Abstract
In mouse models and humans, Helicobacter pylori is associated with an increase in serum gastrin and gastrin-expressing (G) cells with a concomitant decrease in somatostatin-expressing D cells. Inflammation of the gastric mucosa can progress to metaplastic changes in the stomach and to decreased colonization by H. pylori and increased colonization by non-H. pylori organisms. In addition, about 20% of individuals with chronic gastritis are H. pylori negative, suggesting that other organisms may induce gastritis. Consistent with this hypothesis, we report here that Acinetobacter lwoffii causes the same histologic changes as does H. pylori. Gastric epithelial cells were isolated from the entire stomach by an enzymatic method for quantitation by both flow cytometry and morphometric analysis. Two months after mice were inoculated with H. pylori or A. lwoffii, the mucosal T- and B-cell numbers significantly increased. After 4 months of infection, there was a threefold increase in the number of G cells and a doubling in the number of parietal cells. A threefold decrease in the number of D cells occurred in H. pylori- and A. lwoffii-infected mice. Plasma gastrin levels increased after both H. pylori and A. lwoffii infection. Histology revealed the presence of inflammation in the gastric mucosa with both A. lwoffii and H. pylori infection. A periodic acid-Schiff stain-alcian blue stain revealed mucous gland metaplasia of the corpus. Collectively, the results demonstrate that gastritis and hypergastrinemia are not specific for H. pylori but can be induced by other gram-negative bacteria capable of infecting the mouse stomach.
Helicobacter pylori causes chronic atrophic gastritis, and its presence is correlated with the development of peptic ulcer disease and gastric adenocarcinoma (19, 25, 27). However, there is also an association between colonization of the stomach by non-Helicobacter organisms and chronic atrophic gastritis (7, 9, 30). Approximately 25% of gastric cancer patients have no evidence of previous or current H. pylori infection based on serology (12). In addition, during long-term acid suppression, the presence of H. pylori and that of non -H. pylori bacteria are independent risk factors for the development of atrophic gastritis (29). Studies of several animal models and humans have clearly shown that bacteria are important in triggering mucosal damage and inflammation in the stomach (15, 20, 42). In addition, under hypochlorhydric conditions it is known that bacterial overgrowth by non-Helicobacter organisms triggers perturbations in the neuroendocrine and epithelial cell populations (42). The implications are that the pathology observed may not be specific for H. pylori but instead is the general response of the gastric mucosa to colonization by bacteria.
H. pylori is characterized by its ability to survive in the low-pH milieu of the stomach by generating an alkaline microenvironment. With reduced levels of acid (hypochlorhydria or achlorhydria), the competitive niche established by H. pylori dissipates and the human stomach becomes susceptible to colonization by other organisms (9, 18). Gastric colonization by gram-negative bacteria other than H. pylori is common in intensive care unit patients, who often have an alkaline gastric pH due to routine treatment with antacids, proton pump inhibitors, and histamine 2 receptor antagonists. Antiulcer medications are known to increase the gastric pH and permit colonization of the stomach by opportunistic pathogens, such as Acinetobacter baumannii, Klebsiella pneumoniae, and Pseudomonas spp., believed to contribute to the development of nosocomial pneumonia (8, 36). Patients with pernicious anemia are colonized by organisms other than H. pylori and develop atrophic gastritis and elevated levels of gastrin in serum (9, 18). Moreover, 2 weeks of proton pump therapy reduces gastric acid by 75% and is sufficient to permit bacterial colonization of the stomach in healthy volunteers (23). In addition, our recent studies have demonstrated that the rise in plasma gastrin levels in mice with chronic gastritis is the result of inflammation and not gastric pH (42). The genera of bacteria isolated from these stomachs include gram-positive and gram-negative organisms, e.g., Neisseria and Acinetobacter species (34, 35). In addition, about 20% of individuals with chronic gastritis are H. pylori negative, suggesting that organisms other than H. pylori induce changes in the normal stomach.
The study described here tests the hypothesis that inflammation in the stomach may be caused by organisms other than H. pylori. We have recently shown that a mixed microaerophilic culture of Acinetobacter, Pseudomonas, and a gram-positive species stimulates an increase in serum gastrin levels when inoculated into the mouse stomach (26). In addition, live cultures of the bacteria stimulated gastrin and interleukin-8 promoter activity. We found that the protein mediating the promoter activation is a major outer membrane protein (OmpA-like protein) from the Acinetobacter lwoffii family. Therefore, we analyzed whether oral inoculation by A. lwoffii alone is sufficient to trigger gastritis, hypergastrinemia, and the same neuroendocrine cell changes as those observed with H. pylori.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
A stock of H. pylori (SS1 strain, obtained from K. A. Eaton, Ohio State University) was inoculated on 5% sterile horse blood in campylobacter selective agar (Difco) supplemented with trimethoprim (5 mg/ml), vancomycin (10 mg/ml), and nystatin (10 mg/ml) (17). Plates were incubated for 2 days in a humidified microaerophilic chamber (BBL Gas System, with CampyPak Plus packs [Fisher]). H. pylori was harvested and used to inoculate mouse stomachs by oral intubations. A stock of A. lwoffii (obtained from the American Type Culture Collection) expressing OmpA (26) was used to inoculate Leeds Acinetobacter Medium (LAM) plates (11). The plates were incubated overnight at 37°C. The bacteria were harvested and used for oral intubations of mice.
Seven 12-week-old C57BL/6 mice were anesthetized by ether. Mice were pretreated by orally intubating them with streptomycin (5 mg/ml) diluted in brain heart infusion (BHI) for 3 consecutive days. After 48 h, mice were orally inoculated with a catheter three times over a period of 3 days with 108 organisms (per 200 μl) of either A. lwoffii or H. pylori suspended in BHI. Control animals were administered 200 μl of BHI alone. Mice were sacrificed at 2, 3, and 4 months after oral inoculation.
Verification of colonization.
To verify colonization by H. pylori, gastric biopsy samples were collected from the stomach, streaked onto blood plates (as described above), and incubated under microaerophilic conditions at 37°C for 3 to 5 days. Single colonies from these plates were tested for urease (with a drop of urea broth: 10 g of urea, 0.5% [wt/vol] phenol red, 0.22 g of NaH2PO4 · H2O, 0.51 g of Na2HPO4, and 100 mg of NaN3, in 500 ml of distilled water at pH 6.2), catalase (with 3% H2O2 solution), and oxidase (DrySlide; BBL) activity.
To verify colonization by A. lwoffii, gastric biopsy samples were collected from the stomach, streaked onto LAM plates (as described above), and incubated at 37°C overnight. Colonies were checked for up to 5 days of incubation; however, there were no colonies positive for Acinetobacter. Therefore, to verify colonization by A. lwoffii, DNA was extracted from the stomach tissue of inoculated mice with a DNeasy tissue kit (Qiagen) according to the manufacturer's protocol. Oligonucleotide primers were used to amplify a 16S rRNA amplicon of approximately 1,500 bp. The primer sequences were generated according to the method of Vaneechoutte et al. (38) and were as follows: 5′ TGG CTC AGA TTG AAC GCT GGC GGC (forward primer) and 5′ TAC CTT GTT ACG ACT TCA CCC CA (reverse primer). PCR conditions were as previously published (38). Amplified ribosomal DNA restriction analysis (ARDRA) was then carried out to identify the Acinetobacter genomic species isolated from the inoculated mouse stomachs. Restriction was carried out for 1 h at 37°C in 20-μl volumes of incubation buffer containing 5 U of restriction enzyme AluI, CfoI, MspI, MboI, RsaI, or HinfI and 10 μl of 1,500-bp PCR product. Restriction fragment patterns were analyzed by gel electrophoresis and compared to the patterns previously published (38).
Immunohistochemistry.
A longitudinal section of the stomach (spanning both the corpus and antral regions) was fixed in 4% paraformaldehyde-phosphate-buffered saline and embedded in paraffin, and 3-μm sections were prepared. Sections were deparaffinized through an alcohol series and permeabilized in 3% H2O2 and 100% ethanol. Nonspecific antigen sites were blocked with 20% normal goat serum-phosphate-buffered saline-0.1% Triton X-100 for 30 min before incubation for 2 h with either a 1:200 dilution of mouse anti-H+,K+-ATPase β subunit (Medical and Biological Laboratories), rabbit antisomatostatin (Zymed), rabbit antigastrin (Dako), or Ki67 (Novocastra Laboratories) antibody. A 1:500 dilution of the secondary anti-rabbit or anti-mouse immunoglobulin G antibody was added for 30 min and visualized with avidin-biotin complexes with the Vectastain Elite ABC kit and diaminobenzidine as the substrate (Vector Laboratories, Inc., Burlingame, Calif.). Morphometric analysis was performed on sections stained for parietal, G, and D cells and Ki67-positive nuclei. A total of 10 correctly oriented glands in random fields from representative areas of inflammation were counted.
Sections were also stained with hematoxylin and eosin (H&E), periodic acid-Schiff stain (PAS)-alcian blue, or Warthin-Starry stain. Sections stained by H&E were graded on the intensity of gastritis and metaplasia by a pathologist blinded to the treatment according to a grading system developed for the histologic quantification of gastritis in mice (6). Sections were scored as follows: 0 to 1, no inflammation or metaplasia; 2, inflammatory infiltrate sufficient to displace glands; and 3, marked inflammation with metaplasia. Metaplasia is defined as the replacement of normal fundic morphology with mucus-secreting glands (6).
Cell preparation and flow cytometry.
Lymphocytes and epithelial cells were isolated from the gastric mucosa of individual mice according to a previous method (2). Briefly, the stomach was dissected into 2-mm-size pieces. The pieces were first incubated in 20 ml of Hank's balanced salt solution containing 5% bovine serum albumin, 1 mM dithiothreitol, and 1 mM EDTA for 1 h with vigorous shaking at 37°C to release the epithelial cell population. This first cell suspension was passed through a filter (50-μm-pore-size Filcon filter; Dako), collected, and washed twice with RPMI medium containing 5% fetal calf serum. The stripped mucosa was then subjected to enzymatic digestion in 20 ml of RPMI medium containing 1 mg of dispase II (Roche Molecular Biochemicals)/ml for two 30-min incubations at 37°C with vigorous shaking to remove mucosal lymphocytes. The lymphocytes were collected, washed, and surface labeled for flow cytometry. Isolated T cells, B cells, and leukocytes were labeled with fluorescein isothiocyanate-conjugated anti-mouse CD3 (Pharmingen), CD19 (Pharmingen), and phycoerythrin-conjugated anti-mouse CD45 (Pharmingen), respectively. G cells were detected with (Lys3)-bombesin 14 (Bachem, Torrance, Calif.) conjugated to the fluorescent dye Cy3 (Amersham Pharmacia) (31). D cells were detected with a somatostatin antibody (Vector Laboratories) after permeabilization. Parietal cells were detected with an antibody specific for the β subunit of H+,K+-ATPase (Medical and Biological Laboratories) after permeabilization (42). The total epithelial cell population was quantified using a monoclonal anti-human cytokeratin-18 (keratin RCK106) antibody (catalogue no. 11416; Cappel ICN Pharmaceuticals). Fluorescein isothiocyanate-conjugated anti-mouse immunoglobulin (Cappel ICN Pharmaceuticals) was used to detect the antibody to the β subunit and cytokeratin-18 primary antibodies. In each case an isotype control sample which contained the secondary antibody alone was run to eliminate nonspecific cross-reactivity and background. Labeled cells were then analyzed by flow cytometry with a Coulter Elite ESP Cell Sorter (Beckman-Coulter Electronics, Hialeah, Fla.). A total of 10,000 cells were analyzed for all cell types. Changes in T and B cells were calculated as follows: cell number in total cell preparation × % CD45+ cells × % CD3+ or CD19+ cells = number of T or B cells in the mucosa (1).
Peptide assays.
After sacrifice, approximately 1 ml of blood was collected by cardiac puncture, aliquoted into lithium heparinized glass tubes (Becton Dickinson Vacutainer Systems, Rutherford, N.J.), and centrifuged at 3,000 rpm for 15 min at 4°C (Beckman GS-6KC centrifuge). Plasma was collected and immediately stored at −20°C until assayed. Gastrin was directly assayed with 50 μl of plasma by radioimmunoassay using 125I-15Met human gastrin and antiserum 1296 (Center for Ulcer Research and Education, University of California, Los Angeles). Antiserum 1296 detects all carboxy-terminal fragments larger than the pentapeptide and measures both sulfated and nonsulfated forms of gastrin identically. Gastrin-17 (5 pmol/liter) was used to generate the standard curve. The 50% infective dose was 1 fmol/ml, and the inter- and intra-assay coefficients of variation were <2 and 11%, respectively (33).
Statistical analysis.
The results were statistically tested by unpaired t test or one-way analysis of variance as appropriate with available software (GraphPad Prism; GraphPad Software, San Diego, Calif.). A P value of <0.05 was considered significant.
RESULTS
Verifying mouse colonization by H. pylori and A. lwoffii.
A representative Warthin-Starry stain demonstrated the presence of Helicobacter in the gastric crypts of mice 4 months after inoculation (Fig. 1B) compared to a control mouse (Fig. 1A). Colonies isolated from gastric biopsy samples of all mice inoculated with H. pylori were positive for all three enzyme tests (oxidase, catalase, and urease). H. pylori could not be cultured from stomachs of control or A. lwoffii-inoculated mice. No colonies were isolated from mice inoculated with A. lwoffii. Therefore, verification of A. lwoffii colonization was achieved by ARDRA.
FIG. 1.
Verification of H. pylori and A. lwoffii colonization in inoculated mice. (A and B) Warthin-Starry stain of the stomach sections from control (A) and H. pylori-inoculated (B) mice showing the presence of Helicobacter in the gastric crypts of 4-month-infected mice. (C) Amplification of a 1,500-bp amplicon in tissue extracts from A. lwoffii-inoculated mice with 16S rRNA primers specific for Acinetobacter. Lane 1, water control; lane 2, H. pylori DNA; lane 3, A. lwoffii DNA; lanes 4 to 10, control mice; lanes 11 to 17, A. lwoffii isolated from inoculated mice. (D) ARDRA pattern for PCR products amplified from H. pylori (lanes 1), A. lwoffii DNA (lanes 2), or A. lwoffii isolated from inoculated mice (lanes 3). Lanes M, DNA markers.
DNA isolated from A. lwoffii-inoculated mice was amplified for Acinetobacter-specific 16S rRNA. Figure 1C shows PCR products of the expected 1,500-bp fragment size in both A. lwoffii-inoculated mice. ARDRA of DNA isolated from A. lwoffii-inoculated mice demonstrated a restriction fragment pattern identical to that of DNA extracted from the original A. lwoffii culture (Fig. 1D) (38). The restriction digests with either CfoI, AluI, or MspI enzyme showed clear differences between H. pylori and A. lwoffii 16S rRNA. Collectively these results confirm the presence of A. lwoffii in the inoculated mice.
Both H. pylori and A. lwoffii cause gastric mucosal inflammation.
H&E stains show inflammation within the corpus mucosa of both H. pylori- and A. lwoffii-infected mice (Fig. 2B and C, respectively). Mucous gland metaplasia was documented in mice infected for 2 or 3 months with H. pylori or A. lwoffii (Fig. 2E and F, respectively). A PAS-alcian blue stain was used to demonstrate the presence of neutral and acid mucins present in both H. pylori- and A. lwoffii-infected mice (Fig. 2E and F, respectively). Histologic changes were evaluated by a pathologist who scored the slides for intensity of inflammation and metaplasia. Within 2 months of H. pylori infection the histologic scores increased significantly and remained elevated throughout the 4-month infection period (Fig. 3). Although the histologic score increased after a 2-month A. lwoffii infection, inflammation was not as intense as that seen for H. pylori-inoculated mice. At 4 months of infection, the histologic scores for the A. lwoffii-inoculated mice were comparable to those of the H. pylori-inoculated mice (Fig. 3).
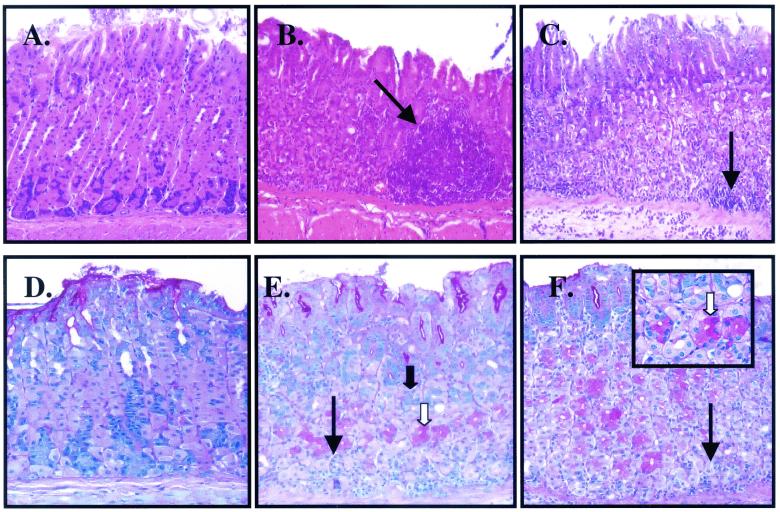
FIG. 2.
Histochemical analysis of tissue extracts from H. pylori- or A. lwoffii-inoculated mice. H&E staining of tissue extracts from control (A) and H. pylori (B)- and A. lwoffii (C)-inoculated mice shows significant inflammation as indicated by the arrows. PAS-alcian blue staining of tissue extracts from control (D) and H. pylori (E)- and A. lwoffii (F)-inoculated mice shows neutral (white arrow) and acid (solid arrow) mucins. Magnification, ×400 (all panels).
FIG. 3.
Histologic scores for H. pylori (HP)- and A. lwoffii (AC)-inoculated mice. Shown are results of histologic evaluation of the level of inflammation and metaplasia over 4 months of infection with H. pylori and A. lwoffii. Scoring was based upon the protocol by Eaton et al. (6). n = 7 for each group.
Lymphocytes were elevated during H. pylori and A. lwoffii inoculation of the gastric mucosa.
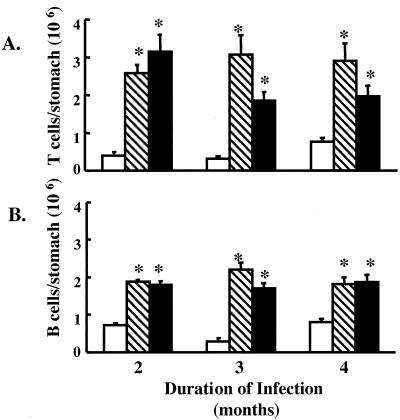
Histologic evaluation was supported by flow cytometry results that revealed an increase in T cells by approximately threefold after 2, 3, and 4 months of infection with H. pylori and A. lwoffii (Fig. 4A). The B-cell population increased after 2, 3, and 4 months of both H. pylori and A. lwoffii infection (Fig. 4B). Collectively, these data show that gastric mucosal inflammation is caused not only by H. pylori infection but also by A. lwoffii infection.
FIG. 4.
Gastric mucosal lymphocytes after bacterial colonization. Histograms for mice analyzed after 4 months of infection are shown. The figure shows the numbers of T (A) and B (B) cells analyzed by flow cytometry of gastric cells isolated from control (open bars) or H. pylori (hatched bars)- or A. lwoffii (solid bars)-inoculated mice. ∗, P < 0.05 compared to control animals; n = 7 animals.
Changes in neuroendocrine and epithelial cell populations.
Morphometric and flow cytometric analyses were used to analyze changes in neuroendocrine cell numbers after bacterial colonization. Immunohistochemical evaluation revealed an increase in the number of H+,K+-ATPase β-subunit-positive cells in the gastric mucosa of 2-month H. pylori- and A. lwoffii-inoculated mice compared to controls (Fig. 5A). When the parietal cells were quantified by morphometry (Fig. 5B) and flow cytometry (Fig. 5C), there was a significant increase in the number of cells after 2, 3, and 4 months of H. pylori and A. lwoffii infection. The percentage of cells staining positive for the epidermal growth factor (EGF) receptor as a general epithelial cell marker also nearly doubled over the same 4-month period, suggesting a general increase in the mucosal population (Fig. 6A). As a measure of the proliferative rate, sections were stained for Ki67. The number of Ki67-positive cells increased approximately twofold in both H. pylori (from 9 ± 1 to 15 ± 2 cells/gland)- and A. lwoffii (from 6 ± 1 to 12 ± 2 cells/gland)-inoculated mice. Figure 6B shows representative stomach sections stained with Ki67 from a 4-month-infected mouse reflecting the twofold increase in the number of positively stained cells.
FIG. 5.
Parietal cell changes after bacterial colonization. (A) Immunohistochemical stain of parietal cells in control (a) and H. pylori (b)- and A. lwoffii (c)-inoculated mice. (B) Morphometric analysis of parietal cells per gland from control (open bars) and H. pylori (hatched bars)- and A. lwoffii (closed bars)-inoculated mice. (C) Flow cytometry was used to analyze the changes in the parietal cell population per stomach. The number of parietal cells was analyzed by flow cytometry with gastric cells isolated from control (open bars) and H. pylori (hatched bars)- or A. lwoffii (closed bars)-inoculated mice. ∗, P < 0.05 compared to control mice; n = 7 animals.
FIG. 6.
Changes in proliferative markers after bacterial colonization. (A) Flow cytometry was used to analyze the changes in cells expressing the EGF receptor (epithelial cell population). The number of EGF receptor-positive cells increased in mice inoculated with H. pylori (hatched bars) or A. lwoffii (closed bars) compared to control animals (open bars). ∗, P < 0.05 compared to controls; n = 7 mice. (B) Representative slides of stomach sections stained with the proliferation marker Ki67 from control (a) and H. pylori (HP)- and A. lwoffii (AC)-inoculated mice infected for 4 months. Arrow indicates BrdU-labeled cells.
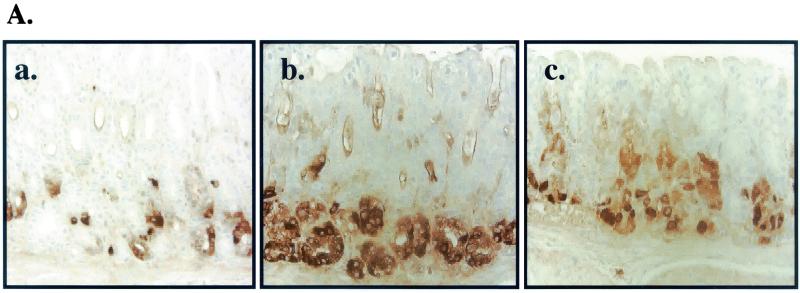
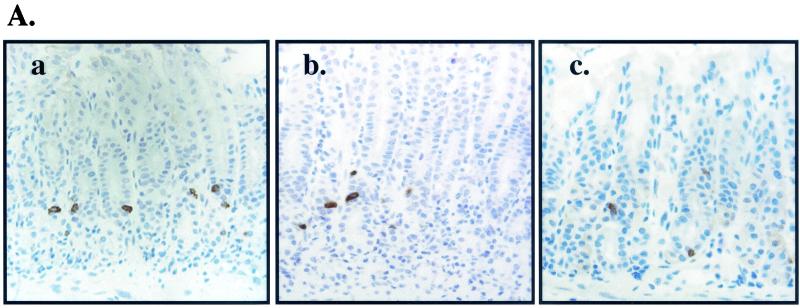
Immunohistochemistry revealed an increase in the number of positive gastrin-immunoreactive cells in both H. pylori- and A. lwoffii-inoculated mice infected for 2 months (Fig. 7). After 2, 3, and 4 months of colonization with H. pylori and A. lwoffii, G cells increased between two- and fivefold as analyzed by morphometry (Fig. 7B) or flow cytometry (Fig. 7C). In contrast to the parietal and G cells, D-cell numbers decreased after 2 months of H. pylori and A. lwoffii infection compared to those for uninoculated mice (Fig. 8). The number of D cells decreased twofold after H. pylori and A. lwoffii infection over the 4-month period in both the fundus and the antrum (Fig. 8B) or when quantified as a percentage of the total stomach (Fig. 8C). Consistently with the changes in the other cell populations, the decrease in D cells was sustained for 4 months.
FIG. 7.
G-cell changes after bacterial colonization. (A) Immunohistochemical stain of G cells in control (a) and H. pylori (b)- and A. lwoffii (c)-inoculated mice. (B) Morphometric analysis of G cells per gland from control (open bars) and H. pylori (hatched bars)- and A. lwoffii (closed bars)-inoculated mice. (C) Flow cytometry was used to analyze the changes in the G-cell population. The number of G cells was analyzed by flow cytometry with gastric cells isolated from control (open bars) or H. pylori (hatched bars)- or A. lwoffii (closed bars)-inoculated mice. ∗, P < 0.05 compared to control mice; n = 7 animals.
FIG. 8.
D-cell changes after bacterial colonization. (A) Immunohistochemical stain of D cells in control (a) and H. pylori (b)- and A. lwoffii (c)-inoculated mice infected for 4 months. (B) Morphometric analysis of fundic and antral D cells per gland of slides collected from control (open bars) and H. pylori (hatched bars)- and A. lwoffii (closed bars)-inoculated mice. (C) Flow cytometry was used to analyze the changes in the D-cell population. The number of D cells was analyzed by flow cytometry with gastric cells isolated from control (open bars) or H. pylori (hatched bars)- or A. lwoffii (closed bars)-inoculated mice. ∗, P < 0.05 compared to control mice; n = 7 animals.
Plasma gastrin concentrations are elevated during H. pylori and A. lwoffii infection.
Table 1 summarizes the plasma gastrin concentrations measured after 2, 3, and 4 months of colonization with either H. pylori or A. lwoffii. After 2, 3, and 4 months of H. pylori infection plasma gastrin concentrations increased by four- to sevenfold (Table 1). Similarly, after 2, 3, and 4 months of A. lwoffii infection plasma gastrin levels increased by three- to sixfold (Table 1).
TABLE 1.
Changes in plasma gastrin concentrations
| Infection (organism and duration) | Plasma gastrin concn (pmol/liter) in:
|
|
|---|---|---|
| Control mice | Inoculated mice | |
| H. pylori | ||
| 2 mo | 56 ± 23 | 383 ± 99a |
| 3 mo | 44 ± 10 | 195 ± 36a |
| 4 mo | 62 ± 21 | 406 ± 42 |
| A. lwoffii | ||
| 2 mo | 50 ± 16 | 153 ± 64a |
| 3 mo | 38 ± 3 | 186 ± 63a |
| 4 mo | 35 ± 3 | 222 ± 55a |
P < 0.05 versus control mice.
DISCUSSION
Conditions such as gastritis, peptic ulcers, and gastric carcinoma are often associated with colonization by H. pylori in the stomach. Colonization by bacteria other than H. pylori has been shown, though the effects on the gastric mucosa have not been studied in detail. The present study demonstrates that bacterial colonization of the mouse stomach with A. lwoffii will cause a pathological response that mimics infection by H. pylori. Colonization of the mouse stomach by A. lwoffii stimulated an increase in T and B lymphocytes after 4 months of A. lwoffii infection. Bacterial infection by either organism increased the number of G and parietal cells with a concomitant decrease in the number of D cells. In addition, after 2 months of infection with H. pylori or A. lwoffii, there was an overall increase in the number of parietal and epithelial cells with the appearance of mucous gland metaplasia. Thus, infection by gram-negative organisms, whether by H. pylori or not, triggers an inflammatory response by the host. The increase in cellular proliferation, e.g., epithelial, parietal, and G cells, is similar to what we observed elsewhere for hypochlorhydric mice exhibiting bacterial overgrowth from non-H. pylori organisms (42). Moreover, the presence of metaplasia with an increase in the number of cells suggests a change in the differentiation pattern rather than a net increase in cell death.
The susceptibility of the stomach to H. pylori versus A. lwoffii infection may depend upon the gastric pH. H. pylori survives in the human stomach at low pH (pH < 3) by creating an alkaline microenvironment with urease production (21). Although Acinetobacter has variable urease activity, this bacterial species has been reported previously to colonize the hypochlorhydric stomach of patients (32, 39). In addition, the mouse stomach, which has a gastric acidic range of approximately 3 to 5 μeq, provides an environment permissive for colonization by non-H. pylori organisms (42). Consistent with the mouse model presented here, prior studies have reported that increasing gastric pH in the human and mouse stomach results in bacterial overgrowth and subsequently the development of gastritis and metaplasia (9, 18, 35). In our study, reducing the gastric acidity to 1 to 2 μeq was sufficient to observe bacterial overgrowth (42).
Unlike with the H. pylori-inoculated mice, we were unable to culture the live A. lwoffii bacteria from the mouse stomach with LAM plates. Yet, A. lwoffii-inoculated mice had significant inflammation and were positive for Acinetobacter 16S rRNA by ARDRA. Therefore, these animals were clearly colonized with A. lwoffii and developed gastritis while the controls did not. Since it has recently been shown that Toll-like receptor 9 (TLR-9) mediates a Th1 inflammatory response from bacterial DNA (10), it is highly possible that the presence of Acinetobacter DNA in the gut was sufficient to induce gastritis, but this requires further analysis.
Acinetobacter has been previously isolated from the gastric contents of patients treated with proton pump inhibitors and is clinically relevant in humans (32). Both A. baumannii and A. lwoffii are known to cause nosocomial infections in ventilated patients (8). In addition, a study using a rat model shows the contribution of gut bacteria, in particular A. lwoffii, in the pathogenesis of hepatic encephalopathy (41). However, our study is the first to correlate Acinetobacter colonization with gastric inflammation and metaplasia. It is known that gastritis coincides with an increase in gastric pH and bacterial overgrowth (9, 18). Furthermore, gastritis predominating in the corpus is thought to be due to an autoimmune phenomenon or pernicious anemia in which the incidence of H. pylori infection in these patients is low (9, 35). The implications of these findings for human disease are that bacterial overgrowth by non-H. pylori organisms, e.g., Acinetobacter, may be one of several highly sought-after triggers for autoimmune (type A) chronic gastritis.
Both H. pylori and A. lwoffii infections caused a significant amount of inflammation. When the quantitative analysis of the inflammation was taken into consideration, e.g., T-cell numbers and gastrin levels, arguably H. pylori appeared to generate a more intense inflammatory response than did A. lwoffii. Inflammation seen after histology was correlated with an increase in the lymphocyte population. Similarly, patients infected with H. pylori eventually develop chronic gastritis, a condition that is characterized by infiltration of the gastric submucosa and lamina propria by T cells, B cells, and macrophages (3, 5, 13, 20, 28, 43). We extend these findings here by showing that this pathology is not specific for H. pylori but can also be caused by non-H. pylori organisms, specifically Acinetobacter.
The proliferative response of the epithelium appears to be initiated by inflammation. In particular, we show an increase in the number of parietal cells with both H. pylori and A. lwoffi infections. Although some human and mouse studies report parietal cell loss (i.e., atrophy), this process appears to be time dependent, occurring after long-standing bacterial infection (Y. Zavros and J. L. Merchant, unpublished observations). In support of our findings the presence of inflammation due to bacterial overgrowth during acid suppression (14, 42) or Helicobacter felis infection (40) is correlated with an increase in parietal cell numbers. Thus, both the increase in parietal cells and the increase in G cells, by H. pylori, A. lwoffii, or bacterial overgrowth, are due to the inflammatory response of the stomach to bacterial infection (42). In contrast, the decrease in the D cells is due to the hypergastrinemia (42). There was an increase in the number of Ki67-positively stained nuclei in infected animals as well as EGF receptor surface-labeled cells, suggesting an increase in the overall proliferative rate. The increase in the EGF receptor is consistent with the fact that transforming growth factor α and heparin-binding EGF levels are elevated in patients with gastric inflammation (4). Moreover, after H. pylori infection, hypergastrinemia up-regulates heparin-binding EGF and transforming growth factor α (16, 22, 24, 37, 40). Collectively, these results would suggest that EGF receptor ligands may be regulating mucosal proliferation.
In conclusion, gastritis is not specific to H. pylori infection. Acinetobacter, a known human pathogen, is able to colonize the mouse stomach and cause gastric inflammation and metaplastic changes in a manner similar to that of H. pylori. This study has clinical relevance to patients with low gastric acid secretion either due to diseases such as autoimmune gastritis and pernicious anemia or due to acid-suppressing drugs. These patients are known to have bacterial overgrowth associated with gastritis. An important question to address in future clinical studies is whether A. lwoffii or other Acinetobacter species and/or other gram-negative organisms are responsible for the gastritis observed for patients serologically negative for H. pylori. Given that Toll-like receptors can recognize bacterial DNA, these organisms may not be cultured out of the stomach (as observed here) but instead may be capable of triggering inflammation simply due to bacterial remnants.
Acknowledgments
Y.Z. is an associate and J.L.M. is an assistant investigator of the Howard Hughes Medical Institute. This work was supported in part by Public Health Service grant DK-45729 (J.L.M.).
We acknowledge the assistance of the Flow Cytometry core in the University of Michigan Cancer Center (CA46952) and the University of Michigan Multipurpose Arthritic Center (AR20557) and the assistance of the radioligand core of the University of Michigan Gastrointestinal Peptide Research Center (DK-34533), particularly Chris Dickinson for preparation of the iodinated gastrin. We thank Andrea Todisco and Linda Samuelson for critical review of the manuscript. We also thank Mary Van Antwerp for her technical assistance.
Editor: J. D. Clements
REFERENCES
- 1.Alderuccio, F., B. H. Toh, P. A. Gleeson, and I. R. van Driel. 1995. A novel method for isolating mononuclear cells from the stomachs of mice with experimental autoimmune gastritis. Autoimmunity 21:215-221. [DOI] [PubMed] [Google Scholar]
- 2.Davies, M. D., and D. M. Parrott. 1981. Cytotoxic T cells in small intestine epithelial, lamina propria and lung lymphocytes. Immunology 44:367-371. [PMC free article] [PubMed] [Google Scholar]
- 3.D'Elios, M. M., A. Amedei, M. Manghetti, F. Costa, C. T. Baldari, A. S. Quazi, J. L. Telford, S. Romagnani, and G. Del Prete. 1999. Impaired T-cell regulation of B-cell growth in Helicobacter pylori-related gastric low-grade MALT lymphoma. Gastroenterology 117:1105-1112. [DOI] [PubMed] [Google Scholar]
- 4.Dempsey, P. J., J. R. Goldenring, C. J. Soroka, I. M. Modlin, R. W. McClure, C. D. Lind, D. A. Ahlquist, M. R. Pittelkow, D. C. Lee, E. P. Sandgren, D. L. Page, and R. J. Coffey. 1992. Possible role of transforming growth factor alpha in the pathogenesis of Ménétrier's disease: supportive evidence from humans and transgenic mice. Gastroenterology 103:1950-1963. [DOI] [PubMed] [Google Scholar]
- 5.Dixon, M. F., R. M. Genta, J. H. Yardley, and P. Correa. 1996. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am. J. Surg. Pathol. 20:1161-1181. [DOI] [PubMed] [Google Scholar]
- 6.Eaton, K. A., S. R. Ringler, and S. J. Danon. 1999. Murine splenocytes induce severe gastritis and delayed-type hypersensitivity and suppress bacterial colonization in Helicobacter pylori-infected SCID mice. Infect. Immun. 67:4594-4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elliott, S. N., A. Buret, W. McKnight, M. J. S. Miller, and J. L. Wallace. 1998. Bacteria rapidly colonize and modulate healing of gastric ulcers in rats. Am. J. Physiol. 275:G425-G432. [DOI] [PubMed] [Google Scholar]
- 8.Garrouste-Orgeas, M., S. Chevret, G. Arlet, O. Marie, M. Rouveau, N. Popoff, and B. Schlemmer. 1997. Oropharyngeal or gastric colonization and nosocomial pneumonia in adult intensive care unit patients. A prospective study based on genomic DNA analysis. Am. J. Respir. Crit. Care Med. 156:1647-1655. [DOI] [PubMed] [Google Scholar]
- 9.Haruma, K., K. Komoto, H. Kawaguchi, S. Okamoto, M. Yoshihara, K. Sumii, and G. Kajiyama. 1995. Pernicious anemia and Helicobacter pylori infection in Japan: evaluation in a country with a high prevalence of infection. Am. J. Gastroenterol. 90:1107-1110. [PubMed] [Google Scholar]
- 10.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira. 2000. A Toll-like receptor recognizes bacterial DNA. Nature 408:740-745. [DOI] [PubMed] [Google Scholar]
- 11.Jawad, A., P. M. Hawkey, J. Heritage, and A. M. Snelling. 1994. Description of Leeds Acinetobacter Medium, a new selective and differential medium for isolation of clinically important Acinetobacter spp., and comparison with Herellea agar and Holton's agar. J. Clin. Microbiol. 32:2353-2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jonkers, D., P. Houben, W. Hameeteman, E. Stobberingh, A. de Bruine, J. W. Arends, I. Biemond, G. Lundqvist, and R. Stockbrugger. 1999. Differential features of gastric cancer patients, either Helicobacter pylori positive or Helicobacter pylori negative. Ital. J. Gastroenterol. Hepatol. 31:836-841. [PubMed] [Google Scholar]
- 13.Judd, L. M., P. A. Gleeson, B. H. Toh, and I. R. van Driel. 1999. Autoimmune gastritis results in disruption of gastric epithelial cell development. Am. J. Physiol. 277:G209-G218. [DOI] [PubMed] [Google Scholar]
- 14.Karam, S. M., and J. G. Forte. 1994. Inhibiting gastric H(+)-K(+)-ATPase activity by omeprazole promotes degeneration and production of parietal cells. Am. J. Physiol. 266:G745-G758. [DOI] [PubMed] [Google Scholar]
- 15.Khanolkar-Gaitonde, S. S., G. K. Reubish, Jr., C. K. Lee, and C. T. Stadtlander. 2000. Isolation of bacteria other than Helicobacter pylori from stomachs of squirrel monkeys (Saimiri spp.) with gastritis. Dig. Dis. Sci. 45:272-280. [DOI] [PubMed] [Google Scholar]
- 16.Kinoshita, Y., and S. Ishihara. 2000. Mechanism of gastric mucosal proliferation induced by gastrin. J. Gastroenterol. Hepatol. 15(Suppl.):D7-D11. [DOI] [PubMed] [Google Scholar]
- 17.Lee, A., J. O'Rourke, M. C. De Ungria, B. Robertson, G. Daskalopoulos, and M. F. Dixon. 1997. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology 112:1386-1397. [DOI] [PubMed] [Google Scholar]
- 18.Lehy, T., A. M. Roucayrol, and M. Mignon. 2000. Histomorphological characteristics of gastric mucosa in patients with Zollinger-Ellison syndrome or autoimmune gastric atrophy: role of gastrin and atrophying gastritis. Microsc. Res. Tech. 48:327-338. [DOI] [PubMed] [Google Scholar]
- 19.Marshall, B. J., and J. R. Warren. 1984. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet i:1311-1315. [DOI] [PubMed]
- 20.Mattapallil, J. J., S. Dandekar, D. R. Canfield, and J. V. Solnick. 2000. A predominant Th1 type of immune response is induced early during acute Helicobacter pylori infection in rhesus macaques. Gastroenterology 118:307-315. [DOI] [PubMed] [Google Scholar]
- 21.Meyer-Rosberg, K., D. R. Scott, D. Rex, K. Melchers, and G. Sachs. 1996. The effect of environmental pH on the proton motive force of Helicobacter pylori. Gastroenterology 111:886-900. [DOI] [PubMed] [Google Scholar]
- 22.Miyazaki, Y., Y. Shinomura, S. Tsutsui, S. Zushi, Y. Higashimoto, S. Kanayama, S. Higashiyama, N. Taniguchi, and Y. Matsuzawa. 1999. Gastrin induces heparin-binding epidermal growth factor-like growth factor in rat gastric epithelial cells transfected with gastrin receptor. Gastroenterology 116:78-89. [DOI] [PubMed] [Google Scholar]
- 23.Mowat, C., C. Williams, D. Gillen, M. Hossack, D. Gilmour, A. Carswell, A. Wirz, T. Preston, and K. E. McColl. 2000. Omeprazole, Helicobacter pylori status, and alterations in the intragastric milieu facilitating bacterial N-nitrosation. Gastroenterology 119:339-347. [DOI] [PubMed] [Google Scholar]
- 24.Murayama, Y., J.-I. Miyagawa, S. Higashiyama, S. Kondo, M. Yabu, K. Isozaki, Y. Kayanoki, S. Kanayama, Y. Shinomura, N. Taniguchi, and Y. Matsuzawa. 1995. Localization of heparin-binding epidermal growth factor-like growth factor in human gastric mucosa. Gastroenterology 109:1051-1059. [DOI] [PubMed] [Google Scholar]
- 25.Nomura, A., G. N. Stemmermann, P.-H. Chyou, I. Kato, G. I. Perez-Perez, and M. J. Blaser. 1991. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N. Engl. J. Med. 325:1132-1136. [DOI] [PubMed] [Google Scholar]
- 26.Ofori-Darko, E., Y. Zavros, G. Rieder, S. A. Tarle, M. Van Antwerp, and J. L. Merchant. 2000. An OmpA-like protein from Acinetobacter spp. stimulates gastrin and interleukin-8 promoters. Infect. Immun. 68:3657-3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parsonnet, J., G. D. Friedman, D. P. Vandersteen, Y. Chang, J. H. Vogelman, N. Orentreich, and R. K. Sibley. 1991. Helicobacter pylori infection and the risk of gastric carcinoma. N. Engl. J. Med. 325:1127-1131. [DOI] [PubMed] [Google Scholar]
- 28.Roth, K. A., S. B. Kapadia, S. M. Martin, and R. G. Lorenz. 1999. Cellular immune responses are essential for the development of Helicobacter felis-associated gastric pathology. J. Immunol. 163:1490-1497. [PubMed] [Google Scholar]
- 29.Sanduleanu, S., D. Jonkers, A. De Bruine, W. Hameeteman, and R. W. Stockbrugger. 2001. Non-Helicobacter pylori bacterial flora during acid-suppressive therapy: differential findings in gastric juice and gastric mucosa. Aliment. Pharmacol. Ther. 15:379-388. [DOI] [PubMed] [Google Scholar]
- 30.Saunders, N. J., J. F. Peden, D. W. Hood, and E. R. Moxon. 1998. Simple sequence repeats in the Helicobacter pylori genome. Mol. Microbiol. 27:1091-1098. [DOI] [PubMed] [Google Scholar]
- 31.Seensalu, R., D. Avedian, R. Barbuti, M. Song, L. Slice, and J. H. Walsh. 1997. Bombesin-induced gastrin release from canine G cells is stimulated by Ca2+ but not by protein kinase C, and is enhanced by disruption of rho/cytoskeletal pathways. J. Clin. Investig. 100:1037-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharma, B. K., I. A. Santana, E. C. Wood, R. P. Walt, M. Pereira, P. Noone, P. L. Smith, C. L. Walters, and R. E. Pounder. 1984. Intragastric bacterial activity and nitrosation before, during, and after treatment with omeprazole. Br. Med. J. Clin. Res. 289:717-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shulkes, A., and M. Read. 1991. Regulation of somatostatin secretion by gastrin- and acid-dependent mechanisms. Endocrinology 129:2329-2334. [DOI] [PubMed] [Google Scholar]
- 34.Stockbruegger, R. W. 1985. Bacterial overgrowth as a consequence of reduced gastric acidity. Scand. J. Gastroenterol. Suppl. 111:7-16. [DOI] [PubMed] [Google Scholar]
- 35.Stockbruegger, R. W., P. B. Cotton, G. G. Menon, J. O. Beilby, B. A. Bartholomew, M. J. Hill, and C. L. Walters. 1984. Pernicious anaemia, intragastric bacterial overgrowth, and possible consequences. Scand. J. Gastroenterol. 19:355-364. [PubMed] [Google Scholar]
- 36.Torres, A., M. El-Ebiary, N. Soler, C. Monton, N. Fabregas, and C. Hernandez. 1996. Stomach as a source of colonization of the respiratory tract during mechanical ventilation: association with ventilator-associated pneumonia. Eur. Respir. J. 9:1729-1735. [DOI] [PubMed] [Google Scholar]
- 37.Tsutsui, S., Y. Shinomura, S. Higashiyama, Y. Higashimoto, Y. Miyazaki, S. Kanayama, S. Hiraoka, T. Minami, S. Kitamura, Y. Murayama, J. Miyagawa, N. Taniguchi, and Y. Matsuzawa. 1997. Induction of heparin binding epidermal growth factor-like growth factor and amphiregulin mRNAs by gastrin in the rat stomach. Biochem. Biophys. Res. Commun. 235:520-523. [DOI] [PubMed] [Google Scholar]
- 38.Vaneechoutte, M., L. Dijkshoorn, I. Tjernberg, A. Elaichouni, P. de Vos, G. Claeys, and G. Verschraegen. 1995. Identification of Acinetobacter genomic species by amplified ribosomal DNA restriction analysis. J. Clin. Microbiol. 33:11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.von Graevenitz, A. 1995. Acinetobacter, Alcaligenes, Moraxella, and other nonfermentative gram-negative bacteria, p. 520-532. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 6th ed. ASM Press, Washington, D.C.
- 40.Wang, T. C., C. A. Dangler, D. Chen, J. R. Goldenring, T. Koh, R. Raychowdhury, R. J. Coffey, S. Ito, A. Varro, G. J. Dockray, and J. G. Fox. 2000. Synergistic interaction between hypergastrinemia and Helicobacter infection in a mouse model of gastric cancer. Gastroenterology 118:36-47. [DOI] [PubMed] [Google Scholar]
- 41.Yurdaydin, C., T. J. Walsh, H. D. Engler, J. H. Ha, Y. Li, E. A. Jones, and A. S. Basile. 1995. Gut bacteria provide precursors of benzodiazepine receptor ligands in a rat model of hepatic encephalopathy. Brain Res. 679:42-48. [DOI] [PubMed] [Google Scholar]
- 42.Zavros, Y., G. Rieder, A. Ferguson, L. Samuelson, and J. L. Merchant. 2002. Genetic or chemical hypochlorhydria is associated with inflammation that modulates parietal and G cell populations. Gastroenterology 122:119-133. [DOI] [PubMed] [Google Scholar]
- 43.Zhang, Q. B., G. Etolhi, J. B. Dawodu, C. G. Gemmell, and R. I. Russell. 1999. Relationship between mucosal levels of Helicobacter pylori-specific IgA, interleukin-8 and gastric inflammation. Clin. Sci. 96:409-414. [PubMed] [Google Scholar]