Abstract
1. I have studied the electrical excitability of outgrowing processes of individual neurones in cultures made from dissociated neural plates of embryos of Xenopus laevis prior to the time of neurite outgrowth in vivo. 2. The electrical excitability of neurites was tested by stimulating them extracellularly and recording responses with an intracellular electrode in their cell bodies; neurites were excitable at all times examined. 3. The ionic basis of the excitability of neurites was tested by recording from cells while changing the composition of the salines perfusing the cultures. 4. In cultures less than 10 hr old, all neurites tested made responses which depended on Ca2+. The action potentials of the cell bodies were also Ca2+-dependent at these times. 5. Between 10 and 12 hr in culture, a time at which the cell bodies still made Ca2+-dependent action potentials, neurites acquired the ability to make Na+-dependent responses. At these times, two-thirds of neurites tested retained the ability to produce divalent cation-dependent action potentials when perfused with solutions of isotonic Ba2+. 6. After 12 hr in culture, no neurites were observed to make Ca2+-or Ba2+-dependent responses; only Na+-dependent responses were observed. Cells continued to initiate and elongate new neurites until about 24 hr in culture. Thus neurites sent out at different times in culture differed in their development of excitability. 7. Cell bodies making exclusively Ca2+-dependent action potentials could be found until about 15 hr in culture, after which time a Na+-dependent component appeared. Cell bodies could then be observed to make action potentials which depended on both Ca2+ and Na+ until about 3 days in culture. After 3 days, most cell bodies made predominately Na+-dependent action potentials. Unlike the neurites, cell bodies retained the ability to make action potentials in isotonic Ba2+ for as long as the cultures were maintained (up to 5 days). 8. The possibility that changes in the ionic basis of action potentials reflected the death of one population of cells and the simultaneous appearance of another population with different properties was eliminated by observing the fate of single cells while changes in the physiological properties were occurring. Such observations showed that the majority of cells in each culture were surviving throughout the period of study. 9. Thus the membranes of the neurites and cell bodies of neurones in these cultures appeared to undergo independently timed changes in the ionic basis of their action potentials.
Full text
PDF



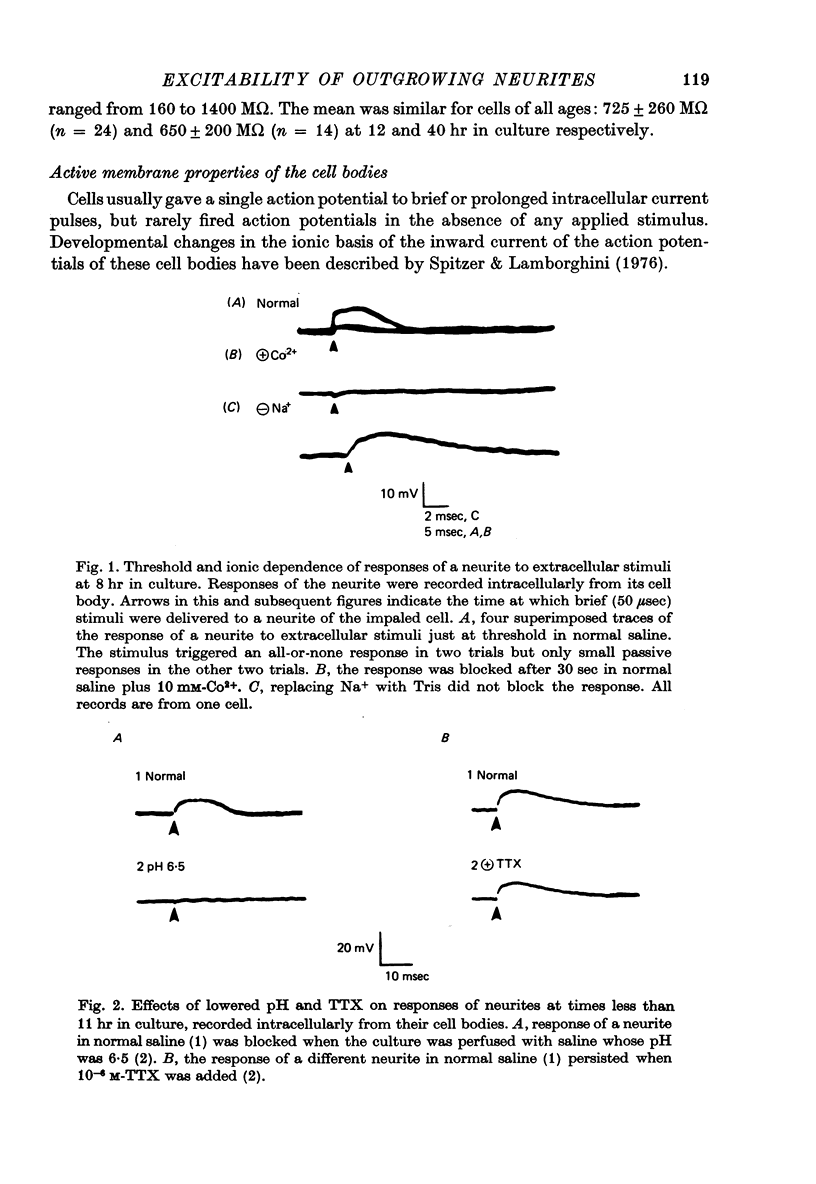

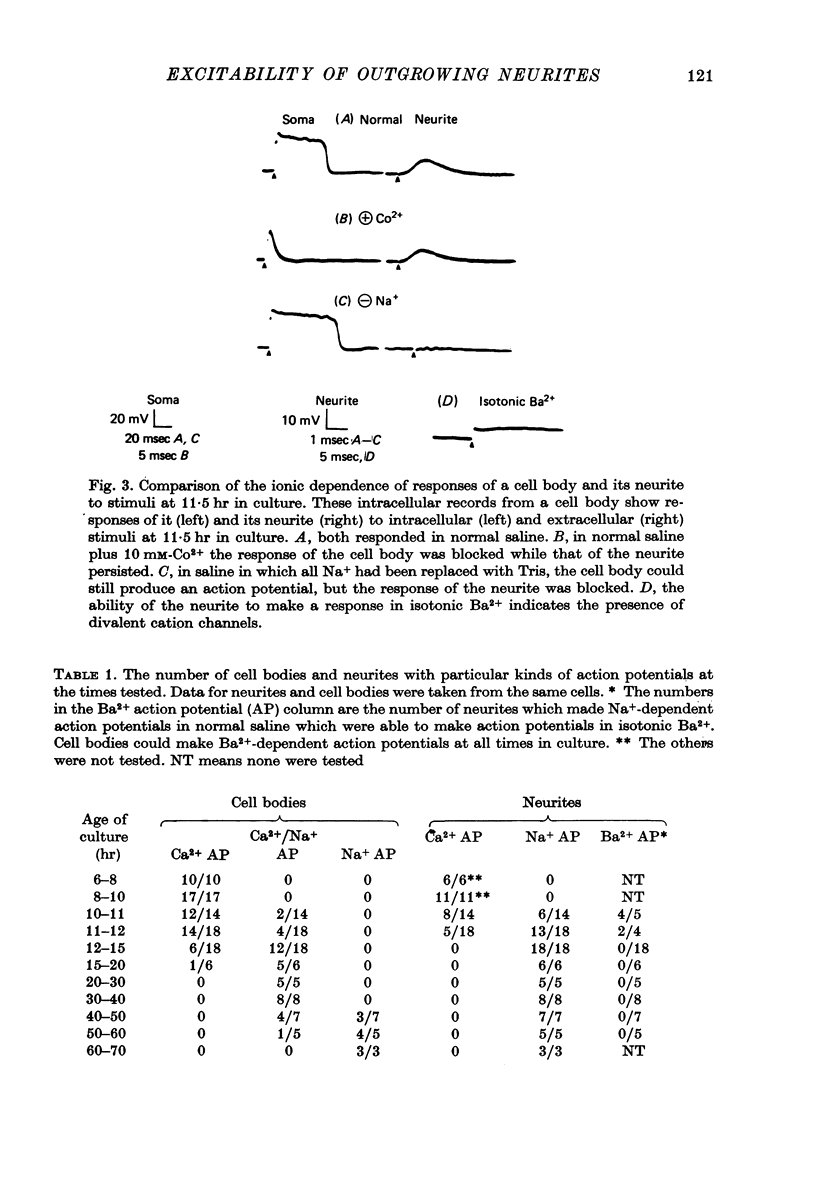
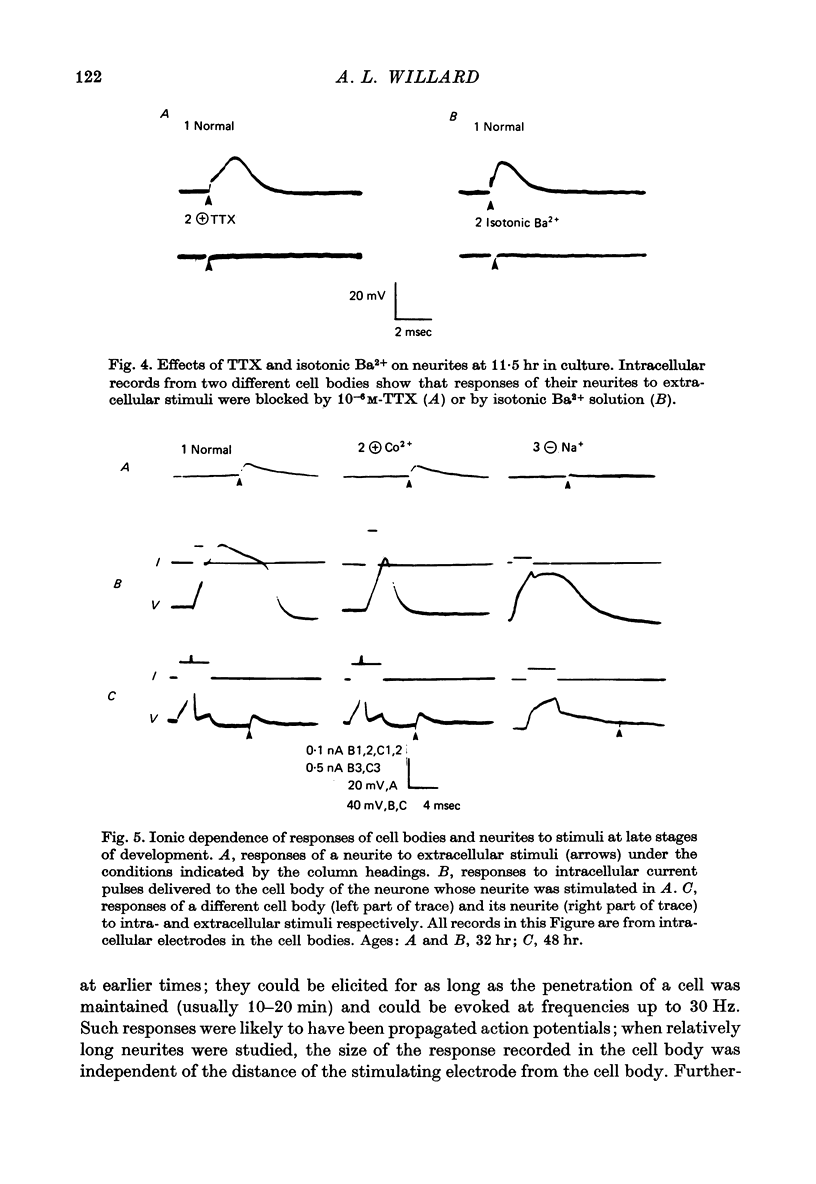
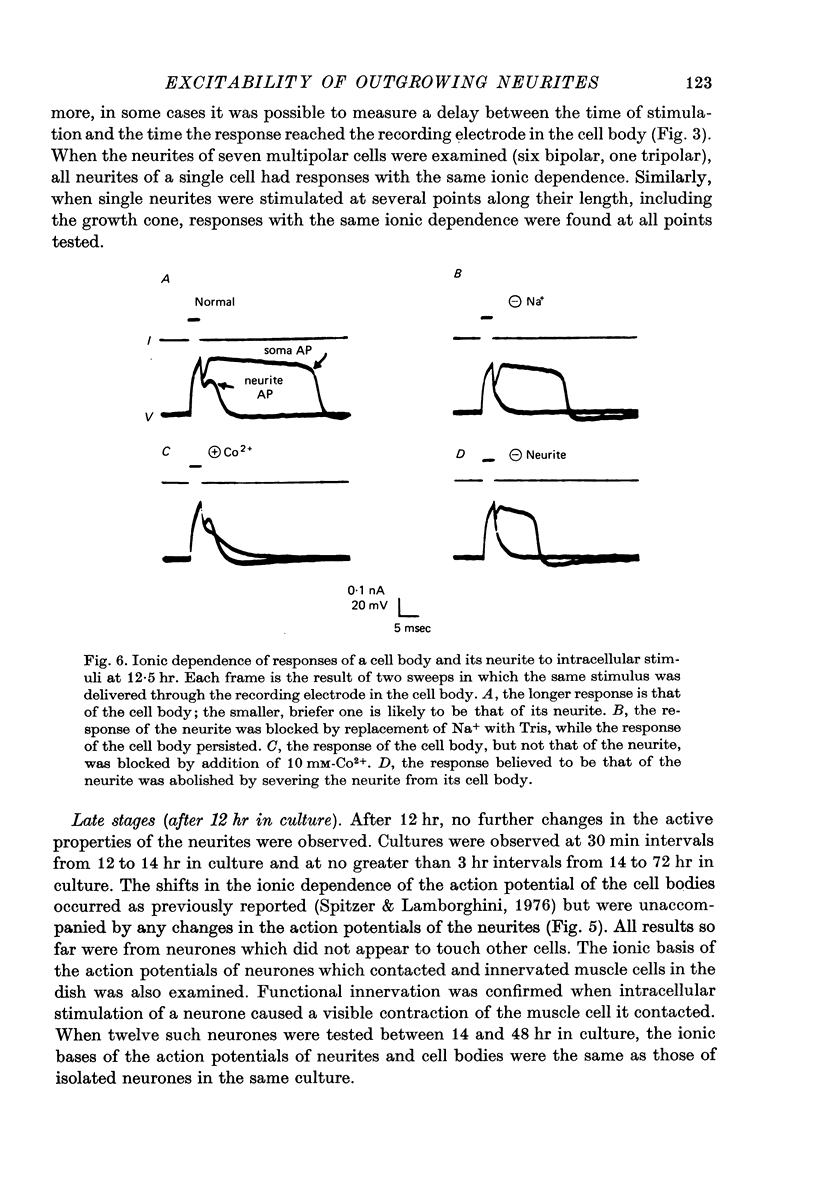





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baccaglini P. I. Action potentials of embryonic dorsal root ganglion neurones in Xenopus tadpoles. J Physiol. 1978 Oct;283:585–604. doi: 10.1113/jphysiol.1978.sp012521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccaglini P. I., Spitzer N. C. Developmental changes in the inward current of the action potential of Rohon-Beard neurones. J Physiol. 1977 Sep;271(1):93–117. doi: 10.1113/jphysiol.1977.sp011992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter M. A., Fischbach G. D. The action potential of chick dorsal root ganglion neurones maintained in cell culture. J Physiol. 1977 May;267(2):281–298. doi: 10.1113/jphysiol.1977.sp011813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S. Ca spike. Adv Biophys. 1973;4:71–102. [PubMed] [Google Scholar]
- Horn R. Propagating calcium spikes in an axon of Aplysia. J Physiol. 1978 Aug;281:513–534. doi: 10.1113/jphysiol.1978.sp012437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki S., Satow Y. Sodium- and calcium-dependent spike potentials in the secretory neuron soma of the X-organ of the crayfish. J Gen Physiol. 1971 Feb;57(2):216–236. doi: 10.1085/jgp.57.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junge D., Miller J. Different spike mechanisms in axon and soma of molluscan neurone. Nature. 1974 Nov 8;252(5479):155–156. doi: 10.1038/252155a0. [DOI] [PubMed] [Google Scholar]
- Kado R. T. Aplysia giant cell: soma-axon voltage clamp current differences. Science. 1973 Nov 23;182(4114):843–845. doi: 10.1126/science.182.4114.843. [DOI] [PubMed] [Google Scholar]
- Kullberg R. W., Lentz T. L., Cohen M. W. Development of the myotomal neuromuscular junction in Xenopus laevis: an electrophysiological and fine-structural study. Dev Biol. 1977 Oct 1;60(1):101–129. doi: 10.1016/0012-1606(77)90113-0. [DOI] [PubMed] [Google Scholar]
- Llinás R., Hess R. Tetrodotoxin-resistant dendritic spikes in avian Purkinje cells. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2520–2523. doi: 10.1073/pnas.73.7.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Steinberg I. Z., Walton K. Presynaptic calcium currents and their relation to synaptic transmission: voltage clamp study in squid giant synapse and theoretical model for the calcium gate. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2918–2922. doi: 10.1073/pnas.73.8.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meech R. W., Standen N. B. Potassium activation in Helix aspersa neurones under voltage clamp: a component mediated by calcium influx. J Physiol. 1975 Jul;249(2):211–239. doi: 10.1113/jphysiol.1975.sp011012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake M. The development of action potential mechanism in a mouse neuronal cell line in vitro. Brain Res. 1978 Mar 24;143(2):349–354. doi: 10.1016/0006-8993(78)90574-7. [DOI] [PubMed] [Google Scholar]
- Ross W. N., Stuart A. E. Voltage sensitive calcium channels in the presynaptic terminals of a decrementally conducting photoreceptor. J Physiol. 1978 Jan;274:173–191. doi: 10.1113/jphysiol.1978.sp012142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer N. C., Baccaglini P. I. Development of the action potential in embryo amphibian neurons in vivo. Brain Res. 1976 May 14;107(3):610–616. doi: 10.1016/0006-8993(76)90148-7. [DOI] [PubMed] [Google Scholar]
- Spitzer N. C., Lamborghini J. E. The development of the action potential mechanism of amphibian neurons isolated in culture. Proc Natl Acad Sci U S A. 1976 May;73(5):1641–1645. doi: 10.1073/pnas.73.5.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer N. C. Low pH selectively blocks calcium action potentials in amphibian neurons developing in culture. Brain Res. 1979 Feb 9;161(3):555–559. doi: 10.1016/0006-8993(79)90687-5. [DOI] [PubMed] [Google Scholar]
- Spitzer N. C. The ionic basis of the resting potential and a slow depolarizing response in Rohon-Beard neurones of Xenopus tadpoles. J Physiol. 1976 Feb;255(1):105–135. doi: 10.1113/jphysiol.1976.sp011272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WERMAN R., GRUNDFEST H. Graded and all-or-none electrogenesis in arthropod muscle. II. The effects of alkali-earth and onium ions on lobster muscle fibers. J Gen Physiol. 1961 May;44:997–1027. doi: 10.1085/jgp.44.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald F. Ionic differences between somatic and axonal action potentials in snail giant neurones. J Physiol. 1972 Jan;220(2):267–281. doi: 10.1113/jphysiol.1972.sp009706. [DOI] [PMC free article] [PubMed] [Google Scholar]



