Abstract
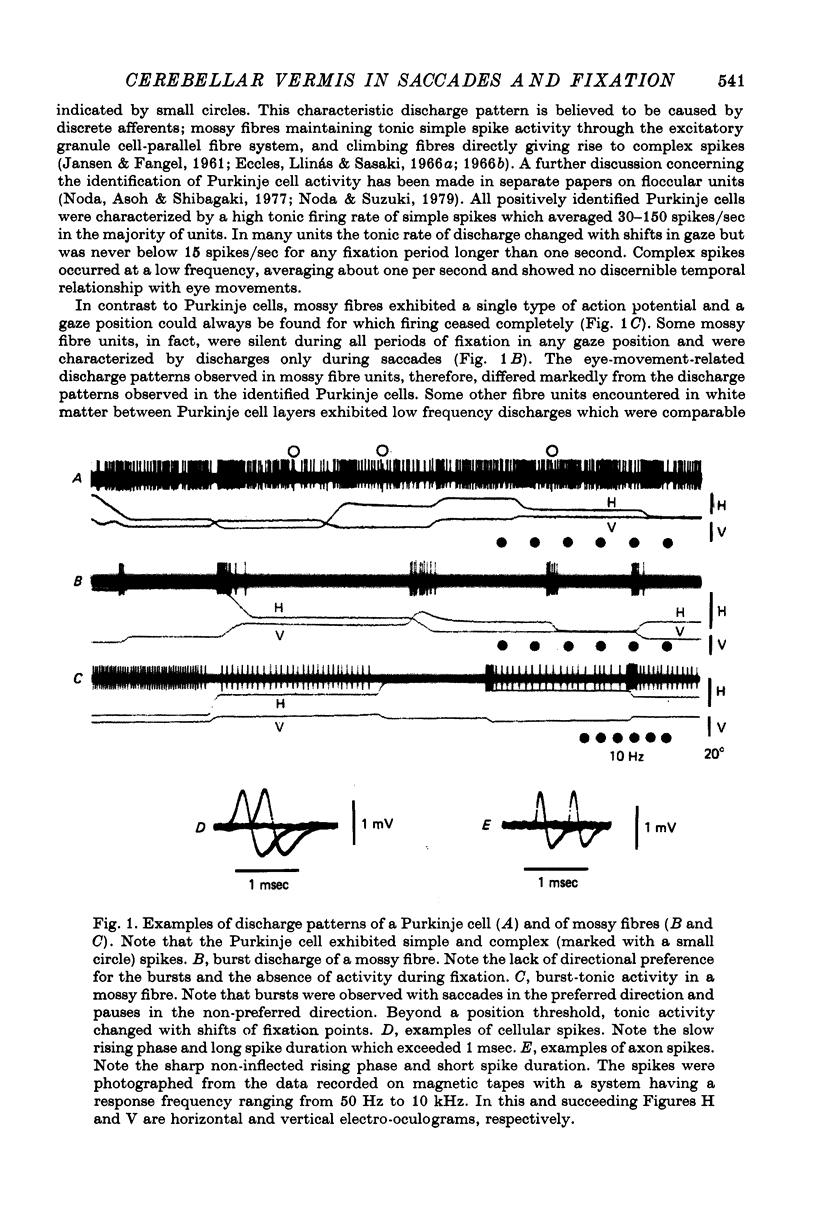
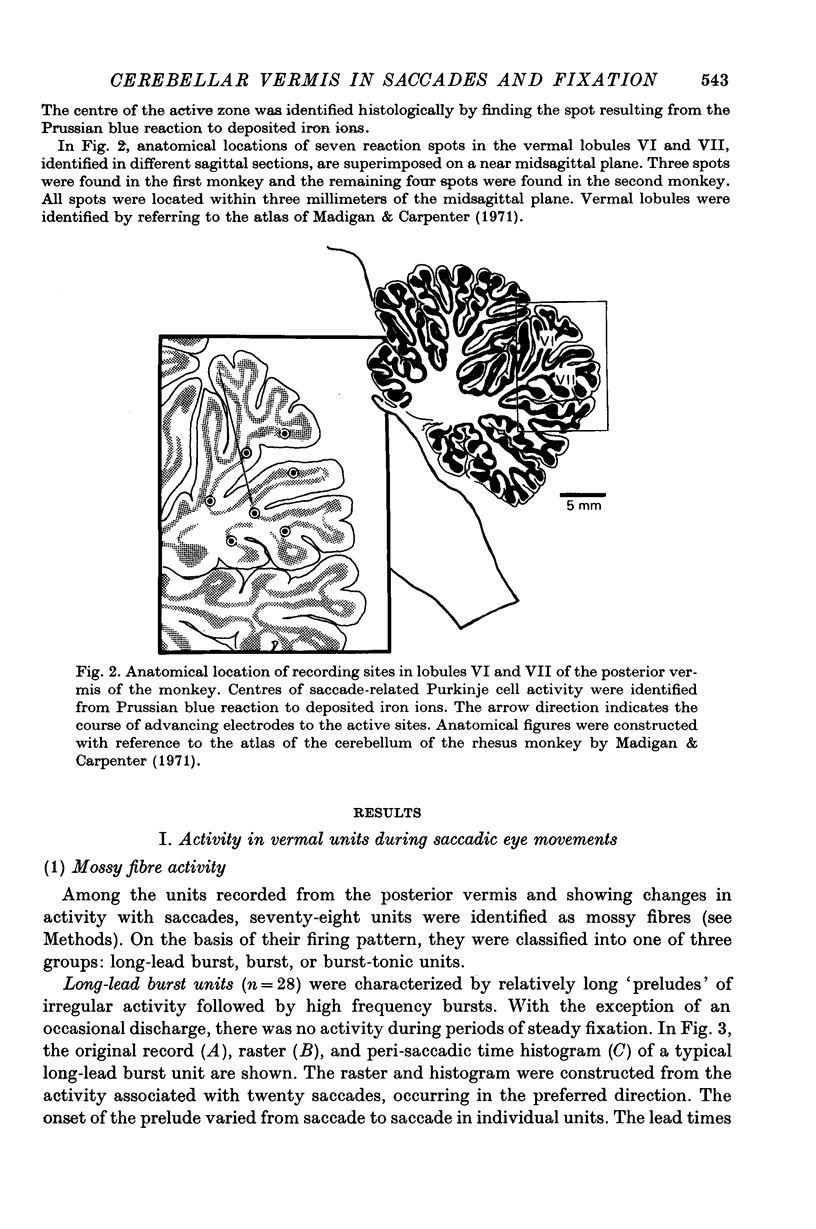
1. Discharges of Purkinje cells and of presumed mossy fibres were extracellularly recorded from vermal lobules VI and VII of two monkeys during saccadic eye movements and fixation. Among the units showing changes in activity in relation to either saccades or eye position, eighty-four units were identified as mossy fibres and ninety-one units were Purkinje cells.
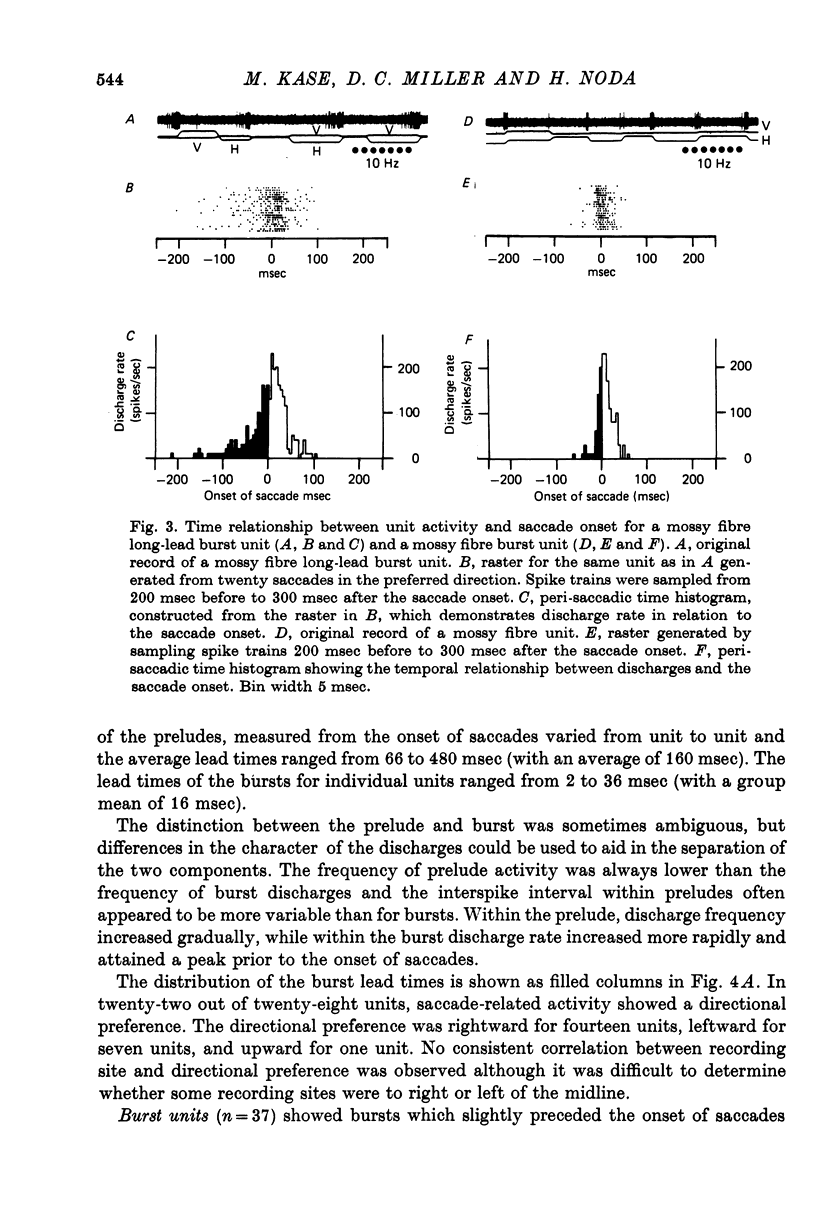
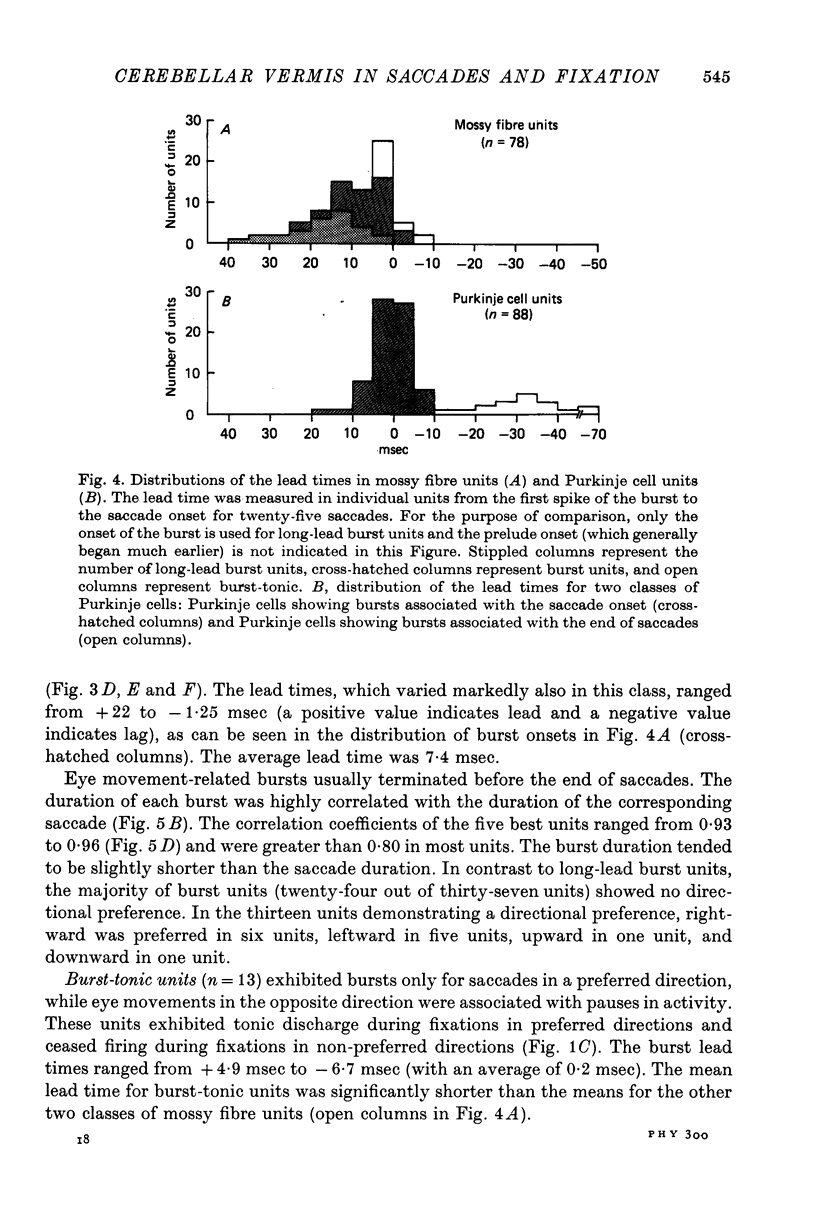
2. Based on the discharge patterns associated with saccades, mossy fibre units were classified into long-lead burst, burst, and burst-tonic units. The long-lead burst units (twenty-eight units) started firing long before the saccades, the discharge consisting of a prelude (average lead time: 160 msec) and a burst (average lead time: 16 msec). In twenty-two units the saccade-related bursts showed a directional preference. The burst units (thirty-seven units) started firing slightly before the saccade onset (average lead time: 7·4 msec) and thirteen units showed directional preference. The bursts in burst-tonic units (thirteen units) had an average lead time of 0·2 msec.
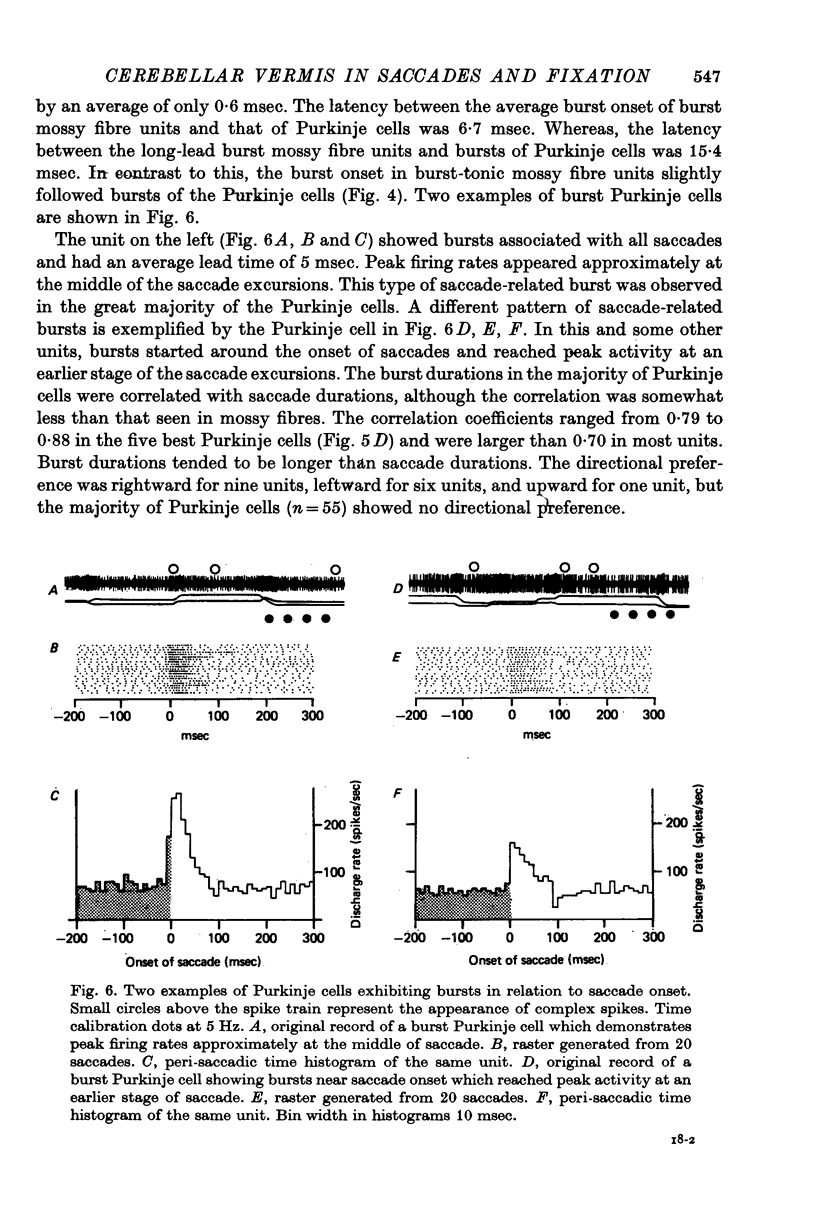
3. Among the ninety-one Purkinje cells, eighty-eight cells showed bursts associated with saccades. Three units paused for all directions of saccades.
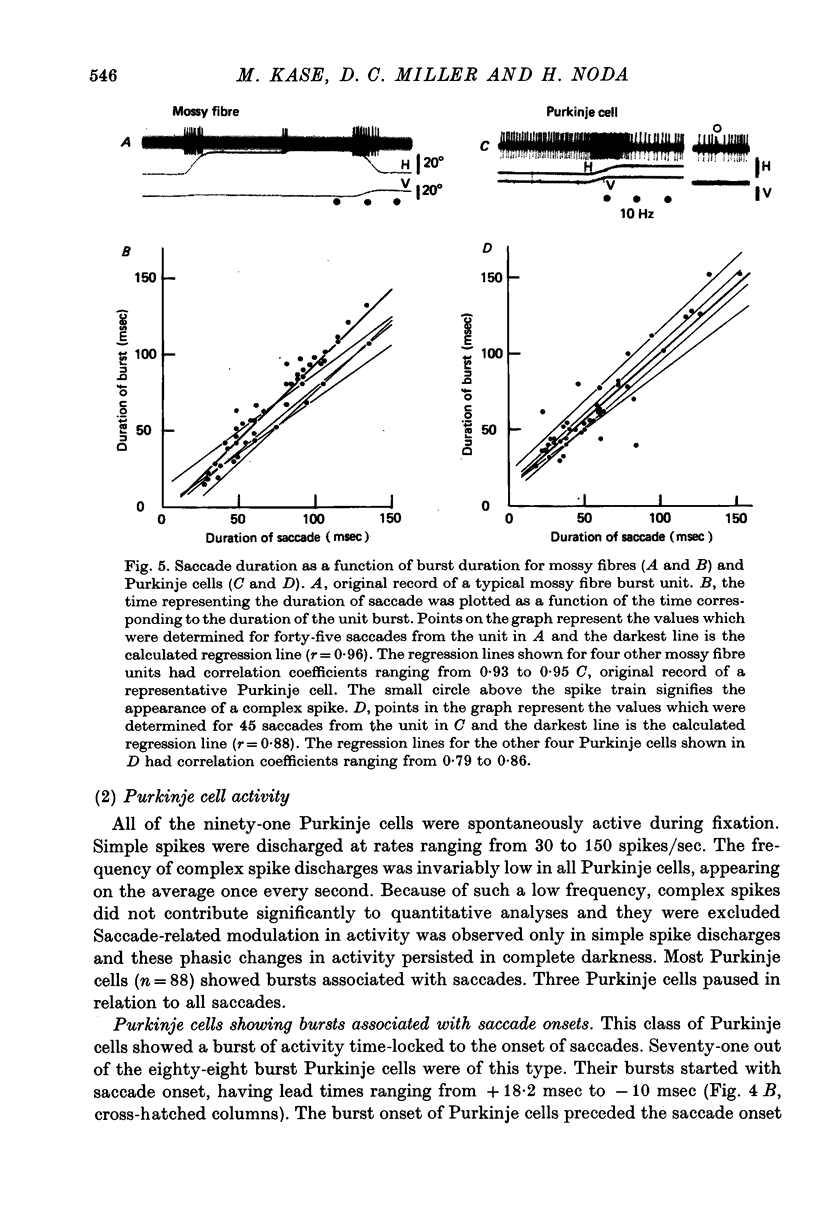
4. Seventy-one units out of the eighty-eight burst Purkinje cells showed bursts beginning approximately at the saccade onset (average lead time: 0·6 msec) and lasting throughout the saccade. The durations of bursts and saccades were highly correlated (correlation coefficients ranging from 0·70 to 0·88).
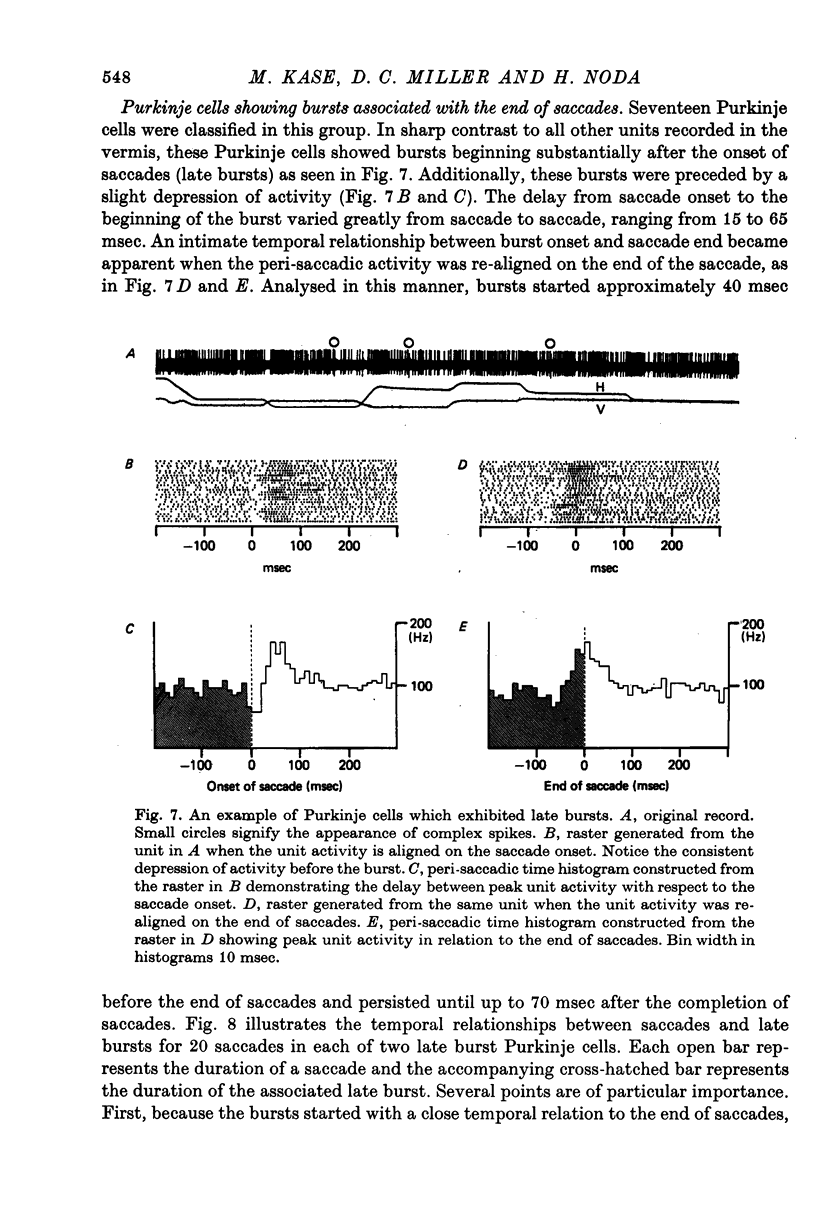
5. In the remaining seventeen burst Purkinje cells, the bursts followed the saccade onset (average delay: 32 msec). The bursts started approximately 40 msec before the end of a saccade and persisted on the average 70 msec after its completion. Peak firing rate occurred with a close temporal relation to the end of the saccade.
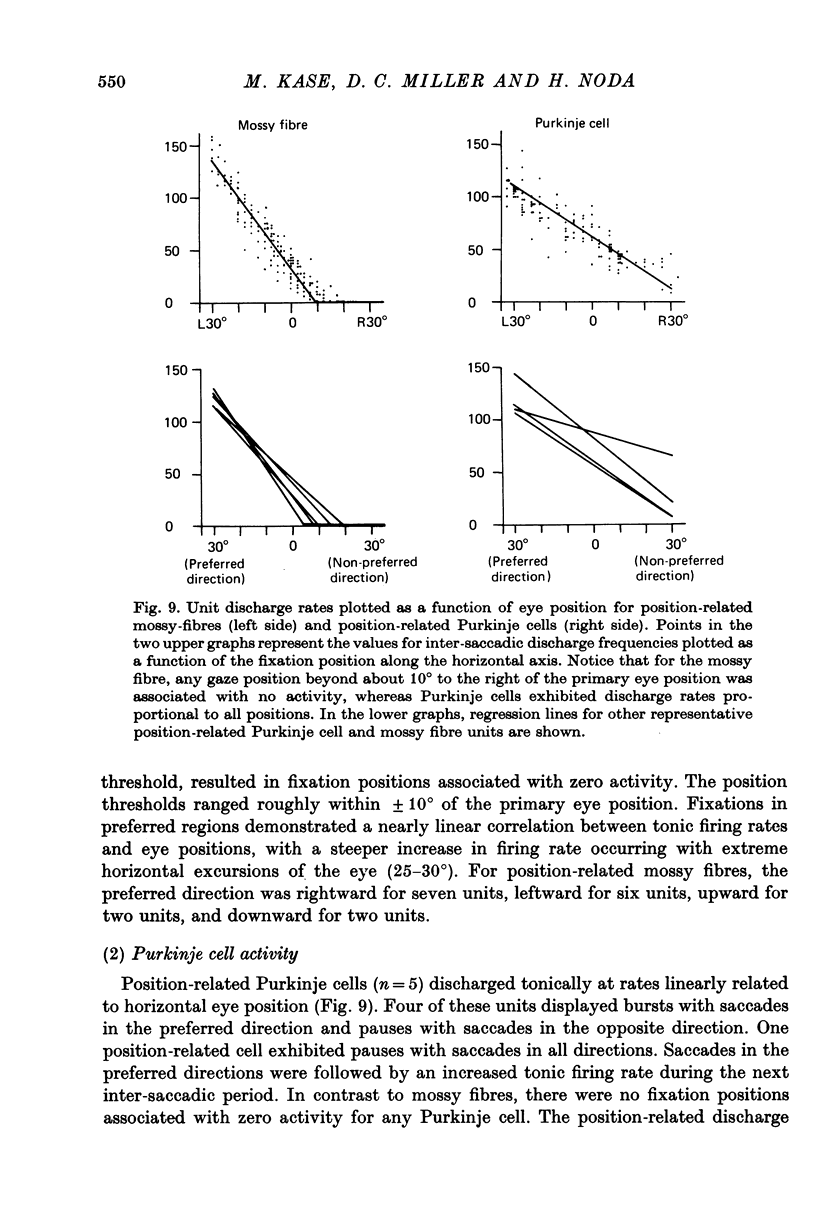
6. The tonic activity in nineteen mossy fibres and five Purkinje cells changed with eye positions. In the nineteen mossy fibres, there were thirteen burst-tonic and six tonic units. The activity in the five Purkinje cells was a linear function of horizontal eye position.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aschoff J. C., Cohen B. Changes in saccadic eye movements produced by cerebellar cortical lesions. Exp Neurol. 1971 Aug;32(2):123–133. doi: 10.1016/0014-4886(71)90056-2. [DOI] [PubMed] [Google Scholar]
- BRODAL A. Experimental demonstration of cerebellar connexions from the perihypoglossal nuclei (nucleus intercalatus, nucleus praepositus hypoglossal and nucleus of roller) in the cat. J Anat. 1952 Apr;86(2):110–129. [PMC free article] [PubMed] [Google Scholar]
- Baker R., Precht W., Llinas R. Mossy and climbing fiber projections of extraocular muscle afferents to the cerebellum. Brain Res. 1972 Mar 24;38(2):440–445. doi: 10.1016/0006-8993(72)90728-7. [DOI] [PubMed] [Google Scholar]
- Batini C., Buisseret-Delmas C., Corvisier J., Hardy O., Jassik-Gerschenfeld D. Brain stem nuclei giving fibers to lobules VI and VII of the cerebellar vermis. Brain Res. 1978 Sep 22;153(2):241–261. doi: 10.1016/0006-8993(78)90405-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batton R. R., 3rd, Jayaraman A., Ruggiero D., Carpenter M. B. Fastigial efferent projections in the monkey: an autoradiographic study. J Comp Neurol. 1977 Jul 15;174(2):281–305. doi: 10.1002/cne.901740206. [DOI] [PubMed] [Google Scholar]
- Cohen B., Goto K., Shanzer S., Weiss A. H. Eye movements induced by electric stimulation of the cerebellum in the alert cat. Exp Neurol. 1965 Oct;13(2):145–162. doi: 10.1016/0014-4886(65)90105-6. [DOI] [PubMed] [Google Scholar]
- Eccles J. C. Circuits in the cerebellar control of movement. Proc Natl Acad Sci U S A. 1967 Jul;58(1):336–343. doi: 10.1073/pnas.58.1.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles J. C., Llinás R., Sasaki K. Parallel fibre stimulation and the responses induced thereby in the Purkinje cells of the cerebellum. Exp Brain Res. 1966;1(1):17–39. doi: 10.1007/BF00235207. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Llinás R., Sasaki K. The excitatory synaptic action of climbing fibres on the Purkinje cells of the cerebellum. J Physiol. 1966 Jan;182(2):268–296. doi: 10.1113/jphysiol.1966.sp007824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs A. F., Kimm J. Unit activity in vestibular nucleus of the alert monkey during horizontal angular acceleration and eye movement. J Neurophysiol. 1975 Sep;38(5):1140–1161. doi: 10.1152/jn.1975.38.5.1140. [DOI] [PubMed] [Google Scholar]
- Fuchs A. F., Kornhuber H. H. Extraocular muscle afferents to the cerebellum of the cat. J Physiol. 1969 Feb;200(3):713–722. doi: 10.1113/jphysiol.1969.sp008718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANIT R., PHILLIPS C. G. Excitatory and inhibitory processes acting upon individual Purkinje cells of the cerebellum in cats. J Physiol. 1956 Sep 27;133(3):520–547. doi: 10.1113/jphysiol.1956.sp005606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould B. B., Graybiel A. M. Afferents to the cerebellar cortex in the cat: evidence for an intrinsic pathway leading from the deep nuclei to the cortex. Brain Res. 1976 Jul 16;110(3):601–611. doi: 10.1016/0006-8993(76)90869-6. [DOI] [PubMed] [Google Scholar]
- Haines D. E. Cerebellar cortical efferents of the posterior lobe vermis in a prosimian primate (Galago) and the tree shrew (Tupaia). J Comp Neurol. 1975 Sep;163(1):21–39. doi: 10.1002/cne.901630103. [DOI] [PubMed] [Google Scholar]
- Harvey R. J., Porter R., Rawson J. A. The natural discharges of Purkinje cells in paravermal regions of lobules V and VI of the monkey's cerebellum. J Physiol. 1977 Oct;271(2):515–536. doi: 10.1113/jphysiol.1977.sp012012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henn V., Cohen B. Quantitative analysis of activity in eye muscle motoneurons during saccadic eye movements and positions of fixation. J Neurophysiol. 1973 Jan;36(1):115–126. doi: 10.1152/jn.1973.36.1.115. [DOI] [PubMed] [Google Scholar]
- Hoddevik G. H., Brodal A., Kawamura K., Hashikawa T. The pontine projection to the cerebellar vermal visual area studied by means of the retrograde axonal transport of horseradish peroxidase. Brain Res. 1977 Mar 11;123(2):209–227. doi: 10.1016/0006-8993(77)90475-9. [DOI] [PubMed] [Google Scholar]
- Hoddevik G. H., Brodal A., Walberg F. The olivocerebellar projection in the cat studied with the method of retrograde axonal transport of horseradish peroxidase. III. The projection to the vermal visual area. J Comp Neurol. 1976 Sep 15;169(2):155–170. doi: 10.1002/cne.901690203. [DOI] [PubMed] [Google Scholar]
- Houk J., Henneman E. Feedback control of skeletal muscles. Brain Res. 1967 Aug;5(4):433–451. doi: 10.1016/0006-8993(67)90014-5. [DOI] [PubMed] [Google Scholar]
- Kawamura K., Brodal A., Hoddevik G. The projection of the superior colliculus onto the reticular formation of the brain stem. An experimental anatomical study in the cat. Exp Brain Res. 1974 Jan 22;19(1):1–19. doi: 10.1007/BF00233392. [DOI] [PubMed] [Google Scholar]
- Kawamura K., Brodal A. The tectopontine projection in the cat: an experimental anatomical study with comments on pathweays for teleceptive impulses to the cerebellum. J Comp Neurol. 1973 Jun 1;149(3):371–390. doi: 10.1002/cne.901490306. [DOI] [PubMed] [Google Scholar]
- Keller E. L. Participation of medial pontine reticular formation in eye movement generation in monkey. J Neurophysiol. 1974 Mar;37(2):316–332. doi: 10.1152/jn.1974.37.2.316. [DOI] [PubMed] [Google Scholar]
- Llinás R., Wolfe J. W. Functional linkage between the electrical activity in the vermal cerebellar cortex and saccadic eye movements. Exp Brain Res. 1977 Aug 8;29(1):1–14. doi: 10.1007/BF00236872. [DOI] [PubMed] [Google Scholar]
- Luschei E. S., Fuchs A. F. Activity of brain stem neurons during eye movements of alert monkeys. J Neurophysiol. 1972 Jul;35(4):445–461. doi: 10.1152/jn.1972.35.4.445. [DOI] [PubMed] [Google Scholar]
- Lynch J. C., Mountcastle V. B., Talbot W. H., Yin T. C. Parietal lobe mechanisms for directed visual attention. J Neurophysiol. 1977 Mar;40(2):362–389. doi: 10.1152/jn.1977.40.2.362. [DOI] [PubMed] [Google Scholar]
- Meyer-Lohmann J., Hore J., Brooks V. B. Cerebellar participation in generation of prompt arm movements. J Neurophysiol. 1977 Sep;40(5):1038–1050. doi: 10.1152/jn.1977.40.5.1038. [DOI] [PubMed] [Google Scholar]
- Miles F. A. Single unit firing patterns in the vestibular nuclei related to voluntary eye movements and passive body rotation in conscious monkeys. Brain Res. 1974 May 17;71(2-3):215–224. doi: 10.1016/0006-8993(74)90963-9. [DOI] [PubMed] [Google Scholar]
- Noda H., Suzuki D. A. The role of the flocculus of the monkey in saccadic eye movements. J Physiol. 1979 Sep;294:317–334. doi: 10.1113/jphysiol.1979.sp012932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Precht W., Volkind R., Blanks R. H. Functional organization of the vestibular input to the anterior and posterior cerebellar vermis of cat. Exp Brain Res. 1977 Feb 16;27(2):143–160. doi: 10.1007/BF00237695. [DOI] [PubMed] [Google Scholar]
- Ritchie L. Effects of cerebellar lesions on saccadic eye movements. J Neurophysiol. 1976 Nov;39(6):1246–1256. doi: 10.1152/jn.1976.39.6.1246. [DOI] [PubMed] [Google Scholar]
- Robinson D. A. Oculomotor unit behavior in the monkey. J Neurophysiol. 1970 May;33(3):393–403. doi: 10.1152/jn.1970.33.3.393. [DOI] [PubMed] [Google Scholar]
- Ron S., Robinson D. A. Eye movements evoked by cerebellar stimulation in the alert monkey. J Neurophysiol. 1973 Nov;36(6):1004–1022. doi: 10.1152/jn.1973.36.6.1004. [DOI] [PubMed] [Google Scholar]
- Schiller P. H., Koerner F. Discharge characteristics of single units in superior colliculus of the alert rhesus monkey. J Neurophysiol. 1971 Sep;34(5):920–936. doi: 10.1152/jn.1971.34.5.920. [DOI] [PubMed] [Google Scholar]
- Schlag J., Lehtinen I., Schlag-Rey M. Neuronal activity before and during eye movements in thalamic internal medullary lamina of the cat. J Neurophysiol. 1974 Sep;37(5):982–995. doi: 10.1152/jn.1974.37.5.982. [DOI] [PubMed] [Google Scholar]
- Schwarz D. W., Tomlinson R. D. Neuronal responses to eye muscle stretch in cerebellar lobule VI of the cat. Exp Brain Res. 1977 Jan 18;27(1):101–111. doi: 10.1007/BF00234828. [DOI] [PubMed] [Google Scholar]
- Thach W. T. Discharge of Purkinje and cerebellar nuclear neurons during rapidly alternating arm movements in the monkey. J Neurophysiol. 1968 Sep;31(5):785–797. doi: 10.1152/jn.1968.31.5.785. [DOI] [PubMed] [Google Scholar]
- Thach W. T. Discharge of cerebellar neurons related to two maintained postures and two prompt movements. II. Purkinje cell output and input. J Neurophysiol. 1970 Jul;33(4):537–547. doi: 10.1152/jn.1970.33.4.537. [DOI] [PubMed] [Google Scholar]
- Wurtz R. H., Goldberg M. E. Activity of superior colliculus in behaving monkey. 3. Cells discharging before eye movements. J Neurophysiol. 1972 Jul;35(4):575–586. doi: 10.1152/jn.1972.35.4.575. [DOI] [PubMed] [Google Scholar]


