Abstract
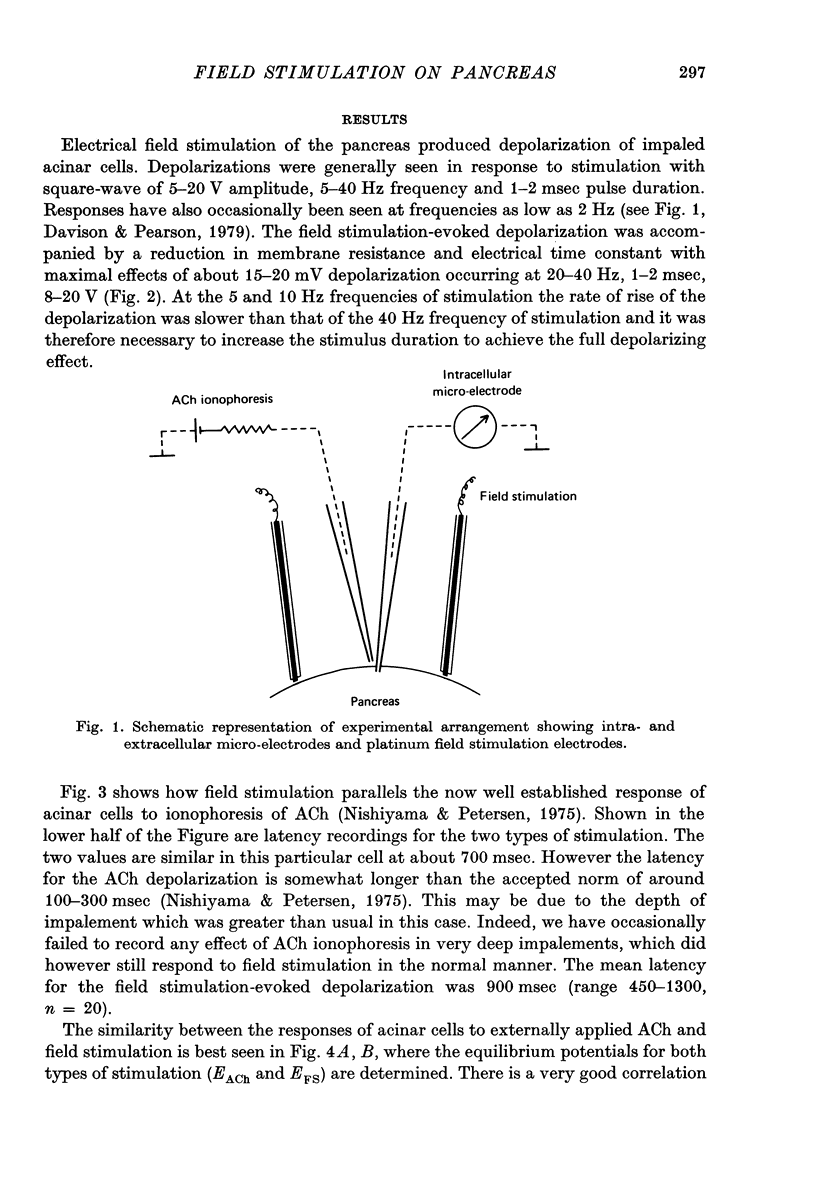
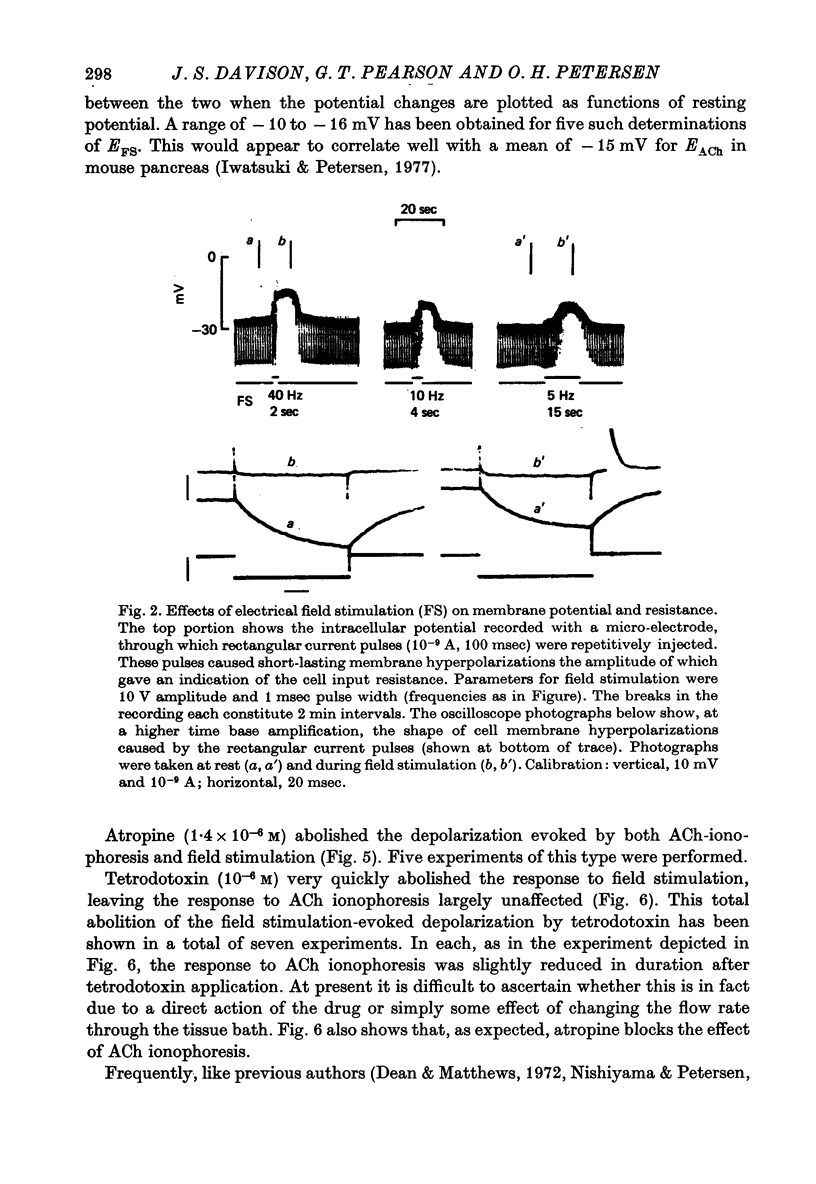
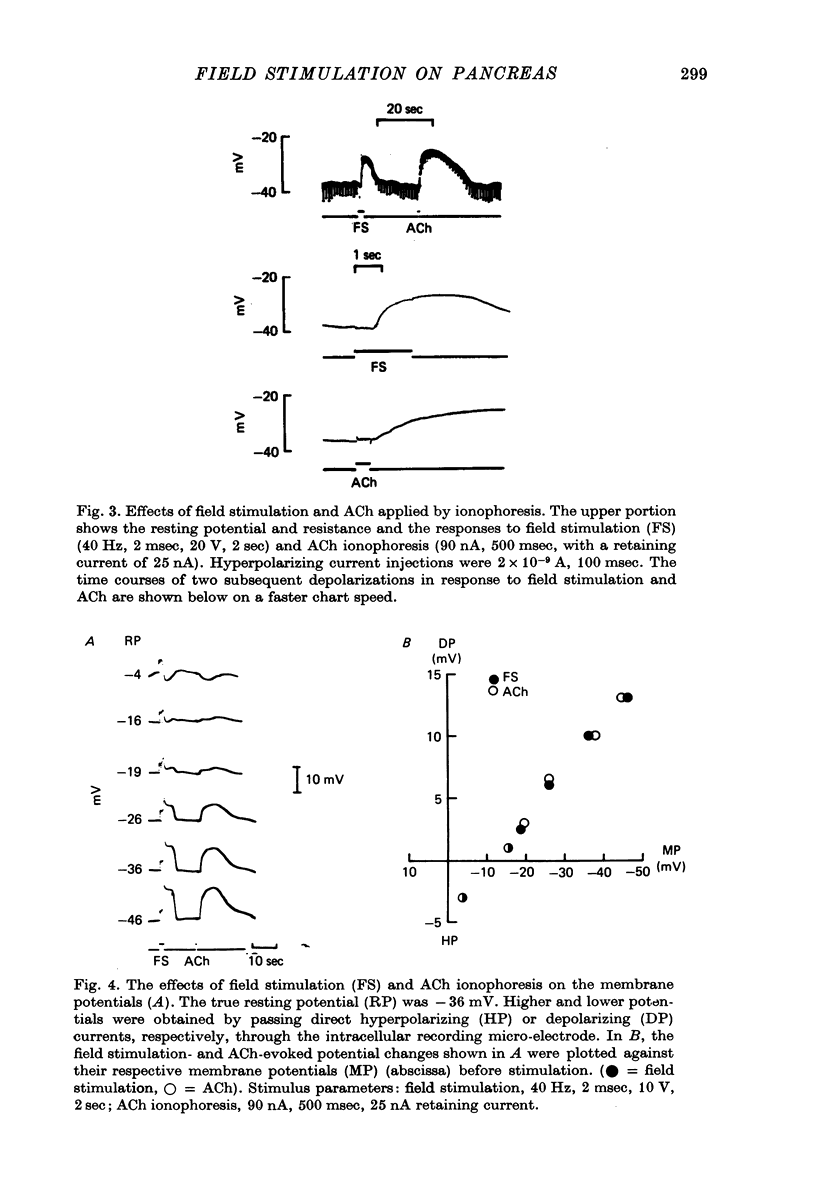
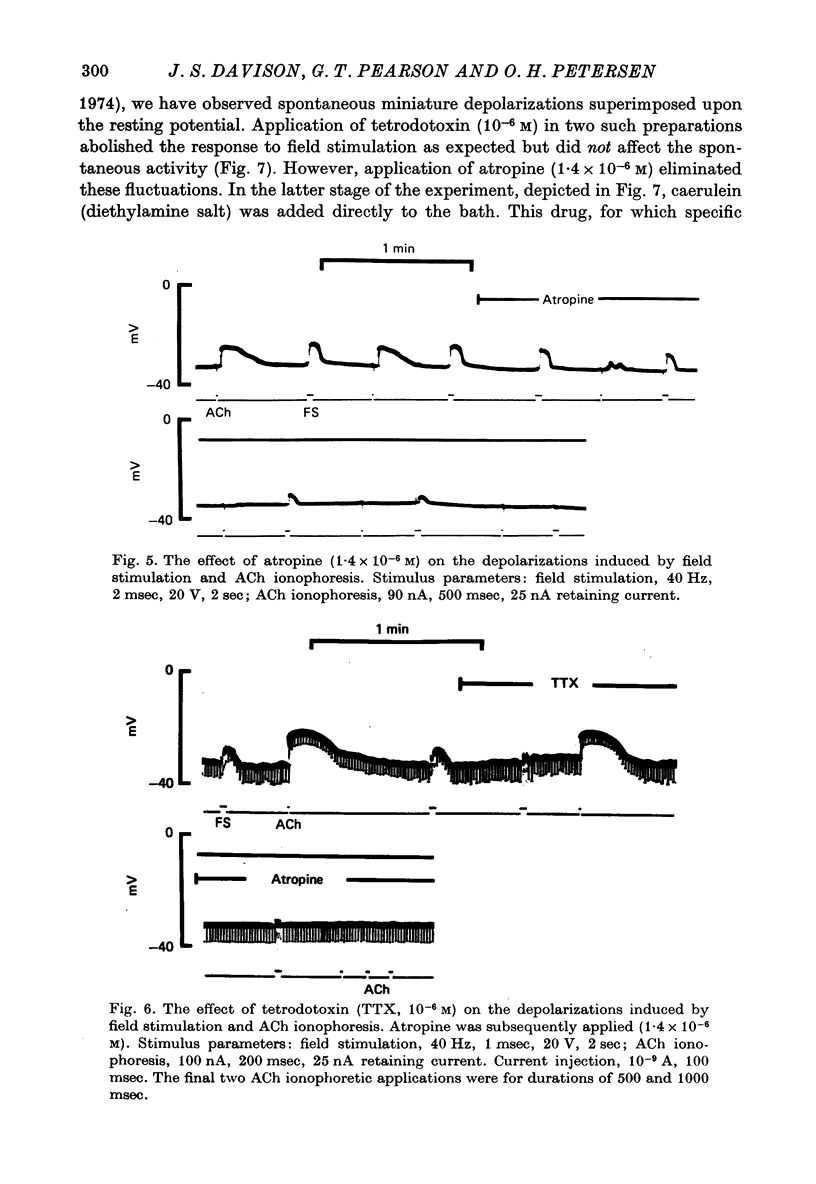
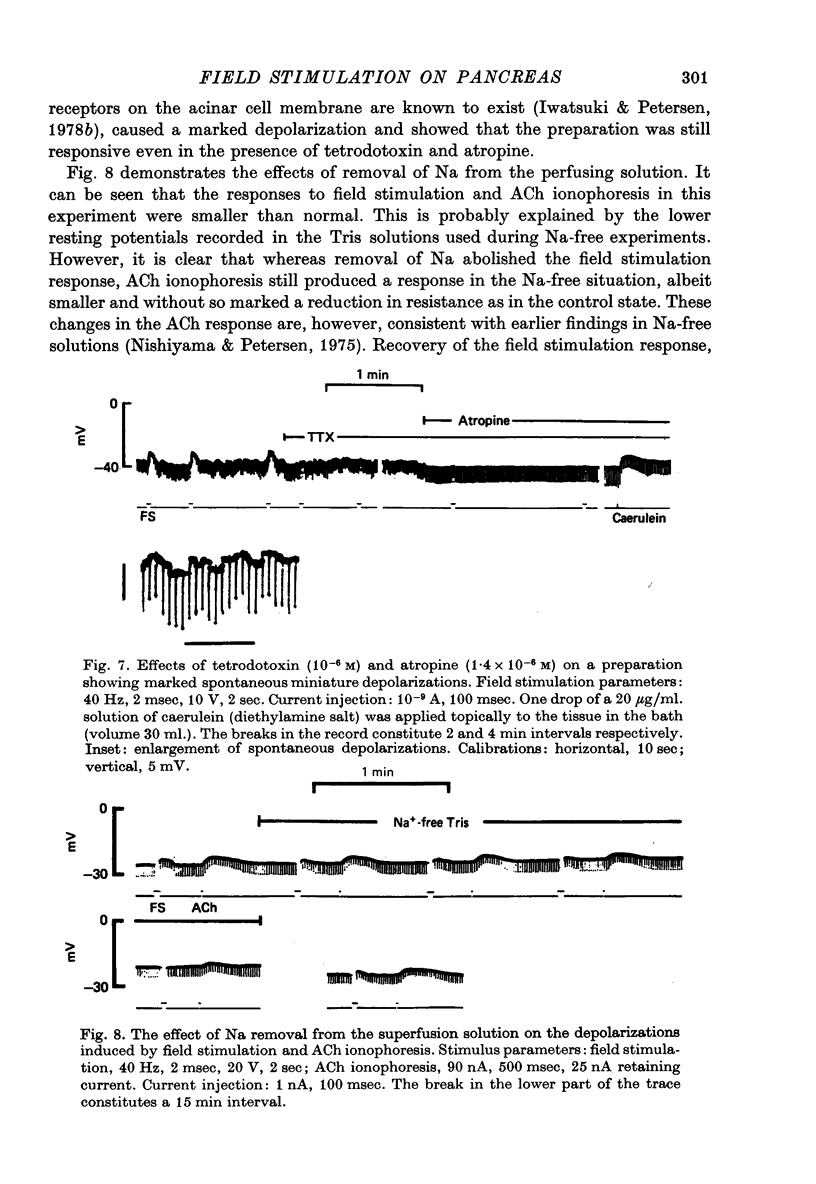
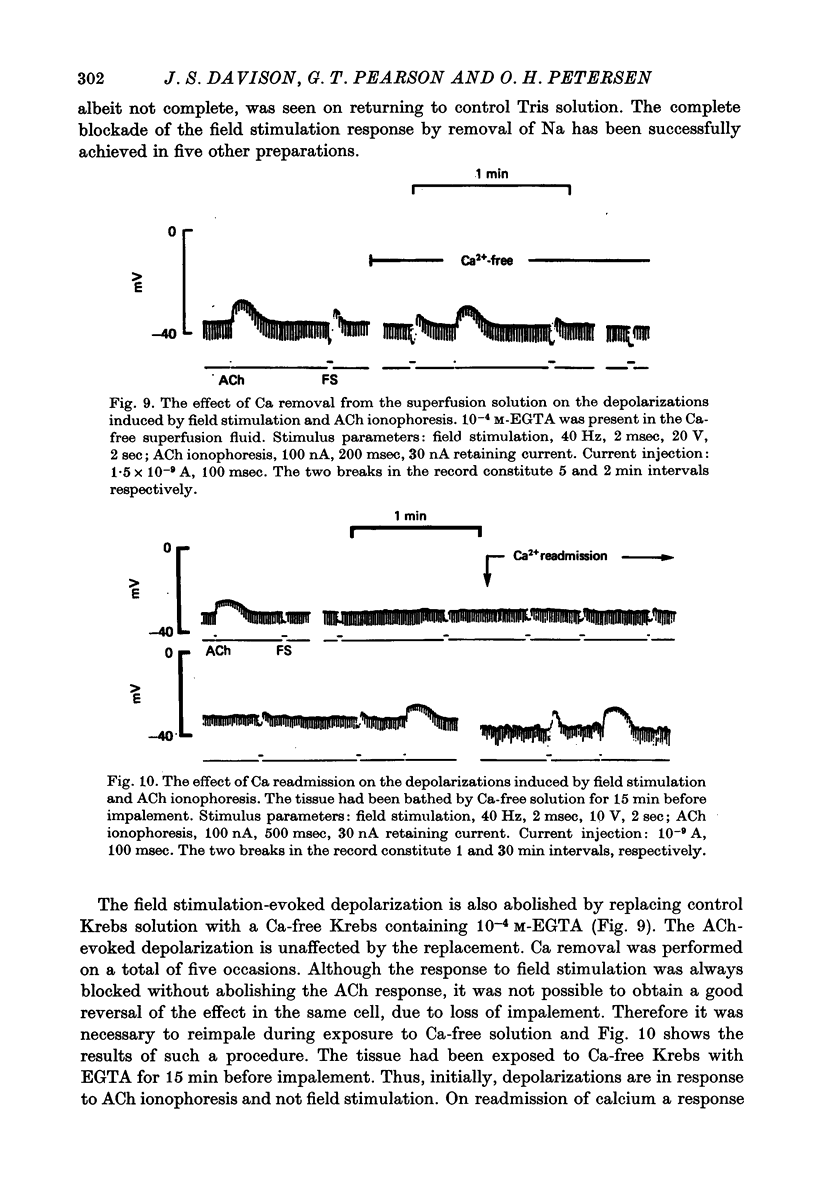
1. Intracellular micro-elctrode recordings of acinar cell membrane potential and resistance were made from the mouse pancreas superfused in vitro. The acinar cells under investigation were stimulated by electrical field stimulation using two platinum wire electrodes and by micro-ionophoretic acetylcholine (ACh) application from an extracellular AChCl-filled micro-electrode. 2. Field stimulation evoked membrane depolarization and reduction in input resistance. Maximal effects were observed at 20-40 Hz frequency, 1-2 msec pulse width and 8-20 V amplitude. The mean latency for the field stimulation-evoked depolarization was 900 msec. Field stimulation responses were seen at low frequency levels of stimulation, the majority of cells responding at 5 Hz and some at 2 Hz. The physiological significance of the low frequency stimulation is discussed. 3. The field stimulation effects resembled those induced by ACh ionophoresis and were abolished by atropine. The equilibrium potentials for both field stimulation and ACh ionophoresis were identical at about -15 mV. The field stimulation response was selectively abolished by tetrodotoxin and by superfusion with Na-free or Ca-free media, while the ACh ionophoretic response persisted. Field stimulation therefore initiated nerve action potentials and consequent ACh release. 4. Spontaneous miniature depolarizations observed in some preparations were not abolished by tetrodotoxin and woult therefore seem to be a result of quantal release of ACh from nerve terminals. 5. There is no indication from the present studies of the existence of neurotransmitters other than ACh. No inhibitory effects have been observed. 6. All preparations studied to date have responded to field stimulation and it is concluded that all acinar cells are potentially under cholinergic neural influence.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Davison J. S., Pearson G. T. Electrophysiological effects of field stimulation on mouse pancreatic acinar cells [proceedings]. J Physiol. 1979 Oct;295:45P–46P. [PubMed] [Google Scholar]
- Dean P. M., Matthews E. K. Pancreatic acinar cells: measurement of membrane potential and miniature depolarization potentials. J Physiol. 1972 Aug;225(1):1–13. doi: 10.1113/jphysiol.1972.sp009926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952 May;117(1):109–128. [PMC free article] [PubMed] [Google Scholar]
- Greenwell J. R., Scratcherd T. The kinetics of pancreatic amylase secretion and its relationship to volume flow and electrical conductance in the anaesthetized cat. J Physiol. 1974 Jun;239(3):443–457. doi: 10.1113/jphysiol.1974.sp010577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwatsuki N., Petersen O. H. Electrical coupling and uncoupling of exocrine acinar cells. J Cell Biol. 1978 Nov;79(2 Pt 1):533–545. doi: 10.1083/jcb.79.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwatsuki N., Petersen O. H. In vitro action of bombesin on amylase secretion, membrane potential, and membrane resistance in rat and mouse pancreatic acinar cells. A comparison with other secretagogues. J Clin Invest. 1978 Jan;61(1):41–46. doi: 10.1172/JCI108923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwatsuki N., Petersen O. H. Pancreatic acinar cells: the acetylcholine equilibrium potential and its ionic dependency. J Physiol. 1977 Aug;269(3):735–751. doi: 10.1113/jphysiol.1977.sp011926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The timing of calcium action during neuromuscular transmission. J Physiol. 1967 Apr;189(3):535–544. doi: 10.1113/jphysiol.1967.sp008183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narahashi T. Chemicals as tools in the study of excitable membranes. Physiol Rev. 1974 Oct;54(4):813–889. doi: 10.1152/physrev.1974.54.4.813. [DOI] [PubMed] [Google Scholar]
- Nishiyama A., Petersen O. H. Pancreatic acinar cells: ionic dependence of acetylcholine-induced membrane potential and resistance change. J Physiol. 1975 Jan;244(2):431–465. doi: 10.1113/jphysiol.1975.sp010807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A., Petersen O. H. Pancreatic acinar cells: membrane potential and resistance change evoked by acetylcholine. J Physiol. 1974 Apr;238(1):145–158. doi: 10.1113/jphysiol.1974.sp010515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen O. H. Electrophysiology of mammalian gland cells. Physiol Rev. 1976 Jul;56(3):535–577. doi: 10.1152/physrev.1976.56.3.535. [DOI] [PubMed] [Google Scholar]


