Abstract
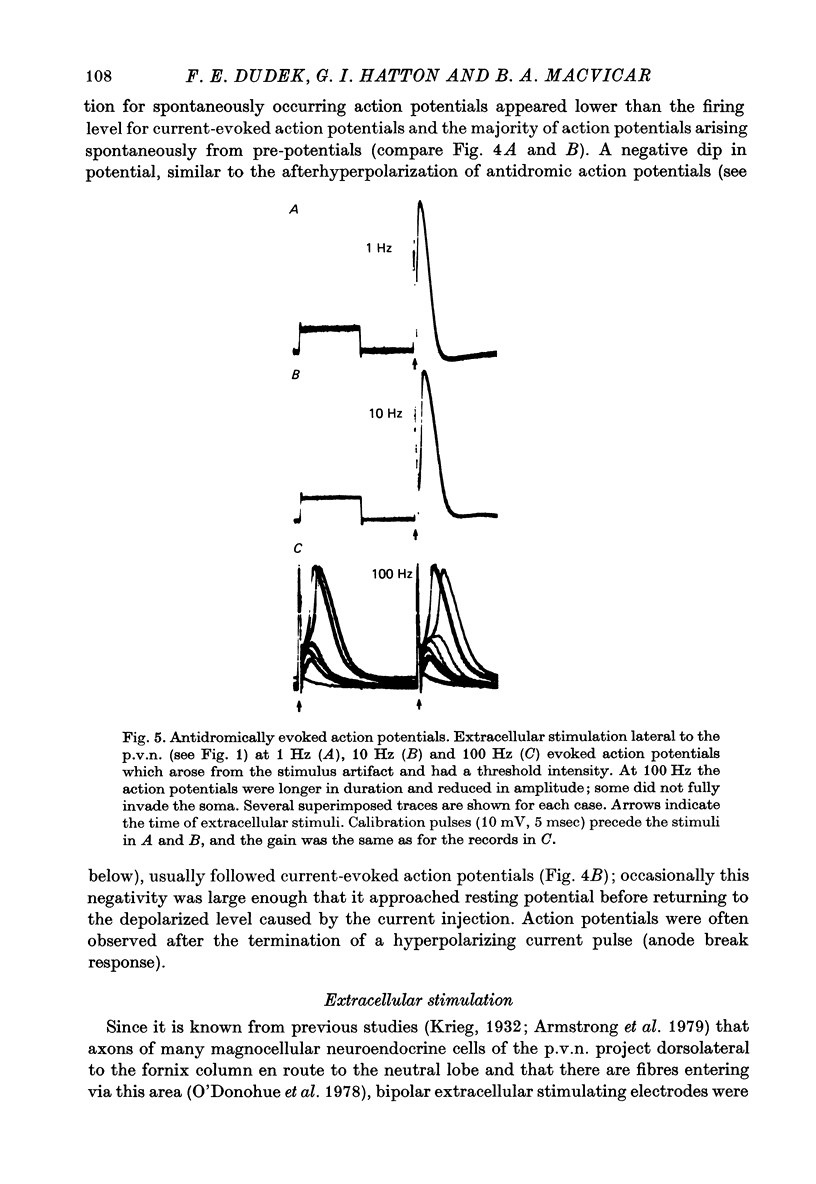
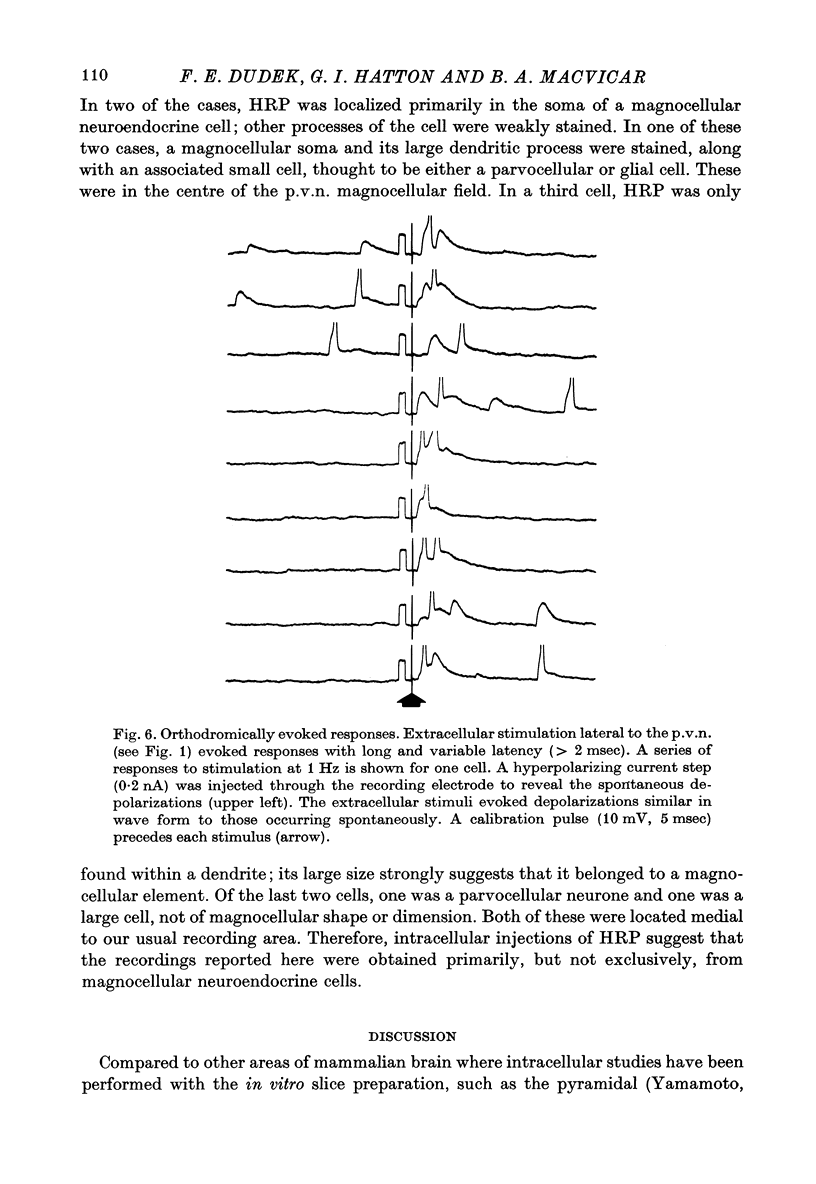
1. The electrical activity of thirty-five neurones in the lateral area of the paraventricular nucleus (p.v.n.) was recorded intracellularly in vitro from slices of rat hypothalamus. 2. Spontaneously occurring action potentials were observed in twenty-four of the neurones. The temporal pattern of action potentials was generally slow and irregular; occasionally some cells fired bursts of action potentials. 3. Depolarizations with a fast rising phase and slow decay occurred spontaneously in most cells. These depolarizations exhibited a wide range of amplitudes in each cell (up to 33 mV), showed temporal summation, and could serve as pre-potentials for spontaneously occurring action potentials. Presumably, these depolarizations were excitatory post-synaptic potentials (e.p.s.p.s.). 4. Depolarizing current injection could evoke action potentials. Extracellular stimuli dorsolateral to the fornix column occasionally elicited action potentials which had a short and invariant latency and which could respond to stimulation rates of 100 Hz. In some cases, extracellular stimuli in the same area evoked depolarizations which had long and variable latency and were similar to those occurring spontaneously. These two types of responses probably represent antidromic and orthodromic activation respectively. 5. Intracellular injections of horseradish peroxidase suggest that these recordings were obtained primarily, but not exclusively, from magnocellular neuroendocrine cells. This is consistent with previous anatomical studies on the location of magnocellular elements in p.v.n.
Full text
PDF

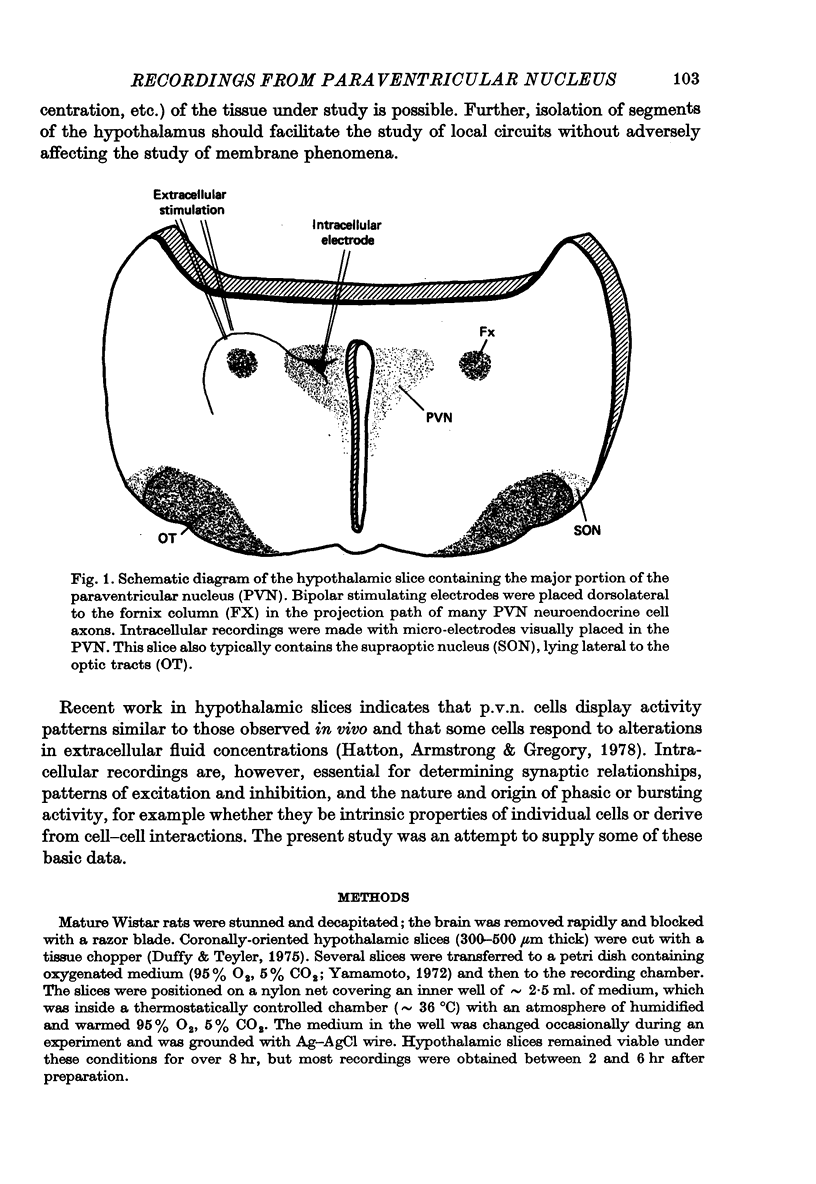

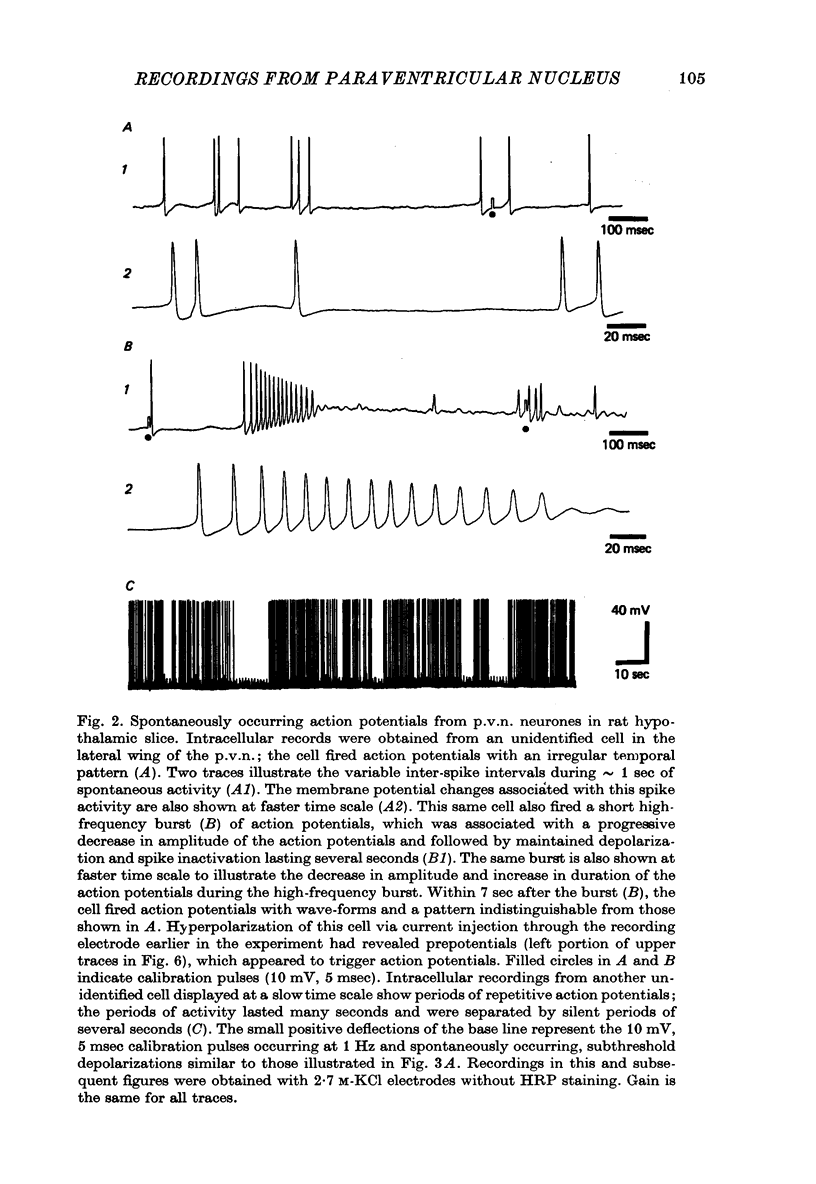
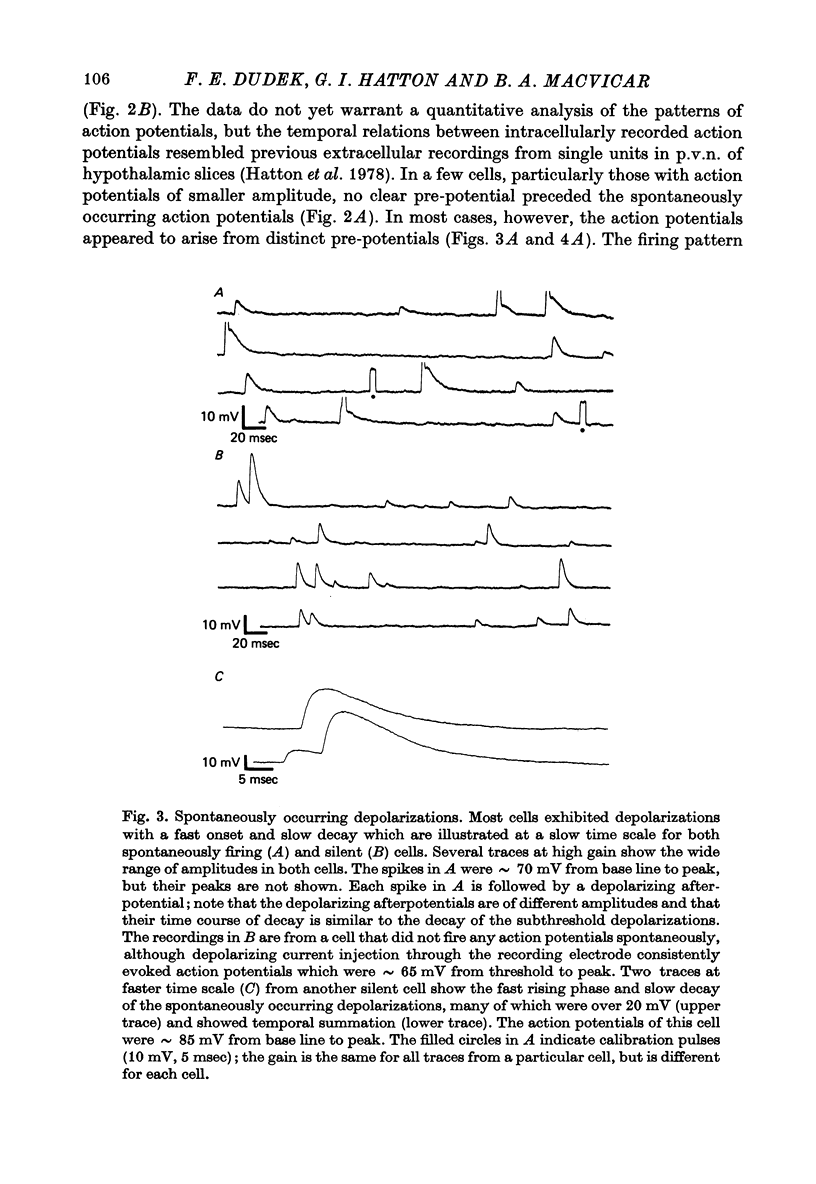
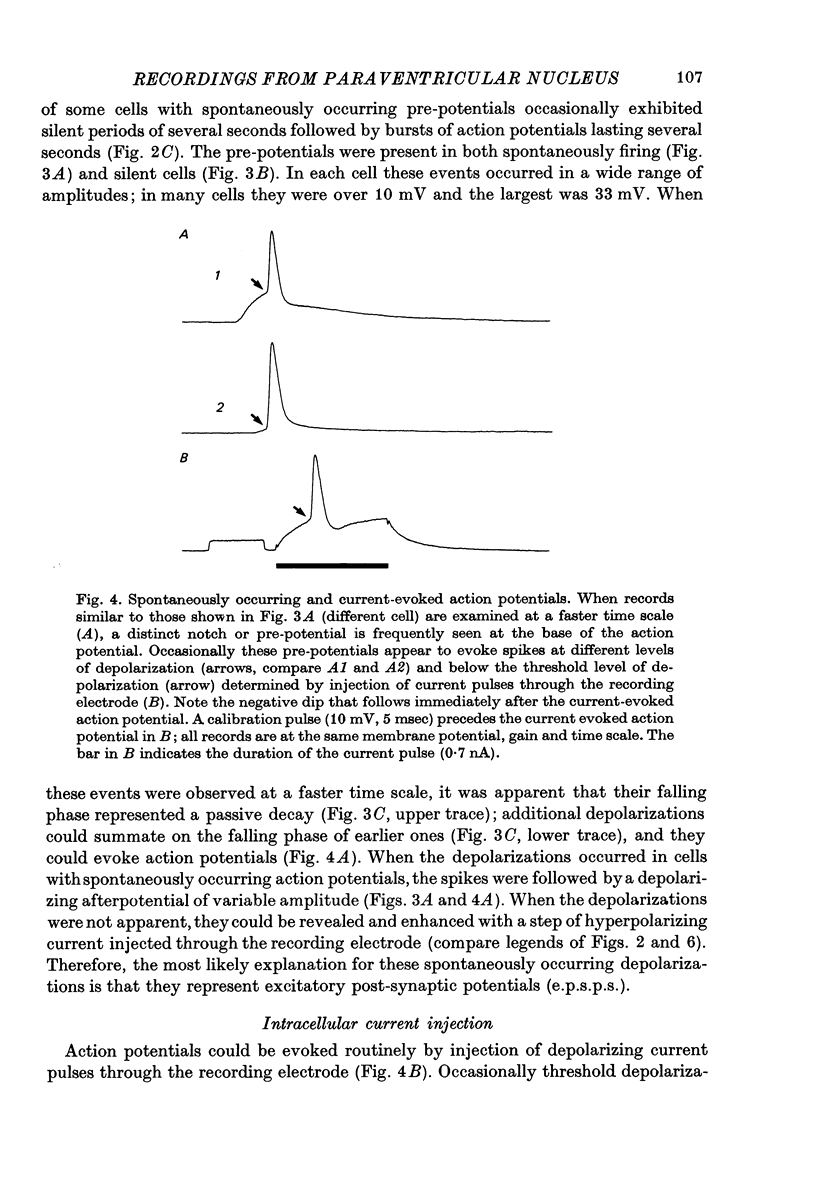





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antunes J. L., Carmel P. W., Zimmerman E. A. Projections from the paraventricular nucleus to the zona externa of the median eminence of the rhesus monkey: an immunohistochemical study. Brain Res. 1977 Nov 25;137(1):1–10. doi: 10.1016/0006-8993(77)91009-5. [DOI] [PubMed] [Google Scholar]
- Barker J. L., Crayton J. W., Nicoll R. A. Noradrenaline and acetylcholine responses of supraoptic neurosecretory cells. J Physiol. 1971 Oct;218(1):19–32. doi: 10.1113/jphysiol.1971.sp009602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship J. E., Kuno M. Analysis of spontaneous subthreshold activity in spinal motoneurons of the cat. J Neurophysiol. 1968 Mar;31(2):195–209. doi: 10.1152/jn.1968.31.2.195. [DOI] [PubMed] [Google Scholar]
- Brimble M. J., Dyball R. E. Characterization of the responses of oxytocin- and vasopressin-secreting neurones in the supraoptic nucleus to osmotic stimulation. J Physiol. 1977 Sep;271(1):253–271. doi: 10.1113/jphysiol.1977.sp011999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brimble M. J., Dyball R. E., Forsling M. L. Oxytocin release following osmotic activation of oxytocin neurones in the paraventricular and supraoptic nuclei. J Physiol. 1978 May;278:69–78. doi: 10.1113/jphysiol.1978.sp012293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy V. J., Watkins W. B. Immunocytochemical study of the hypothalamo-neurohypophysial system. II. Distribution of neurophysin, vasopressin and oxytocin in the normal and osmotically stimulated rat. Cell Tissue Res. 1977 Jun 13;180(4):467–490. doi: 10.1007/BF00220169. [DOI] [PubMed] [Google Scholar]
- Conrad L. C., Pfaff D. W. Efferents from medial basal forebrain and hypothalamus in the rat. II. An autoradiographic study of the anterior hypothalamus. J Comp Neurol. 1976 Sep 15;169(2):221–261. doi: 10.1002/cne.901690206. [DOI] [PubMed] [Google Scholar]
- Defendini R., Zimmerman E. A. The magnocellular neurosecretory system of the mammalian hypothalamus. Res Publ Assoc Res Nerv Ment Dis. 1978;56:137–154. [PubMed] [Google Scholar]
- Dudek F. E., Blankenship J. E. Neuroendocrine cells of Aplysia brasiliana. I. Bag cell action potentials and afterdischarge. J Neurophysiol. 1977 Nov;40(6):1301–1311. doi: 10.1152/jn.1977.40.6.1301. [DOI] [PubMed] [Google Scholar]
- Dudek F. E., Deadwyler S. A., Cotman C. W., Lynch G. Intracellular responses from granule cell layer in slices of rat hippocampus: perforant path synapse. J Neurophysiol. 1976 Mar;39(2):384–393. doi: 10.1152/jn.1976.39.2.384. [DOI] [PubMed] [Google Scholar]
- Duffy C. J., Teyler T. J. A simple tissue slicer. Physiol Behav. 1975 Apr;14(04):525–526. doi: 10.1016/0031-9384(75)90023-2. [DOI] [PubMed] [Google Scholar]
- FUXE K. EVIDENCE FOR THE EXISTENCE OF MONOAMINE NEURONS IN THE CENTRAL NERVOUS SYSTEM. IV. DISTRIBUTION OF MONOAMINE NERVE TERMINALS IN THE CENTRAL NERVOUS SYSTEM. Acta Physiol Scand Suppl. 1965:SUPPL 247–247:37+. [PubMed] [Google Scholar]
- Finlayson L. H., Osborne M. P. Secretory activity of neurons and related electrical activity. Adv Comp Physiol Biochem. 1975;6:165–258. doi: 10.1016/b978-0-12-011506-8.50009-3. [DOI] [PubMed] [Google Scholar]
- Freund-Mercier M. J., Richard P., Miro J. L. Données fonctionnelles en faveur de l'existence de trois types de cellules dans le noyau paraventriculaire chez la ratte. Brain Res. 1975 May 16;89(1):149–154. doi: 10.1016/0006-8993(75)90143-2. [DOI] [PubMed] [Google Scholar]
- Geller H. M. Phasic discharge of neurons in long-term cultures of tuberal hypothalamus. Brain Res. 1975 Aug 15;93(3):511–515. doi: 10.1016/0006-8993(75)90191-2. [DOI] [PubMed] [Google Scholar]
- Gähwiler B. H., Sandoz P., Dreifuss J. J. Neurones with synchronous bursting discharges in organ cultures of the hypothalamic supraoptic nucleus area. Brain Res. 1978 Aug 4;151(2):245–253. doi: 10.1016/0006-8993(78)90882-x. [DOI] [PubMed] [Google Scholar]
- Hatton G. I., Armstrong W. E., Gregory W. A. Spontaneous and osmotically-stimulated activity in slices of rat hypothalamus. Brain Res Bull. 1978 Sep-Oct;3(5):497–508. doi: 10.1016/0361-9230(78)90079-5. [DOI] [PubMed] [Google Scholar]
- Hatton G. I., Hutton U. E., Hoblitzell E. R., Armstrong W. E. Morphological evidence for two populations of magnocellular elements in the rat paraventricular nucleus. Brain Res. 1976 May 21;108(1):187–193. doi: 10.1016/0006-8993(76)90176-1. [DOI] [PubMed] [Google Scholar]
- Hayward J. N. Functional and morphological aspects of hypothalamic neurons. Physiol Rev. 1977 Jul;57(3):574–658. doi: 10.1152/physrev.1977.57.3.574. [DOI] [PubMed] [Google Scholar]
- Hayward J. N. Physiological and morphological identification of hypothalamic magnocellular neuroendocrine cells in goldfish preoptic nucleus. J Physiol. 1974 May;239(1):103–124. doi: 10.1113/jphysiol.1974.sp010558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANDEL E. R. ELECTRICAL PROPERTIES OF HYPOTHALAMIC NEUROENDOCRINE CELLS. J Gen Physiol. 1964 Mar;47:691–717. doi: 10.1085/jgp.47.4.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi K., Yamashita H. Studies of antidromically identified neurosecretory cells of the hypothalamus by intracellular and extracellular recordings. J Physiol. 1972 Mar;221(3):683–705. doi: 10.1113/jphysiol.1972.sp009776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss R. L., Dyball R. E., Cross B. A. Responses of antidromically identified supraoptic and paraventricular units to acetylcholine, noradrenaline and glutamate applied iontophoretically. Brain Res. 1971 Dec 24;35(2):573–575. doi: 10.1016/0006-8993(71)90504-x. [DOI] [PubMed] [Google Scholar]
- O'Donohue T. L., Crowley W. R., Jacobwitz D. M. Changes in ingestive behavior following interruption of a noradrenergic projection to the paraventricular nucleus: histochemical and neurochemical analyses. Pharmacol Biochem Behav. 1978 Jul;9(1):99–105. doi: 10.1016/0091-3057(78)90018-7. [DOI] [PubMed] [Google Scholar]
- Parent A., Butcher L. L. Organization and morphologies of acetylcholinesterase-containing neurons in the thalamus and hypothalamus of the rat. J Comp Neurol. 1976 Nov 15;170(2):205–225. doi: 10.1002/cne.901700206. [DOI] [PubMed] [Google Scholar]
- Saper C. B., Loewy A. D., Swanson L. W., Cowan W. M. Direct hypothalamo-autonomic connections. Brain Res. 1976 Nov 26;117(2):305–312. doi: 10.1016/0006-8993(76)90738-1. [DOI] [PubMed] [Google Scholar]
- Sar M., Stumpf W. E., Miller R. J., Chang K. J., Cuatrecasas P. Immunohistochemical localization of enkephalin in rat brain and spinal cord. J Comp Neurol. 1978 Nov 1;182(1):17–37. doi: 10.1002/cne.901820103. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin P. A. Characteristics of CA1 neurons recorded intracellularly in the hippocampal in vitro slice preparation. Brain Res. 1975 Mar 7;85(3):423–436. doi: 10.1016/0006-8993(75)90817-3. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin P. A. Further characteristics of hippocampal CA1 cells in vitro. Brain Res. 1977 Jun 3;128(1):53–68. doi: 10.1016/0006-8993(77)90235-9. [DOI] [PubMed] [Google Scholar]
- Swaab D. F., Pool C. W., Nijveldt F. Immunofluorescence of vasopressin and oxytocin in the rat hypothalamo-neurohypophypopseal system. J Neural Transm. 1975;36(3-4):195–215. doi: 10.1007/BF01253126. [DOI] [PubMed] [Google Scholar]
- Swanson L. W., Connelly M. A., Hartman B. K. Further studies on the fine structure of the adrenergic innervation of the hypothalamus. Brain Res. 1978 Jul 28;151(1):165–174. doi: 10.1016/0006-8993(78)90960-5. [DOI] [PubMed] [Google Scholar]
- Swanson L. W., Connelly M. A., Hartman B. K. Ultrastructural evidence for central monoaminergic innervation of blood vessels in the paraventricular nucleus of the hypothalamus. Brain Res. 1977 Nov 4;136(1):166–173. doi: 10.1016/0006-8993(77)90142-1. [DOI] [PubMed] [Google Scholar]
- Swanson L. W., Hartman B. K. The central adrenergic system. An immunofluorescence study of the location of cell bodies and their efferent connections in the rat utilizing dopamine-beta-hydroxylase as a marker. J Comp Neurol. 1975 Oct 15;163(4):467–505. doi: 10.1002/cne.901630406. [DOI] [PubMed] [Google Scholar]
- Swanson L. W. Immunohistochemical evidence for a neurophysin-containing autonomic pathway arising in the paraventricular nucleus of the hypothalamus. Brain Res. 1977 Jun 10;128(2):346–353. doi: 10.1016/0006-8993(77)91000-9. [DOI] [PubMed] [Google Scholar]
- Vandesande F., Dierickx K., De Mey J. The origin of the vasopressinergic and oxytocinergic fibres of the external region of the median eminence of the rat hypophysis. Cell Tissue Res. 1977 Jun 13;180(4):443–452. doi: 10.1007/BF00220167. [DOI] [PubMed] [Google Scholar]
- Wakerley J. B., Poulain D. A., Brown D. Comparison of firing patterns in oxytocin- and vasopressin-releasing neurones during progressive dehydration. Brain Res. 1978 Jun 16;148(2):425–440. doi: 10.1016/0006-8993(78)90730-8. [DOI] [PubMed] [Google Scholar]
- Yagi K. Electrophysiology of the neurosecretory cell. Int Rev Cytol. 1977;48:141–186. doi: 10.1016/s0074-7696(08)61744-1. [DOI] [PubMed] [Google Scholar]
- Yamamoto C. Activation of hippocampal neurons by mossy fiber stimulation in thin brain sections in vitro. Exp Brain Res. 1972;14(4):423–435. doi: 10.1007/BF00235037. [DOI] [PubMed] [Google Scholar]
- Yamashita H., Koizumi K., Brooks C. M. Electrophysiological studies of neurosecretory cells in the cat hypothalamus. Brain Res. 1970 Jun 15;20(3):462–466. doi: 10.1016/0006-8993(70)90176-9. [DOI] [PubMed] [Google Scholar]


