Abstract
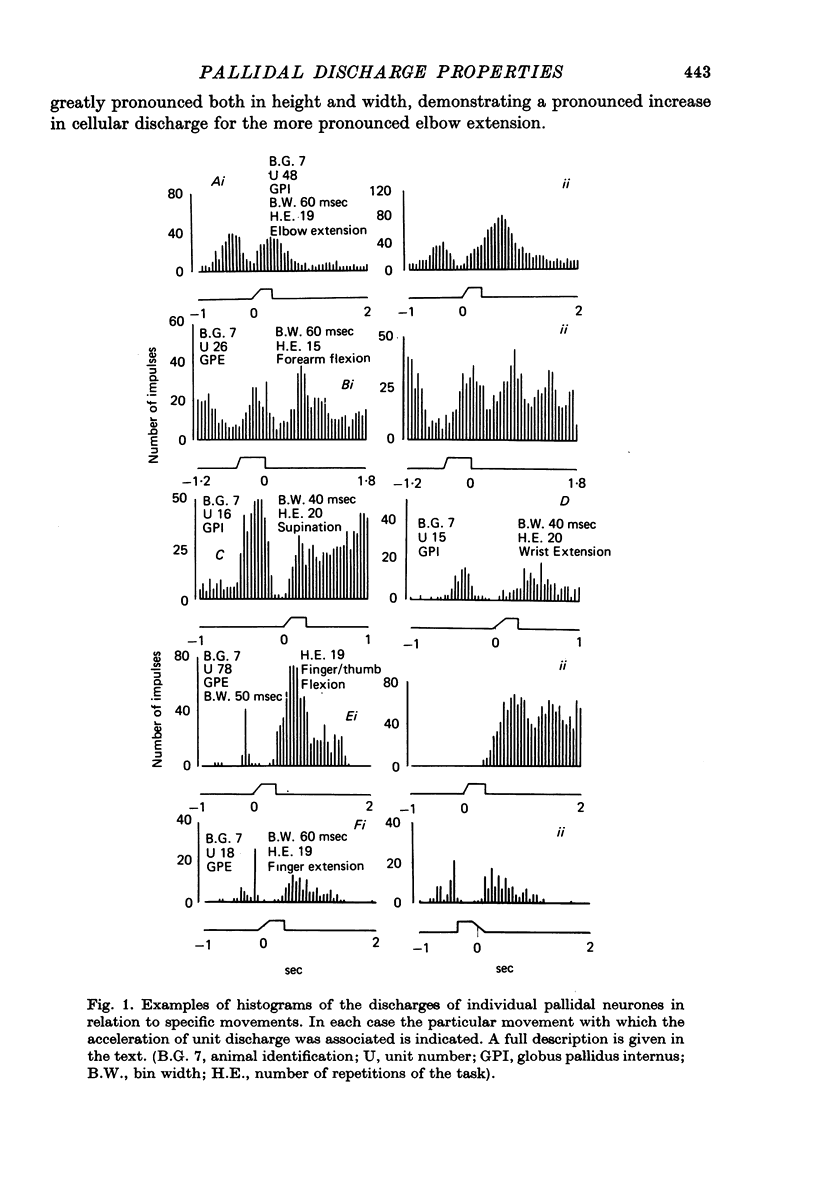
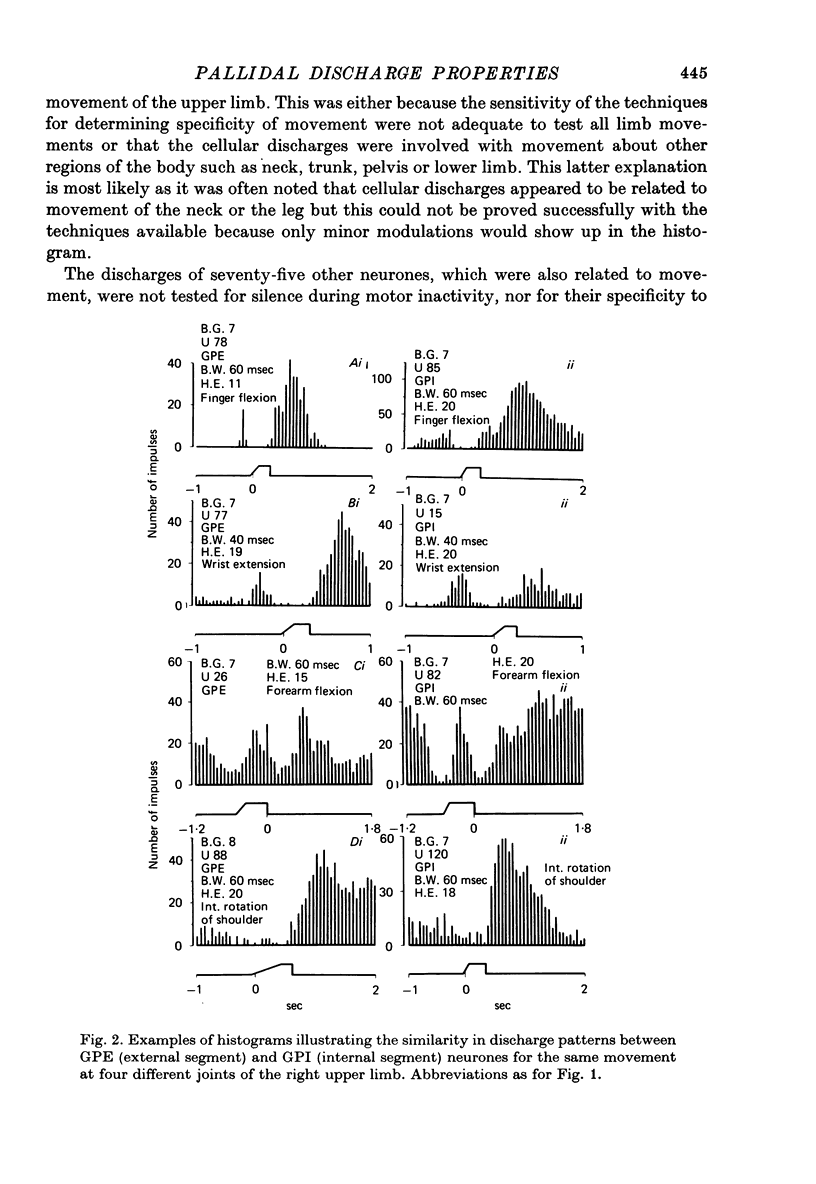
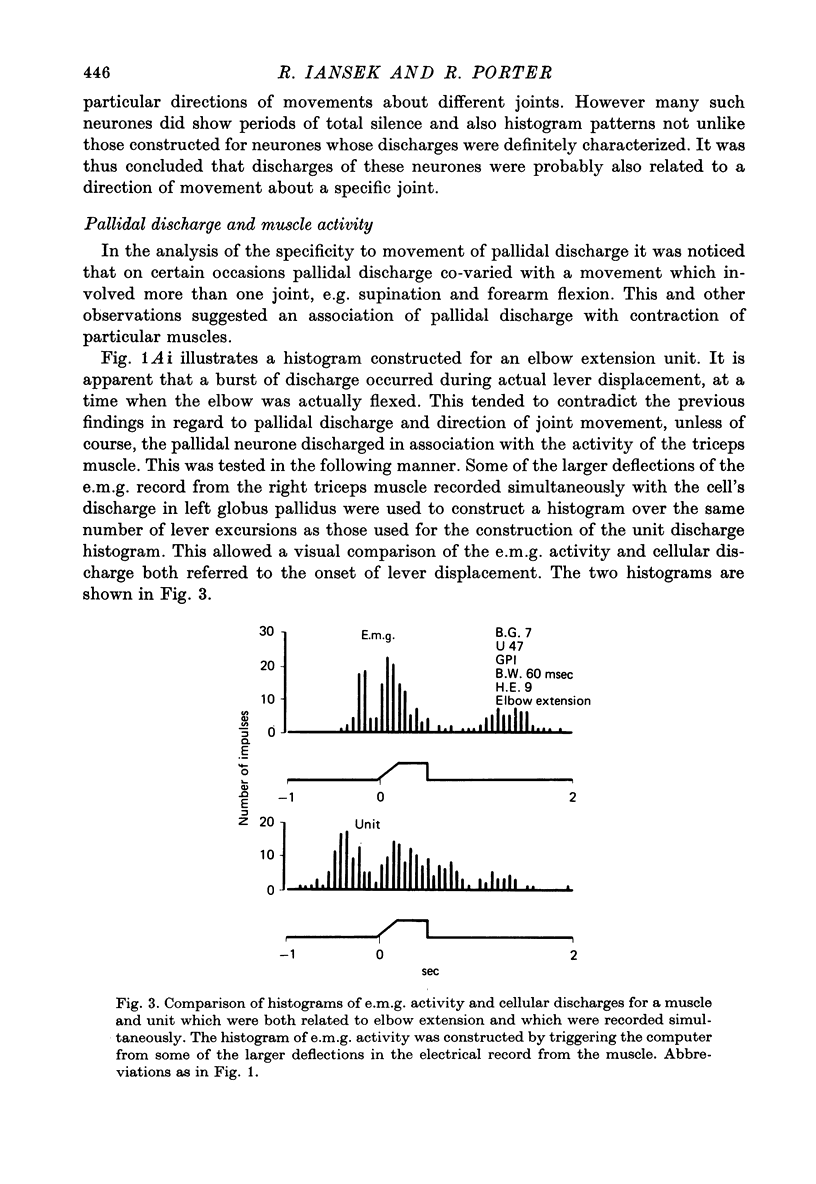
1. Recordings were made of the natural discharges of 388 pallidal neurones in awake, free-to-move monkeys in order to describe the discharge properties of such neurones in relation to normal movement performance. 2. Of the 388 neurones, 156 discharged only in association with one direction of movement of the forelimb about a specific joint. If that movement was not taking place the neurone would not discharge. 3. All joints and directions of movement for the upper limb were represented by clusters of cells within the pallidal population. 4. Twenty-nine per cent of neurones co-varied with movement of both contralateral and ipsilateral limb for the same direction of movement about a given joint; distal movements were represented with similar frequency to proximal movements in this group. 5. Afferent information provided by natural stimulation of peripheral receptors did not directly influence either the discharging or non-discharging pallidal neurones. 6. Movement related neurones were regionally organized and were found in the posterior part of the pallidum.
Full text
PDF




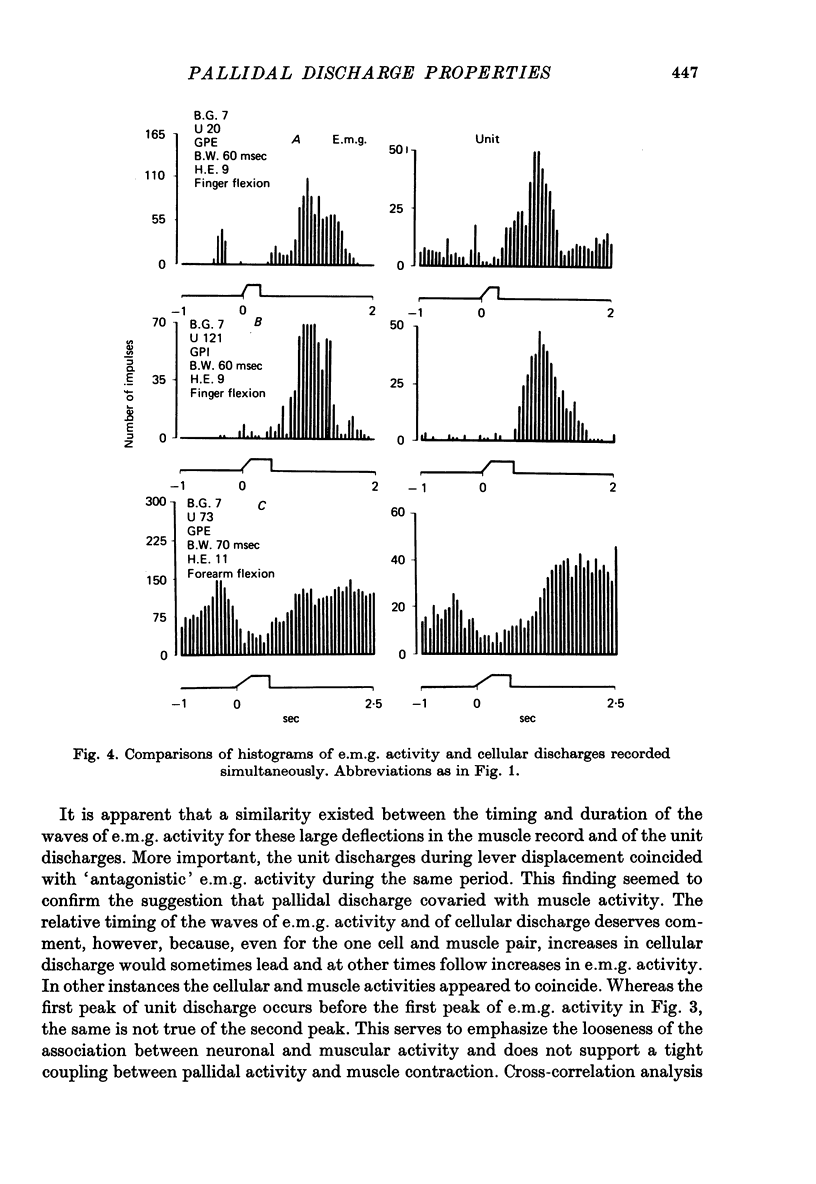
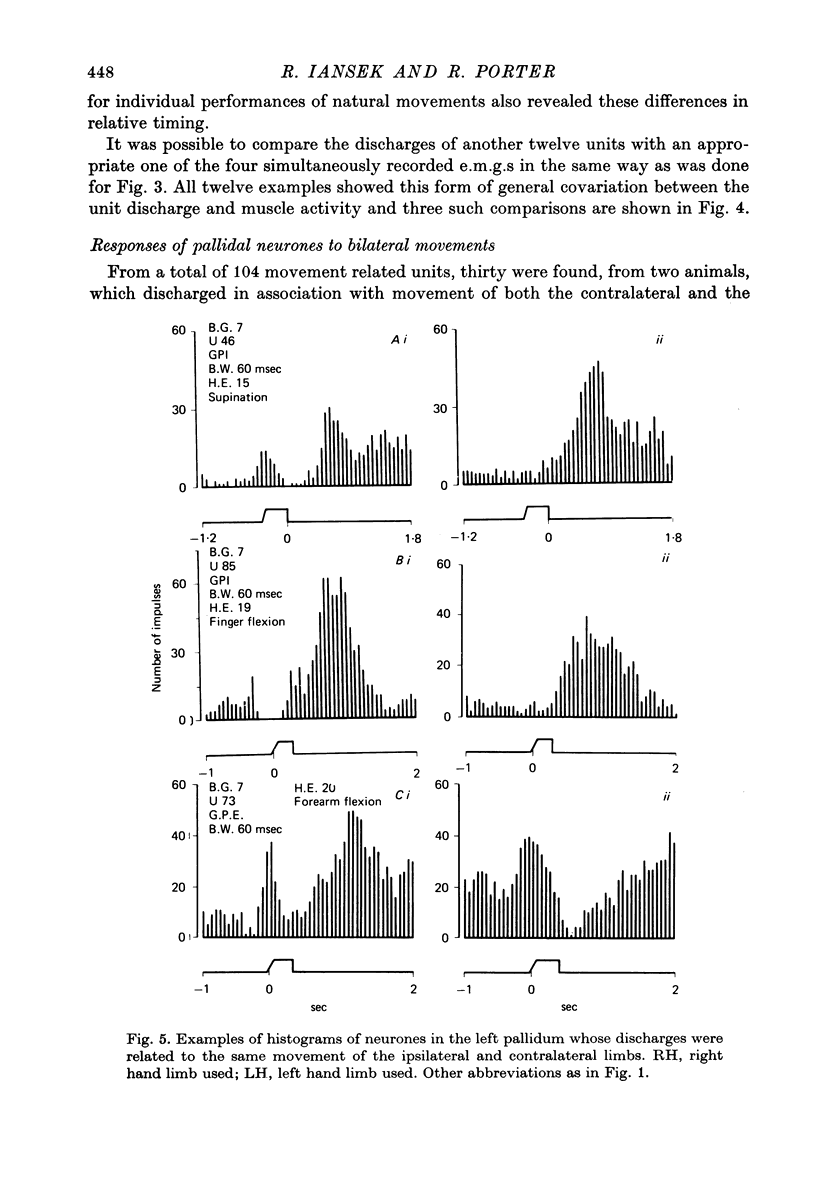

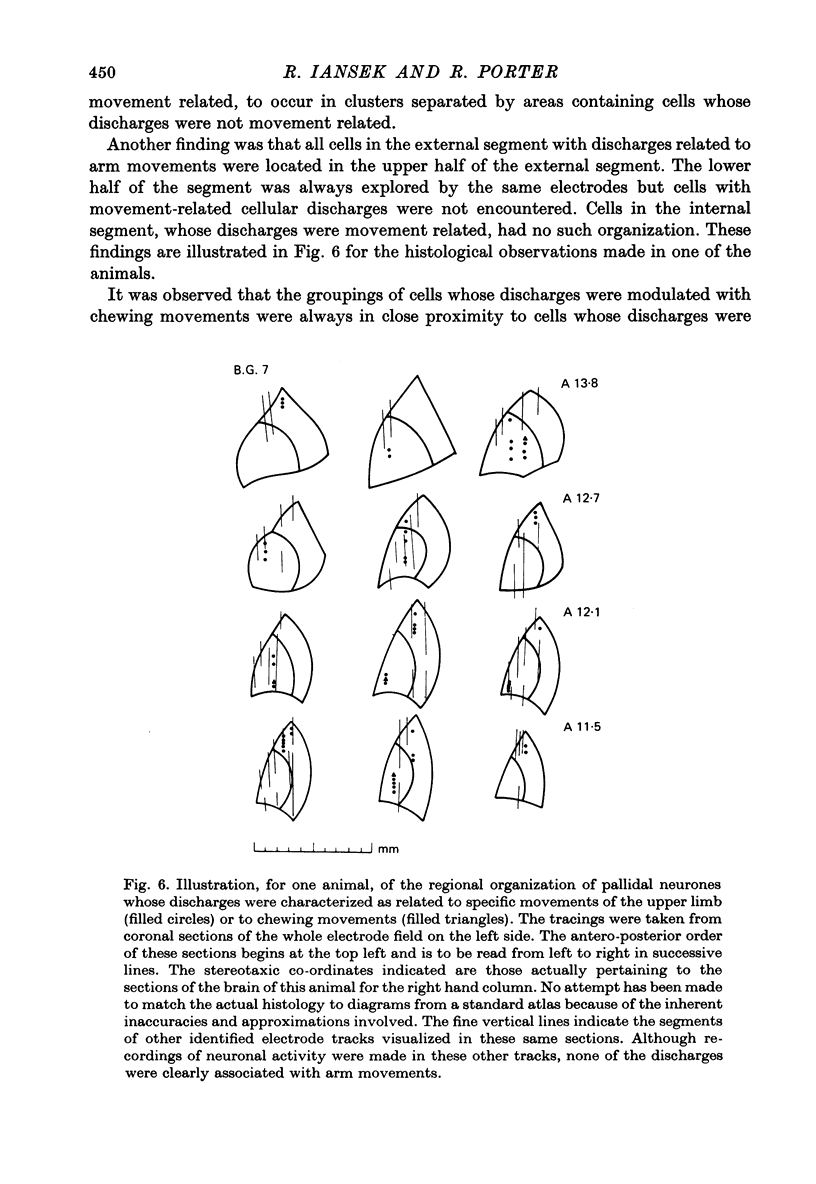





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. E. Discharge patterns of basal ganglia neurons during active maintenance of postural stability and adjustment to chair tilt. Brain Res. 1978 Mar 24;143(2):325–338. doi: 10.1016/0006-8993(78)90572-3. [DOI] [PubMed] [Google Scholar]
- Brinkman J., Bush B. M., Porter R. Deficient influence of peripheral stimuli on precentral neurones in monkeys with dorsal column lesions. J Physiol. 1978 Mar;276:27–48. doi: 10.1113/jphysiol.1978.sp012218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buser P., Pouderoux G., Mereaux J. Single unit recording in the caudate nucleus during sessions with elaborate movements in the awake monkey. Brain Res. 1974 May 17;71(2-3):337–344. doi: 10.1016/0006-8993(74)90976-7. [DOI] [PubMed] [Google Scholar]
- CARPENTER M. B., WHITTIER J. R., METTLER F. A. Analysis of choreoid hyperkinesia in the Rhesus monkey; surgical and pharmacological analysis of hyperkinesia resulting from lesions in the subthalamic nucleus of Luys. J Comp Neurol. 1950 Jun;92(3):293–331. doi: 10.1002/cne.900920303. [DOI] [PubMed] [Google Scholar]
- Carpenter M. B. Anatomical organization of the corpus striatum and related nuclei. Res Publ Assoc Res Nerv Ment Dis. 1976;55:1–36. [PubMed] [Google Scholar]
- Cowan W. M., Powell T. P. Strio-pallidal projection in the monkey. J Neurol Neurosurg Psychiatry. 1966 Oct;29(5):426–439. doi: 10.1136/jnnp.29.5.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong M. R. Activity of pallidal neurons during movement. J Neurophysiol. 1971 May;34(3):414–427. doi: 10.1152/jn.1971.34.3.414. [DOI] [PubMed] [Google Scholar]
- DeLong M. R. Putamen: activity of single units during slow and rapid arm movements. Science. 1973 Mar 23;179(4079):1240–1242. doi: 10.1126/science.179.4079.1240. [DOI] [PubMed] [Google Scholar]
- Fox C. A., Andrade A. N., Lu Qui I. J., Rafols J. A. The primate globus pallidus: a Golgi and electron microscopic study. J Hirnforsch. 1974;15(1):75–93. [PubMed] [Google Scholar]
- Jones E. G., Coulter J. D., Burton H., Porter R. Cells of origin and terminal distribution of corticostriatal fibers arising in the sensory-motor cortex of monkeys. J Comp Neurol. 1977 May 1;173(1):53–80. doi: 10.1002/cne.901730105. [DOI] [PubMed] [Google Scholar]
- Kievit J., Kuypers H. G. Organization of the thalamo-cortical connexions to the frontal lobe in the rhesus monkey. Exp Brain Res. 1977 Sep 28;29(3-4):299–322. doi: 10.1007/BF00236173. [DOI] [PubMed] [Google Scholar]
- Kim R., Nakano K., Jayaraman A., Carpenter M. B. Projections of the globus pallidus and adjacent structures: an autoradiographic study in the monkey. J Comp Neurol. 1976 Oct 1;169(3):263–290. doi: 10.1002/cne.901690302. [DOI] [PubMed] [Google Scholar]
- Kitsikis A., Angyan L., Buser P. Basal ganglia unitary activity during a motor performance in monkeys. Physiol Behav. 1971 May;6(5):609–611. doi: 10.1016/0031-9384(71)90215-0. [DOI] [PubMed] [Google Scholar]
- Kuo J. S., Carpenter M. B. Organization of pallidothalamic projections in the rhesus monkey. J Comp Neurol. 1973 Oct 1;151(3):201–236. doi: 10.1002/cne.901510302. [DOI] [PubMed] [Google Scholar]
- Künzle H. Bilateral projections from precentral motor cortex to the putamen and other parts of the basal ganglia. An autoradiographic study in Macaca fascicularis. Brain Res. 1975 May 2;88(2):195–209. doi: 10.1016/0006-8993(75)90384-4. [DOI] [PubMed] [Google Scholar]
- Künzle H. Projections from the primary somatosensory cortex to basal ganglia and thalamus in the monkey. Exp Brain Res. 1977 Dec 19;30(4):481–492. doi: 10.1007/BF00237639. [DOI] [PubMed] [Google Scholar]
- LAURSEN A. M. An experimental study of pathways from the basal ganglia. J Comp Neurol. 1955 Feb;102(1):1–25. doi: 10.1002/cne.901020102. [DOI] [PubMed] [Google Scholar]
- Lemon R. N., Hanby J. A., Porter R. Relationship between the activity of precentral neurones during active and passive movements in conscious monkeys. Proc R Soc Lond B Biol Sci. 1976 Oct 29;194(1116):341–373. doi: 10.1098/rspb.1976.0083. [DOI] [PubMed] [Google Scholar]
- Porter R., Lewis M. M., Horne M. Analysis of patterns of natural activity of neurones in the precentral gyrus of conscious monkeys. Brain Res. 1971 Nov;34(1):99–113. doi: 10.1016/0006-8993(71)90353-2. [DOI] [PubMed] [Google Scholar]