SSCP analysis. SSCP analysis has the advantage of identifying the expansion of the reactive T-cell population, with the expanded T-cell clones detected by the emergence of distinct bands on the gel (39). Furthermore, T-cell clones using the same Vβ gene but different CDR3 regions can be distinguished by different positions of the bands. Expansion of T-cell clones reactive to human hsp60 and P. gingivalis GroEL was observed more often in periodontitis patients than in control subjects, as demonstrated by SSCP analysis with a higher frequency for human hsp60-reactive T cells. The latter result is consistent with the higher proliferative response to hsp60 in periodontitis patients but is inconsistent with the theory that cross-reactive T-cell epitopes shared by pathogen and host may induce autoimmune phenomena (34), since the number of T-cell clones reactive to P. gingivalis GroEL was smaller than the number responsive to human hsp60. As a well-balanced network of potentially self-reactive T cells that have evaded deletion processes exists in healthy individuals, activation of self-hsp60-reactive T cells seems to occur preferentially during inflammatory responses (1). In support of this concept, we demonstrated that the number of T-cell clones reactive to both human hsp60 and P. gingivalis GroEL was very small in control subjects.
Petit et al. (22) reported a lower proliferative response to human hsp60 in periodontitis patients than in gingivitis patients and speculated that this poor reactivity to hsp's may be a susceptibility factor for destructive periodontal disease. They also examined the cytokine profile following stimulation with hsp60 and demonstrated that the level of IFN-γ production was significantly lower in the periodontitis group than in the control group. In the present study, however, hsp60 significantly stimulated IFN-γ production in periodontitis patients, suggesting that the proliferative responses to human hsp60 are probably a selective stimulation of specific T cells with a type 1 cytokine profile. Although the precise reason is not known, the contradiction between these two results may come from differences in the source of PBMC, i.e., differences with regard to particular patients' race, severity of the disease, and age. Details of clinical assessment were not given in their report; however, it can be considered that disease severity and age were higher for our patients than for their patients.
It has been demonstrated that eukaryotic hsp60 enhanced and accelerated antigen-specific IFN-γ secretion of CD4+ T cells undergoing primary stimulation and that the IFN-γ production depended strictly on the IL-12 that was produced by antigen-presenting cells (6). Since we confirmed the stimulatory effect of human hsp60 but not that of P. gingivalis GroEL on macrophages by measuring tumor necrosis factor alpha production, the Th1 stimulation ability observed in our study could have been at least in part due to IL-12 produced by monocytes in the culture (33). There is evidence of production of both Th1 and Th2 cytokines in periodontitis lesions (9, 40), and a subtle imbalance in the cytokine profile may induce tissue destruction. Therefore, up-regulation of hsp60 expression in gingival lesions during the immune reaction to periodontopathic bacteria may induce Th1 polarization.
The role of the immune response to hsp60 in infectious diseases and autoimmune diseases is still controversial. In rheumatoid arthritis, a protective role for hsp60-specific T cells has been suggested. This is thought to occur as a result of stimulation of suppressive T-cell responses (36) and maintenance of regulatory T-cell networks (35). Although the role of hsp60-reactive T cells in periodontitis remains to be determined, an elevated proliferative response may also be indicative of subsequent disease remission. Alternatively, it is conceivable that hsp60-specific T cells may be involved in tissue destruction via the production of macrophage proinflammatory cytokines and other mediators such as prostaglandin E2 (10). Finally, the reason for the higher reactivity to hsp60 in periodontitis patients remains unknown. Further studies are required to elucidate the role of hsp60-specific T cells in chronic inflammations such as periodontitis.
Acknowledgments
We are indebted to Cornelia M. Weyand (Department of Medicine, Division of Rheumatology, Mayo Clinic and Foundation) for critical reading of the manuscript. We also thank Takako Nakajima and Toshihiko Aoyagi for their assistance.
This work was supported by grants from the Ministry of Education, Science, Sports and Culture of Japan (10470458 and 13470462) and the Fund for Scientific Promotion of Tanaka Industries Co., Ltd., Niigata, Japan.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Anderton, S. M., R. van der Zee, and J. A. Goodacre. 1993. Inflammation activates self hsp60-specific T cells. Eur. J. Immunol. 23:33-38. [DOI] [PubMed] [Google Scholar]
- 2.Anusaksathien, O., and A. E. Dolby. 1990. Autoimmunity and periodontal disease. J. Oral Pathol. Med. 20:101-107. [DOI] [PubMed] [Google Scholar]
- 3.Aoyagi, T., K. Yamazaki, Y. Kabasawa-Katoh, T. Nakajima, N. Yamashita, H. Yoshie, and K. Hara. 2000. Elevated CTLA-4 expression on CD4 T cells from periodontitis patients stimulated with Porphyromonas gingivalis outer membrane antigen. Clin. Exp. Immunol. 119:280-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asano, M., M. Toda, N. Sakaguchi, and S. Sakaguchi. 1996. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J. Exp. Med. 184:387-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brandtzaeg, P., and F. W. Kraus. 1965. Autoimmunity and periodontal disease. Odontol. Tidskr. 73:285-293. [PubMed] [Google Scholar]
- 6.Breloer, M., B. Dorner, S. H. Moré, T. Roderian, B. Fleischer, and A. von Bonin. 2001. Heat shock proteins as “danger signals”: eukaryotic Hsp60 enhances and accelerates antigen-specific IFN-γ production in T cells. Eur. J. Immunol. 31:2051-2059. [DOI] [PubMed] [Google Scholar]
- 7.Choi, Y., B. Kotzin, L. Herron, J. Callahan, P. Marrack, and J. Kappler. 1989. Interaction of Staphylococcus aureus toxin “superantigens” with human T cells. Proc. Natl. Acad. Sci. USA 86:8941-8945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferber, I., G. Schonrich, J. Schenke, A. L. Mellor, G. J. Hammerling, and B. Arnold. 1994. Levels of peripheral T cell tolerance induced by different doses of tolerogen. Science 263:674-676. [DOI] [PubMed] [Google Scholar]
- 9.Fujihashi, K., M. Yamamoto, T. Hiroi, T. V. Bamberg, J. R. McGhee, and H. Kiyono. 1996. Selected Th1 and Th2 cytokine mRNA expression by CD4+ T cells isolated from inflamed human gingival tissues. Clin. Exp. Immunol. 103:422-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gemmell, E., R. I. Marshall, and G. J. Seymour. 1997. Cytokines and prostaglandins in immune homeostasis and tissue destruction in periodontal disease. Periodontology 2000 14:112-143. [DOI] [PubMed] [Google Scholar]
- 11.Hinode, D., M. Yoshioka, S. Tanabe, O. Miki, K. Masuda, and R. Nakamura. 1998. The GroEL-like protein from Campylobacter rectus: immunological characterization and interleukin-6 and -8 induction in human gingival fibroblast. FEMS Microbiol. Lett. 167:1-6. [DOI] [PubMed] [Google Scholar]
- 12.Hirsch, H. Z., A. Tarkowski, E. J. Miller, S. Gay, W. J. Koopman, and J. Mestecky. 1988. Autoimmunity to collagen in adult periodontal disease. J. Oral Pathol. 17:456-459. [DOI] [PubMed] [Google Scholar]
- 13.Hotokezaka, H., H. Hayashida, N. Ohara, H. Nomaguchi, K. Kobayashi, and T. Yamada. 1994. Cloning and sequencing of the groESL homologue from Porphyromonas gingivalis. Biochim. Biophys. Acta 1219:175-178. [DOI] [PubMed] [Google Scholar]
- 14.Jones, L. A., L. T. Chin, G. R. Merriam, L. M. Nelson, and A. M. Kruisbeek. 1990. Failure of clonal deletion in neonatally thymectomized mice: tolerance is preserved through clonal anergy. J. Exp. Med. 172:1277-1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaufmann, S. H. E., U. Vath, J. E. R. Thole, J. D. A. van Embden, and F. Emmrich. 1987. Enumeration of T cells reactive with Mycobacterium tuberculosis organisms and specific for the recombinant mycobacterial 64-kDa protein. Eur. J. Immunol. 17:351-357. [DOI] [PubMed] [Google Scholar]
- 16.Kiessling, R., A. Gronberg, J. Ivanyi, K. Soderstrom, M. Ferm, S. Kleinau, E. Nilsson, and L. Klareskog. 1991. Role of hsp60 during autoimmune and bacterial inflammation. Immunol. Rev. 121:91-112. [DOI] [PubMed] [Google Scholar]
- 17.Lamb, J. R., V. Bal, P. Mendez-Samperio, A. Mehlert, J. Rothbard, S. Jindal, R. A. Young, and D. B. Young. 1989. Stress proteins may provide a link between the immune response to infection and autoimmunity. Int. Immunol. 1:191-196. [DOI] [PubMed] [Google Scholar]
- 18.Macht, L. M., C. J. Elson, J. R. Kirwan, J. S. H. Gaston, A. G. Lamont, and J. M. Thompson. 2000. Relationship between disease severity and responses by blood mononuclear cells from patients with rheumatoid arthritis to human heat-shock protein 60. Immunology 99:208-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maeda, H., M. Miyamoto, H. Hongyo, A. Nagai, H. Kurihara, and Y. Murayama. 1994. Heat shock protein 60(GroEL) from Porphyromonas gingivalis: molecular cloning and sequence analysis of its gene and purification of the recombinant protein. FEMS Microbiol. Lett. 119:129-136. [DOI] [PubMed] [Google Scholar]
- 20.Munk, M. E., B. Schoel, S. Modrow, R. W. Karr, R. A. Young, and S. H. E. Kaufmann. 1989. T lymphocytes from healthy individuals with specificity to self-epitopes shared by the mycobacterial and human 65-kilodalton heat shock protein. J. Immunol. 143:2844-2849. [PubMed] [Google Scholar]
- 21.Nakano, Y., Y. Inai, Y. Yamashita, S. Nagaoka, T. Kasuzaki-Nagira, T. Nishihara, N. Okahashi, and T. Koga. 1995. Molecular and immunological characterization of a 64-kDa protein of Actinobacillus actinomycetemcomitans. Oral Microbiol. Immunol. 10:151-159. [DOI] [PubMed] [Google Scholar]
- 22.Petit, M. D. A., A. Wassenaar, U. van der Velden, W. van Eden, and B. G. Loos. 1999. Depressed responsiveness of peripheral blood mononuclear cells to heat-shock proteins in periodontitis patients. J. Dent. Res. 78:1393-1400. [DOI] [PubMed] [Google Scholar]
- 23.Quayle, A., K. B. Wilson, S. G. Li, J. Kjeldsen-Kragh, F. Oftung, T. Shinnick, M. Sioud, /O. F/orre, J. D. Capra, and J. B. Natvig. 1992. Peptide recognition, T cell receptor usage and HLA restriction elements of human heat-shock protein (hsp) 60 and mycobacterial 65-kDa hsp-reactive T-cell clones from rheumatoid synovial fluid. Eur. J. Immunol. 22:1315-1322. [DOI] [PubMed] [Google Scholar]
- 24.Read, S., V. Malmstrom, and F. Powrie. 2000. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25+ CD4+ regulatory cells that control intestinal inflammation. J. Exp. Med. 192:295-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salomon, B., D. J. Lenschow, L. Rhee, N. Ashourian, B. Singh, A. Sharpe, and J. A. Bluestone. 2000. B7/CD28 costimulation is essential for the homeostasis of the CD4+ CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity 12:431-440. [DOI] [PubMed] [Google Scholar]
- 26.Schei, O., J. Waerhaug, A. Lovdal, and A. Arno. 1959. Alveolar bone loss as related to oral hygiene and age. J. Periodontol. 30:7-16. [Google Scholar]
- 27.Schonrich, G., U. Kalinke, F. Momburg, M. Malissen, A.-M. Schmitt-Verhulst, B. Malissen, G. J. Hammerling, and B. Arnold. 1991. Downregulation of T cell receptors on self-reactive T cells as novel mechanism for extrathymic tolerance induction. Cell 65:293-304. [DOI] [PubMed] [Google Scholar]
- 28.Seymour, G. J., E. Gemmell, R. A. Reinhardt, J. Eastcott, and M. A. Taubman. 1993. Immunopathogenesis of chronic inflammatory periodontal disease: cellular and molecular mechanisms. J. Periodont. Res. 28:478-486. [DOI] [PubMed] [Google Scholar]
- 29.Tabeta, K., K. Yamazaki, H. Hotokezaka, H. Yoshie, and K. Hara. 2000. Elevated humoral immune response to heat shock protein 60 (hsp60) family in periodontitis patients. Clin. Exp. Immunol. 120:285-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takahashi, T., Y. Kuniyasu, M. Toda, N. Sakaguchi, M. Itoh, M. Iwata, J. Shimizu, and S. Sakaguchi. 1998. Immunologic self-tolerance maintained by CD25+ CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int. Immunol. 10:1969-1980. [DOI] [PubMed] [Google Scholar]
- 31.Takahashi, T., T. Tagami, S. Yamazaki, T. Uede, J. Shimizu, N. Sakaguchi, T. W. Mak, and S. Sakaguchi. 2000. Immunologic self-tolerance maintained by CD25+ CD4+ regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J. Exp. Med. 192:303-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thornton, A. E., and E. M. Shevach. 1998. CD4+ CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J. Exp. Med. 188:287-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ueki, K., K. Tabeta, H. Yoshie, and K. Yamazaki. 2002. Self-heat shock protein 60 induces tumor necrosis factor-α in monocyte-derived macrophages: possible role in chronic inflammatory periodontal disease. Clin. Exp. Immunol., in press. [DOI] [PMC free article] [PubMed]
- 34.van Eden, W. 1991. Heat-shock proteins as immunogenic bacterial antigens with the potential to induce and regulate autoimmune arthritis. Immunol. Rev. 121:5-21. [DOI] [PubMed] [Google Scholar]
- 35.van Eden, W., R. van der Zee, A. G. A. Paul, B. Prakken, U. Wendling, S. M. Anderton, and M. H. M. Wauben. 1998. Do heat shock proteins control the balance of T-cell regulation in inflammatory diseases? Immunol. Today 19:303-307. [DOI] [PubMed] [Google Scholar]
- 36.van Roon, J. A. G., W. van Eden, J. L. A. M. van Roy, F. J. P. G. Lafeber, and J. W. J. Bijlsma. 1997. Stimulation of suppressive T cell responses by human but not bacterial 60-kD heat-shock protein in synovial fluid of patients with rheumatoid arthritis. J. Clin. Investig. 100:459-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vayssier, C., D. Mayrand, and D. Grenier. 1994. Detection of stress proteins in Porphyromonas gingivalis and other oral bacteria by Western immunoblotting analysis. FEMS Microbiol. Lett. 121:303-307. [DOI] [PubMed] [Google Scholar]
- 38.Wassenaar, A., C. Reinhardus, T. Thepen, L. Abraham-Inpijn, and F. Kievits. 1995. Cloning, characterization, and antigen specificity of T-lymphocyte subsets extracted from gingival tissue of chronic adult periodontitis patients. Infect. Immun. 63:2147-2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamamoto, K., H. Sakoda, T. Nakajima, T. Kato, M. Okubo, M. Dohi, Y. Mizushima, K. Ito, and K. Nishioka. 1992. Accumulation of multiple T cell clonotypes in the synovial lesions of patients with rheumatoid arthritis revealed by a novel clonality analysis. Int. Immunol. 4:1219-1223. [DOI] [PubMed] [Google Scholar]
- 40.Yamazaki, K., T. Nakajima, and K. Hara. 1995. Immunohistological analysis of T cell functional subsets in chronic inflammatory periodontal disease. Clin. Exp. Immunol. 99:384-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamazaki, K., T. Nakajima, Y. Ohsawa, K. Tabeta, H. Yoshie, K. Sakurai, and G. J. Seymour. 2000. Selective expansion of T cells in gingival lesions of patients with chronic inflammatory periodontal disease. Clin. Exp. Immunol. 120:154-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Young, D. B., and T. R. Garbe. 1991. Heat shock proteins and antigens of Mycobacterium tuberculosis. Infect. Immun. 59:3086-3093. [DOI] [PMC free article] [PubMed] [Google Scholar]