Abstract
In this work we analyzed the roles of meningococcal lipooligosaccharide (LOS) and capsule expression in the interaction of Neisseria meningitidis with human dendritic cells (DC). Infection of DC with serogroup B wild-type meningococci induced a strong burst of the proinflammatory cytokines and chemokines tumor necrosis factor alpha, interleukin-6 (IL-6), and IL-8. In contrast, a serogroup B mutant strain lacking LOS expression barely led to cytokine induction, demonstrating that meningococcal LOS is the main mediator of the proinflammatory response in human DC. Sialylation of meningococcal LOS did not influence cytokine secretion by DC. However, we found the phagocytosis of N. meningitidis by human DC to be inhibited by LOS sialylation. In addition, the expression of the meningococcal serogroup A, B, and C capsules dramatically reduced DC adherence of N. meningitidis and phagocytosis to some extent. Hence, LOS sialylation and capsule expression are independent mechanisms protecting N. meningitidis from the phagocytic activity of human DC.
Initiating a specific immune response to bacterial pathogens requires that bacterial antigens are captured, processed, and presented by antigen-presenting cells. Dendritic cells (DC) are one of the most potent types of antigen-presenting cells of the immune system and are thought to be crucial for the initiation of primary T-cell-mediated responses to foreign antigens (3). DC progenitors arise in the bone marrow, enter the blood, and seed nonlymphoid tissues, where they localize to epithelia, including skin epidermis, gut and airway epithelia, lung parenchyma, and the interstitial spaces of many solid organs (2). These DC are considered to be immature in that they are optimized for antigen uptake and processing, but not for the ability to initiate primary T-cell responses. Immature DC can capture antigens by phagocytosis (18), macropinocytosis (35), and endocytosis (34, 35). Exposure of DC to inflammatory stimuli converts these cells from an antigen-capturing to an antigen-presenting mode, which is defined as DC maturation (3). After antigen exposure, the DC leave the periphery and migrate via the afferent lymph or blood to tissue-draining secondary lymphoid organs such as lymph nodes or spleen. During this migration, DC undergo a process of maturation such that upon arrival in the lymphoid organs, they have a greatly diminished capacity for antigen uptake and processing but have gained the ability to present antigens efficiently for priming T cells in the context of major histocompatibility complex class I, major histocompatibility complex class II, and costimulatory molecules such as CD40, CD54, CD80, and CD 86 (3).
Neisseria meningitidis, a gram-negative diplococcus, is an invasive human pathogen causing sepsis and meningitis. The severity of meningococcal disease is related to the level of endotoxin in plasma and cerebrospinal fluid, which in turn determines the intensity of the host's proinflammatory response. The lethality of the infection is independent of bacterial viability but is induced by overproduction of the cytokines tumor necrosis factor alpha (TNF-α), interleukin-1 (IL-1), and IL-6 and the chemokine IL-8 (29, 42, 46). Infiltrating monocytes are one of the most important sources of these cytokines and chemokines during meningococcal infection (24, 29, 42, 46). Thorough analysis of the interaction of N. meningitidis with human monocytes like macrophages and DC is therefore an important step in understanding the pathogenesis of meningococcal disease.
We recently analyzed the interaction of N. meningitidis serogroup B with human DC and demonstrated a strong phagocytosis and rapid phagosomal killing of meningococci by DC. The serogroup B capsule significantly impaired neisserial adherence to DC and killing of the bacteria in the phagosome (22). In addition, we found serogroup B meningococci to induce DC maturation and strong secretion of the cytokines TNF-α, IL-6 and IL-8 by DC, suggesting that activated DC may be an important source of high levels of proinflammatory cytokines in neisserial infection and thereby may contribute to the pathology of meningococcal disease (22). The main mediator of this cytokine induction is probably the meningococcal lipooligosaccharide (LOS), as shown for killed serogroup B meningococci (9) as well as purified LOS (22). In the present study, we extended these observations to serogroup A and C meningococci and clarified the mechanism of neisserial killing in the presence of human DC. Furthermore, we analyzed the role of neisserial LOS in cytokine induction in DC by infections with live bacteria and assessed the implications of LOS sialylation for adherence, phagocytosis, and the proinflammatory response in DC.
MATERIALS AND METHODS
Bacteria.
The meningococcal strains used in this study were piliated N. meningitidis serogroup A strain A2044, serogroup B strains MC58 and H44/76, and serogroup C strain C1701. Strain MC58 was isolated from a clinical case in the United Kingdom in 1985 (43); strain H44/76 was isolated in Norway in 1978 (17). Both strains are B:15:P1.7,16, immunotype L3, and belong to the ET-5 complex. Meningococcal isolates A2044 and C1701 were kindly provided by U. Berger (Institute for Hygiene, Heidelberg, Germany). Isolate A2044 belongs to the clonal subgroup II/III; C1701 is an ET-37 complex isolate. By using a set of monoclonal and polyclonal antibodies obtained from W. Zollinger (Walter Reed Army Research Institute, Washington, D.C.), the LOS immunotype of strain A2044 was determined to be L3,7,9; the immunotype of strain C1701 was determined to be L2. All immunotypes were assessed as described below.
Capsule-deficient mutants of strains A2044 and C1701 were generated by transformation with plasmid pMF121 harboring region E and a minor part of the transport region B of the cps locus with replacement of a 18.5-kb deleted EcoRV fragment by an erythromycin resistance gene (12), resulting in strains A2044 cps and C1701 cps. In addition to the loss of capsule expression, this mutagenesis strategy resulted in deletion of the galE gene and hence in the expression of a truncated LOS. Capsule-deficient mutant strains of the serogroup B strains MC58 and H44/76 were described previously (22). Capsule mutant strains were assessed for capsule expression by enzyme-linked immunosorbent assay (ELISA) as described below and were found to have a capsule-negative phenotype.
Isogenic α-2,3-sialyltransferase (lst) deletion mutant strains (44) were constructed as follows. The upstream flanking sequence of the lst gene of serogroup B strain MC58 was amplified by PCR with the primer pair lstupF (5′-TTTTTGAGTCATAGGTACCTTCTCTTGTAGGTT-3′) and lstupR (5′-ATATCCCTAAAACTCGAGTCCGACAAATTGAAC-3′), incorporating restriction sites for restriction enzymes XhoI and KpnI. The PCR product was inserted into XhoI- and KpnI-precleaved vector pTL1, resulting in vector pAS6. Vector pTL1 is a derivative of vector pBluescript (Invitrogen, Groningen, The Netherlands) carrying the Neisseria uptake sequence (5′-TCGAGAAGTCTGCCG-3′) (23) in the single SacI restriction site of pBluescript, allowing the transformation of N. meningitidis. Subsequently, the lst downstream sequence was amplified using primers lstdownF (5′-GACGATAAAAATCTCGAGCATAGCAAATCAAAAT-3′) and lstdownR (5′-GATGCCCGAATTCACACGCATGGGCA-3′) with restriction sites for the enzymes XhoI and EcoRI. The product was inserted into XhoI- and EcoRI-precleaved vector pAS6, resulting in vector pAS7. The correct sequence of vector pAS7 was verified by sequencing the vector. The wild-type strain N. meningitidis MC58 as well as the isogenic α-2,8-polysialyltransferase (siaD) mutant were transformed with vector pAS7 according to the protocol of Gunn and Stein (14), and transformants were screened by PCR for deletion of the lst gene with primers lstF (5′-AATTTGTCGGAATGGAGCATAG-3′) and lstR (5′-CGTTCAAATCCATGATGCCC-3′), leading to strains MC58 lst and MC58 lst siaD. Both strains were subsequently analyzed by Southern blotting, verifying the deletion of the complete lst gene. The phenotypes of both strains were analyzed by LOS purification followed by Tricine gel analysis and silver staining as described below, demonstrating a higher mobility and hence lower molecular weight of the LOSs of the strains MC58 lst and MC58 lst siaD in comparison to the LOSs of strains MC58 and MC58 siaD.
The lpxA mutant of strain H44/76 has been described previously (38).
All strains were analyzed by Western blotting for expression of pili, Opa, and Opc. Isogenic mutants compared in the experimental approaches always expressed identical amounts of pili, Opa, and Opc.
LOS immunotyping.
LOS immunotyping was performed by ELISA using a panel of antibodies kindly provided by W. Zollinger (Walter Reed Army Institute). Polystyrole microtiter plates (Greiner, Frickenhausen, Germany) were coated with 100 μl of poly-d-lysine (5 μg/ml in phosphate-buffered saline [PBS]). Whole bacterial cells (5 × 106 in 20 μl of PBS) were added, left for 1 h at room temperature, and subsequently fixed with 0.05% glutaraldehyde in PBS. After three washes with PBS, probes were saturated with 150 μl of 5% (wt/vol) fat-free milk powder in 100 mM Tris-HCl and 150 mM NaCl. Antibodies in appropriate dilutions were added and left for 2 h at room temperature, and binding was detected using peroxidase-labeled anti-mouse antibody (Dako, Hamburg, Germany) as a secondary antibody.
LOS analysis.
LOS was analyzed by electrophoretic separation on 20% Tricine-buffered sodium dodecyl sulfate-polyacrylamide gels and silver staining as described previously (40).
Detection of capsule polysaccharide.
ELISAs were performed using monoclonal antibody (MAb) 735 against meningococcal B capsule polysaccharide as described previously (11) and MAb 924 against meningococcal C capsule polysaccharide and MAb 932 against meningococcal A capsule polysaccharide (M. Frosch, unpublished data).
Generation of human DC from peripheral blood monocytes.
Human DC were produced from human peripheral blood mononuclear cells (PBMCs) by a standard protocol (32). PBMCs were isolated from citrate-buffered leukocyte-enriched buffy coats of healthy adult donors by Histopaque (1.077 g/ml; Sigma, Deisenhofen, Germany) density gradient centrifugation at 400 × g and room temperature. After depletion of T lymphocytes with neuraminidase-treated sheep erythrocytes, the remaining mononuclear cells were plated on tissue culture dishes (3003; Falcon Labware, Oxnard, Calif.) at a density of 5 × 106 cells/ml in RPMI 1640 medium (Gibco Life Technologies, Karlsruhe, Germany), supplemented with l-glutamine (2 mM) and 1% autologous human plasma, for 60 min at 37°C. Nonadherent cells were washed free with PBS, and adherent cells were cultured for 7 days without antibiotics in RPMI 1640 medium supplemented with 10% fetal calf serum (PAN Biotech, Aidenbach, Germany), 2 mM l-glutamine, 1,000 U of recombinant human IL-4 (PBH, Hannover, Germany) per ml, and 800 U of recombinant human granulocyte-macrophage colony-stimulating factor (Leukomax; Sandoz, Basel, Switzerland) per ml. Cytokines were replenished every other day.
Infection of DC.
On day 7, nonadherent DC were collected prior to infection by moderately vigorous aspiration and transferred to new 24-well plates at a density of 5 × 105 cells/ml (21). Bacteria were grown overnight in 5% CO2 on gonococcal complex (GC) agar with 1% supplement and used to inoculate supplemented proteose peptone medium (PPM+), in which they were grown to mid-log phase for infection. After being washed twice with PBS, the bacteria were diluted in RPMI 1640 medium and added at a multiplicity of infection (MOI) of about 1 to each well. The cultures were incubated in RPMI 1640 medium with 1% autologous human plasma (unless otherwise indicated) at 37°C for different time periods before numbers of nonadherent, adherent, and invasive bacteria were assessed. Numbers of nonadherent bacteria were determined by plating serial dilutions of DC supernatant on GC agar plates, followed by incubation at 37°C in 5% CO2 for 24 h. For assessment of intracellular bacteria, cells were washed three times with PBS, followed by incubation of DC for 1 h in fresh RPMI 1640 medium containing 100 μg of gentamicin (Gibco Life Technologies) per ml and 2% autologous human plasma. The cells were then washed three times with PBS, followed by addition of 1% saponin in PBS to lyse DC. CFU were determined by plating appropriate dilutions of the lysates on GC agar. For assessment of cell-associated (adherent plus intracellular) bacteria per well, the assay was performed as described above except that the incubation step with gentamicin was omitted. All samples were tested in triplicate, and experiments were repeated at least twice.
Assessment of DC supernatants for bactericidal substances.
To test whether human DC secrete bactericidal substances, DC were cultured at a cell density of 5 × 105 in RPMI 1640 containing 1% autologous human plasma for 8 h. The DC supernatant was collected, DC were removed by centrifugation, and the supernatant was directly used for assessment as growth medium for wild-type MC58 and capsule-deficient MC58 siaD strains without freezing. To this end, the bacteria were inoculated into (i) DC supernatant mixed with PPM+ at a 1:1 ratio, (ii) RPMI 1640 mixed with PPM+, and (iii) pure PPM+. At the start of the experiment, all cultures exhibited an optical density at 600 nm of 0.15. Bacterial growth was assessed at 50 and 100 min postinoculation.
To further investigate whether DC secrete bactericidal substances upon infection by either wild-type or capsule-deficient meningococci, DC at a cell density of 5 × 105 in RPMI 1640 containing 1% autologous human plasma were infected with either wild-type MC58 bacteria or the MC58 siaD strain for 8 h. Subsequently, DC supernatants were collected and centrifuged for removal of DC and meningococci. The DC supernatants were subjected to three cycles of freezing at −70°C and thawing at 37°C to remove remaining meningococci. The absence of viable meningococci was confirmed by plating on GC agar. Subsequently, DC supernatants were assessed for bactericidal substances as described above for supernatants of uninfected DC.
Cytokine assessment by ELISA of DC supernatants.
To assess the amounts of cytokines and chemokines secreted by DC after infection with N. meningitidis, DC were infected and supernatants were sampled at 6 h postinfection (p.i.). Supernatants were snap frozen in liquid nitrogen and stored at −80°C. The concentrations of TNF-α, IL-1β, IL-6, and IL-8 were determined twice for each supernatant by using the OptEIA human cytokine sets (BD Pharmingen, Heidelberg, Germany). Test sets were established according to the manufacturer's instructions to a sensitivity of 4 pg/ml. For all ELISA systems, the TMB substrate reagent set (BD Pharmingen) was used to detect the horseradish peroxidase reaction. All supernatants were analyzed twice.
RESULTS
Infection of human DC with N. meningitidis serogroup A, B, and C wild-type strains and capsule-deficient mutant strains.
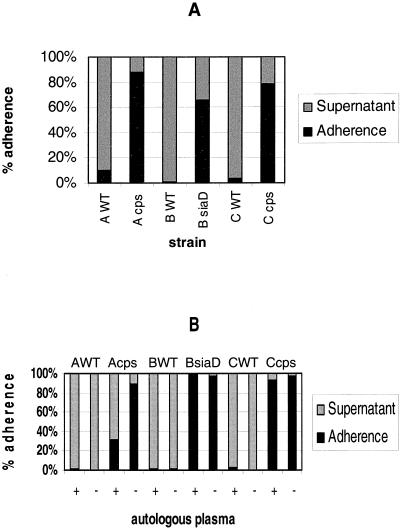
Immature DC were infected with N. meningitidis serogroup A strain A2044, serogroup B strain H44/76, serogroup C strain C1701, and the isogenic unencapsulated mutant strains A2044 cps, H44/76 siaD, and C1701 cps in the presence of 1% autologous human plasma. At 6 h p.i., cell association was determined for all strains. Unencapsulated bacteria of all strains adhered more efficiently than the capsule-expressing wild-type strains (Fig. 1A). For another donor, we made identical observations (data not shown). We next determined the influence of heat-inactivated autologous human plasma on adherence of all strains. For all wild-type strains, the presence of human plasma led to an only slightly higher adherence to the DC (Fig. 1B), while the adherence of the capsule-deficient serogroup B and C strains was not altered and the proportion of cell-adherent serogroup A bacteria was even reduced.
FIG. 1.
Adherence of N. meningitidis serogroups A, B, and C to DC. (A) Adherence of capsulate and noncapsulate N. meningitidis strains A2044, H44/76, and C1701 to human DC was determined. DC (5 × 105 per well) were infected at an MOI of 1 in RPMI 1640 medium containing 1% autologous human plasma. Adherence was determined at 6 h p.i. The proportion of cell-adherent bacteria was calculated by dividing the number of DC-adherent meningococci by the combined numbers of DC-adherent bacteria and meningococci in the supernatant. WT, wild type. (B) The influence of the presence of plasma on adherence of N. meningitidis to DC was assessed. DC (5 × 105) were infected with wild-type strains A2044, H44/76, and C1701 and their isogenic capsule-deficient mutant strains. DC were infected at an MOI of 1 in RPMI containing 1% heat-inactivated autologous human plasma (+) or RPMI without plasma (−). At 6 h p.i., neisserial adherence to DC was determined.
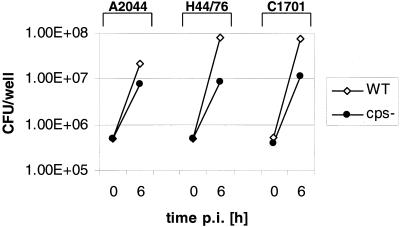
When we assessed the replication of meningococci in the presence of human DC during infection, we found strong growth of all wild-type strains (mainly in the DC supernatant), with the bacterial numbers being 2 orders of magnitude higher after 6 h in comparison to the starting point of the experiment. Surprisingly, the capsule-deficient bacteria were maximally 1 order of magnitude higher at 6 h (Fig. 2). This growth inhibition could be due to either strong phagocytosis and killing of the capsule-deficient bacteria or bactericidal substances which are secreted to the medium by DC. We had previously observed the efficient phagocytic killing of unencapsulated serogroup B meningococci by human DC (22). Here we made similar observations in gentamicin killing assays: at 6 h p.i., we found significantly higher numbers of intracellular bacteria for all capsule-deficient strains in comparison to wild-type bacteria, as determined with DC from two different donors (Table 1). Infection in the absence of human plasma did not significantly alter the numbers of live intracellular meningococci for wild-type and capsule-deficient strains (data not shown). When we assessed the numbers of total intracellular bacteria by Giemsa staining and transmission electron microscopy, we found much higher numbers of intracellular meningococci for all strains than were revealed by the gentamicin killing assays, especially for the capsule-deficient strains (data not shown), suggesting strong phagocytic killing.
FIG. 2.
Replication of N. meningitidis in the presence of DC. DC (5 × 105 per well) were infected at an MOI of 1 with capsulate and noncapsulate N. meningitidis strains A2044, H44/76, and C1701 in RPMI 1640 medium containing 1% autologous human plasma. Total numbers of CFU per well were determined by assessing and combining numbers of DC-associated meningococci and meningococci in the supernatant of DC at 0 and 6 h p.i. WT, wild type.
TABLE 1.
Phagocytosis of N. meningitidis by DC
| Strain | Invasive CFU/well at 6 h p.i.
|
|
|---|---|---|
| Donor 11 | Donor 12 | |
| A2044 (wild type) | 10 | 60 |
| A2044 cps | 23,000 | 38,000 |
| H44/76 (wild type) | 40 | 180 |
| H44/76 siaD | 12,000 | 50,000 |
| C1701 (wild type) | 60 | 870 |
| C1701 cps | 14,000 | 5,000 |
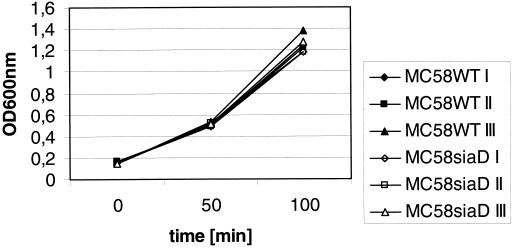
To test whether human DC secrete bactericidal substances, we employed DC supernatant as growth medium for wild-type and capsule-deficient N. meningitidis MC58 strains. For neither the MC58 wild-type strain nor the isogenic α-2,8-polysialyltransferase (siaD) mutant strain was there an inhibition of bacterial growth in DC supernatant in comparison to RPMI 1640 or pure PPM+, suggesting the absence of bactericidal substances in the supernatant of DC (Fig. 3). When the same experiment was performed with supernatants of DC that had previously been infected with either MC58 wild-type bacteria or the MC58 siaD strain for 8 h before collection of DC supernatants, we again did not observe growth differences between wild-type MC58 and MC58 siaD bacteria (data not shown). While it may be that we cannot detect unstable bactericidal substances such as reactive oxygen or nitrogen intermediates with these assays, our data nevertheless strongly suggest that the weak replication of the N. meningitidis capsule-deficient strains during DC infection is mainly due to the stronger phagocytosis and killing of these bacteria by DC and probably not due to the secretion of bactericidal substances
FIG. 3.
Assessment of DC supernatants for bactericidal substances. Supernatant of uninfected DC was collected and directly used for assessment as growth medium for wild-type (WT) and capsule-deficient MC58 strains. To this end, MC58 wild-type and MC58 siaD bacteria were inoculated into DC supernatant mixed with PPM+ at a 1:1 ratio (I), into RPMI 1640 mixed with PPM+ (II), and into pure PPM+ (III). At the start of the experiment, all cultures exhibited an optical density at 600 nm (OD600nm) of 0.15. Bacterial growth was assessed at 50 and 100 min postinoculation.
Sialylation of neisserial LOS inhibits phagocytosis of N. meningitidis by DC.
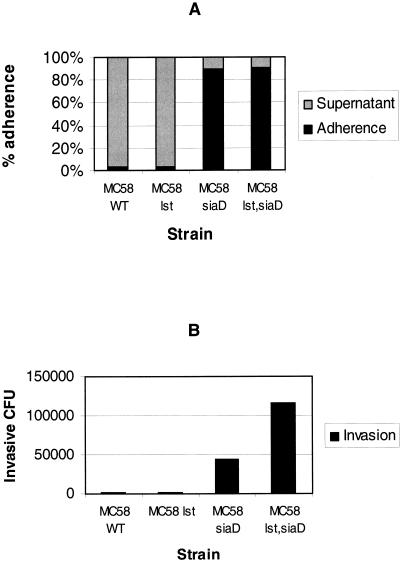
We analyzed the effect of LOS sialylation on adherence and invasion of human DC by N. meningitidis. To this end, an isogenic α-2,3-sialyltransferase (lst) mutant strain, strain MC58 lst, was constructed. In addition, the lst gene was deleted in the MC58 siaD mutant strain, leading to strain MC58 lst siaD. Human DC were infected with wild-type strain MC58 and strains MC58 lst, MC58 siaD, and MC58 lst siaD. At 6 h p.i., cell association and numbers of colony-forming intracellular bacteria were determined for all strains. Infections were performed in the presence of 1% autologous human plasma and 2 μg of CMP-N-acetylneuraminic acid (CMP-NANA) per ml. The exogenous CMP-NANA ensures an optimal LOS sialylation of wild-type MC58 bacteria during cell culture infections, while MC58 lst mutants continue to have a nonsialylated LOS even in the presence of CMP-NANA (15, 44). The encapsulated strains MC58 and MC58 lst both exhibited weak adherence of only about 3% at 6 h p.i. (Fig. 4A). In contrast, the unencapsulated strains MC58 siaD and MC58 lst siaD were highly adherent (90% adherence for MC58 siaD and 91% adherence for strain MC58 lst siaD) (Fig. 4A). Hence, the sialylation of the neisserial LOS does not seem to influence the adherence of N. meningitidis to human DC.
FIG. 4.
Effect of sialylation of neisserial LOS on adherence and invasion of DC by N. meningitidis. Adherence and invasion of human DC by wild-type (WT) strain MC58 and the MC58 lst, MC58 siaD, and MC58 lst siaD mutant strains were determined. DC (5 × 105 per well) were infected at an MOI of 1 in RPMI 1640 medium containing 1% autologous human plasma and CMP-NANA (2 μg/ml). (A) Adherence was determined at 6 h p.i. The proportion of cell-adherent bacteria was calculated by dividing the number of DC-adherent meningococci by the combined numbers of DC-adherent bacteria and meningococci in the supernatant. (B) For determination of invasion, gentamicin was added at 5 h p.i. and numbers of colony-forming intracellular bacteria were determined at 6 h p.i.
The situation is different for the invasion of DC. Infected DC were treated with gentamicin at 5 h p.i., and numbers of invasive colony-forming meningococci were determined at 6 h p.i. (Fig. 4B). While the numbers of living intracellular bacteria were quite low and almost identical for the capsule-expressing strains MC58 and MC58 lst (2,000 and 2,100 per 5 × 105 infected DC, respectively), we found 44,000 intracellular CFU for strain MC58 siaD and even 116,000 for strain MC58 lst siaD. For another donor, we found similar data for adherence and phagocytosis (data not shown). This demonstrates that in addition to capsule expression, the sialylation of LOS prevents the phagocytosis and killing of N. meningitidis by DC.
Infection with serogroup A, B, and C meningococci induces strong cytokine secretion by human DC.
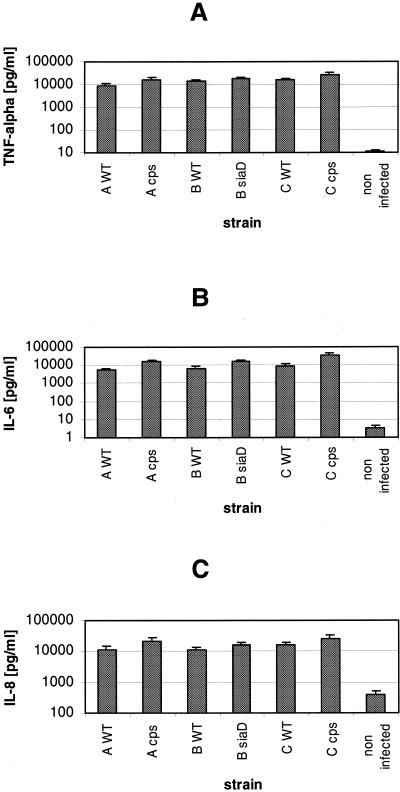
As previously shown for N. meningitidis serogroup B wild-type and unencapsulated bacteria, neisserial infection induces strong cytokine production by human DC (22). Here, we analyzed the cytokine secretion by human DC upon infection with serogroup A, B, and C meningococci. Immature DC were infected with N. meningitidis serogroup A strain A2044, serogroup B strain H44/76, and serogroup C strain C1701 and in parallel with the isogenic unencapsulated mutant strains. Infections were performed in the presence of 1% autologous human plasma, and at 6 h p.i., supernatants were sampled and assayed for cytokine concentrations by ELISA. All strains induced strong production of the cytokines TNF-α and IL-6 and the chemokine IL-8 (Fig. 5). As we had previously observed for N. meningitidis serogroup B, an even stronger production of these immune modulators was observed after infection with the capsule-deficient strains of all serogroups in comparison to the isogenic wild-type strains (Fig. 5). For the cytokine IL-1, we observed only insignificant secretion after 6 h of infection with all strains (data not shown), as we had previously found for serogroup B infection (22). For different donors, we likewise found strong induction of TNF-α, IL-6, and IL-8 and reproduced the slightly weaker induction by capsule-expressing wild-type strains (data not shown).
FIG. 5.
Cytokine production by DC after infection with N. meningitidis serogroups A, B, and C. DC (5 × 105 per well) were infected at an MOI of 1 in RPMI 1640 medium containing 1% autologous human plasma. Cells were infected with capsulate and noncapsulate N. meningitidis strains A2044, H44/76, and C1701. DC supernatants were sampled at 6 h p.i. and assessed for cytokines TNF-α (A) and IL-6 (B) and chemokine IL-8 (C) by ELISA. WT, wild type. Error bars indicate standard deviations.
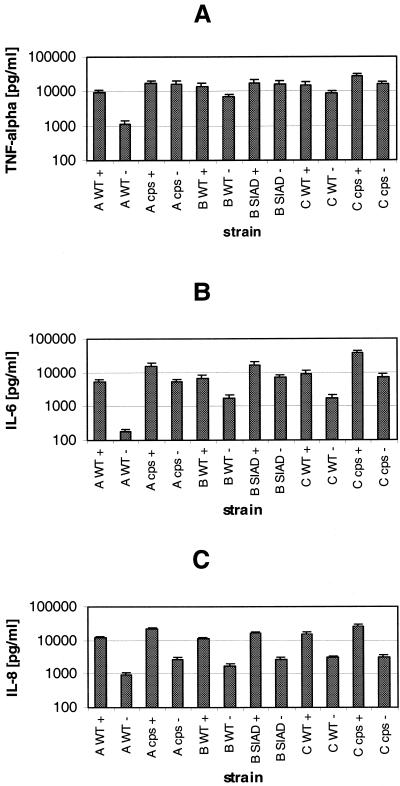
Human plasma increases cytokine production by DC after infection by N. meningitidis.
We next assessed the influence of human plasma on the cytokine production by DC after infection with N. meningitidis. DC were infected with N. meningitidis serogroup A, B, and C strains as well as their isogenic unencapsulated mutant strains in the presence or absence of autologous human plasma. For all strains, we found a significantly stronger induction of TNF-α, IL-6, and IL-8 (Fig. 6) in the presence of human plasma. This is not due to high cytokine levels in the plasma used, since the plasma had previously been assessed for TNF-α, IL-6, and IL-8 and the levels were shown to be insignificant, nor did plasma addition to the cells induce cytokine secretion (data not shown). For all serogroups, the unencapsulated strains induced stronger cytokine production in the presence and absence of plasma than the isogenic wild-type strains.
FIG. 6.
Influence of human plasma on cytokine production by DC infected with N. meningitidis. DC (5 × 105 per well) were infected with wild-type (WT) strains A2044, H44/76, and C1701 and their isogenic capsule-deficient mutant strains. DC were infected at an MOI of 1 in RPMI 1640 medium containing 1% heat-inactivated autologous human plasma (+) or in RPMI 1640 medium without plasma (−). At 6 h p.i., DC supernatants were sampled and assessed for TNF-α (A), IL-6 (B), and IL-8 (C) by ELISA. Error bars indicate standard deviations.
However, the stronger cytokine production observed for all strains in the presence of plasma does not seem to be due to stronger adherence to the DC. While the wild-type strains exhibit a somewhat higher level of adherence in the presence of plasma, the capsule-deficient strains were less adherent when plasma was present in the medium (Fig. 1B). Nevertheless, they induced stronger cytokine and chemokine production in the presence than in the absence of plasma. Again, the levels of IL-1 induced were not significant (data not shown). For different donors, the higher cytokine production in the presence of human plasma was confirmed for all strains (data not shown).
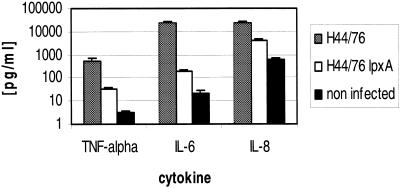
Meningococcal LOS is the main mediator of cytokine production by human DC.
To analyze the role of neisserial LOS in cytokine induction, human DC were infected with either wild-type H44/76 meningococci or the isogenic H44/76 lpxA mutant strain. The lpxA gene codes for the protein LpxA, which is involved in the lipid A biosynthesis pathway. Supernatants were sampled for assessment of cytokine levels at 6 h p.i. During this period of time, both strains exhibited similar levels of replication, adherence to DC, and invasion (data not shown). We observed a striking difference in cytokine production between the wild-type bacteria and the lpxA mutant for TNF-α, IL-6, and IL-8 (Fig. 7), clearly demonstrating that the LOS produced by live bacteria during infection is indeed the main mediator of cytokine induction. Interestingly, however, the DC infected with the lpxA mutant produced more cytokines than cells which were not infected (Fig. 7). Hence, neisserial LOS is an important mediator of the proinflammatory response, but other factors produced by N. meningitidis may also induce cytokine production in human DC. The dependence of the cytokine induction pattern on neisserial LOS was also found for different donors (data not shown).
FIG. 7.
Cytokine production by DC after infection with N. meningitidis serogroup B strains H44/76 and H44/76 lpxA. DC (5 × 105) per well were infected at an MOI of 1 in RPMI 1640 medium containing 1% autologous human plasma. Cells were infected with capsulate N. meningitidis serogroup B strains H44/76 and H44/76 lpxA. DC supernatants were sampled at 6 h p.i. and assessed for TNF-α, IL-6, and IL-8 by ELISA. Error bars indicate standard deviations.
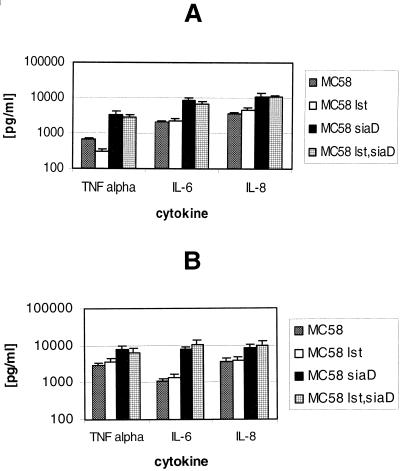
We next assessed the influence of sialylation of neisserial LOS on cytokine induction. Human DC were infected with wild-type strain MC58 and mutant strains MC58 lst, MC58 siaD, and MC58 lst siaD. Infections were performed in the presence of 1% autologous human plasma and 2 μg of CMP-NANA per ml. At 6 h p.i., supernatants were sampled and assessed for cytokine levels. The encapsulated strains (wild-type MC58 and MC58 lst) elicited similar levels of TNF-α, IL-6, and IL-8 (Fig. 8A). While strains MC58 siaD and MC58 lst siaD led to a higher level of cytokine production in human DC than the capsule-expressing strains (MC58 and MC58 lst), there were no major differences between the two capsule-deficient strains (Fig. 8A). For another donor, we could confirm the almost identical cytokine induction patterns of strains MC58 and MC58 lst and also of strains MC58 siaD and MC58 lst siaD (Fig. 8B). Hence, sialylation of neisserial LOS does not seem to have an impact on the proinflammatory response induced by meningococci.
FIG. 8.
Effect of sialylation of neisserial LOS on cytokine production by DC. DC (5 × 105 per well) were infected at an MOI of 1 with wild-type strain MC58 and the MC58 lst, MC58 siaD, and MC58 lst siaD mutant strains. Infections were performed in RPMI 1640 medium containing 1% autologous human plasma and CMP-NANA (2 μg/ml). DC supernatants were sampled at 6 h p.i. and assessed for TNF-α, IL-6, and IL-8 by ELISA. Error bars indicate standard deviations.
DISCUSSION
DC were shown to phagocytose a variety of bacterial pathogens, including Mycobacterium tuberculosis (16), Listeria monocytogenes (13, 21), Salmonella enterica serovar Typhimurium (8, 39), Escherichia coli (31), Yersinia pseudotuberculosis (8), Shigella flexneri (8), Borrelia burgdorferi (10), and Streptococcus gordonii (5), and we recently reported the phagocytosis of N. meningitidis serogroup B bacteria by DC (22). In most cases, the phagocytosed bacteria were found to be rapidly killed by the DC. Several reports suggest that certain intracellular pathogens such as human immunodeficiency virus and Leishmania major may exploit the migratory capacity of DC to penetrate or disseminate within the host by using DC as a Trojan horse (26, 28). However, for N. meningitidis serogroup B bacteria we had observed strong phagocytic killing of the bacteria by human DC (22), making a Trojan horse function for meningococci unlikely. In addition, we found the serogroup B capsule to inhibit adherence to and phagocytic killing of the bacteria by human DC. Here, we extended these observations to strains expressing serogroup A and C capsules. Both capsule types efficiently inhibited adherence to human DC and phagocytosis of the meningococci. Like for the serogroup B strains, the influence of human plasma on the adherence and phagocytosis of the bacteria was negligible. This is in contrast to the phagocytosis of L. monocytogenes in human DC, which is highly dependent on the presence of human plasma. Interestingly, the wild-type bacteria exhibit a significantly higher replication in the presence of human DC than capsule-deficient meningococci. This is apparently due to the more efficient phagocytic killing of the unencapsulated bacteria. We could not detect the release of bactericidal substances to the supernatant of uninfected or N. meningitidis-infected human DC.
Next to the expression of the neisserial capsule polysaccharide, sialylation of the LOS is an important mechanism to escape innate immune responses (45). We therefore analyzed the effect of LOS sialylation on adherence and invasion of human DC by N. meningitidis. The enzyme α-2,3-sialyltransferase, which is encoded by the lst gene, catalyzes the terminal linkage of sialic acid to the lacto-N-neotetraose epitope of meningococcal LOS with immunotype L3,7,9 (44). Capsule-expressing and capsule-deficient lst deletion mutants of serogroup B strain MC58 were assessed for their interaction with DC in comparison to wild-type MC58 and MC58 siaD bacteria. In the presence of the serogroup B capsule, no difference was observed between wild-type bacteria and an lst deletion mutant. However, in capsule-deficient strains we found the sialylation of the neisserial LOS to inhibit the phagocytosis of meningococci by DC. This was not due to inhibition of neisserial adherence, since strains MC58 siaD and MC58 lst siaD were found to be equally efficient in adherence to DC. Therefore it can be assumed that sialylation of the LOS masks neisserial surface molecules (e.g., Opa or Opc) which may mediate phagocytosis of N. meningitidis by DC (27). In addition, neisserial capsule expression and sialylation of LOS seem to be independent mechanisms which inhibit neisserial killing by human DC. While the capsule mainly inhibits adherence to DC and inhibits phagocytosis only to some extent, sialylation of LOS seems to be important to prevent phagocytosis and subsequent phagocytic killing. The existence of two independent mechanisms to escape innate immune responses may be crucial for meningococcal survival, especially in situations in which the neisserial capsule is not present, as seems to be the case during infection and crossing of epithelia (6, 15).
While human DC may play a beneficial role in neisserial meningitis by phagocytosis of the bacterial pathogens, they may represent a sword that cuts two ways. Upon neisserial infection, human DC produce the cytokines associated with fatal meningococcal disease. As we had previously observed for N. meningitidis serogroup B, all meningococcal strains analyzed in this study induced strong cytokine production by human DC. The stronger cytokine induction of capsule-deficient meningococci is a general feature and may be explained by the more efficient adherence of the capsule-lacking bacteria to the DC. For all strains, we found a significantly higher induction of TNF-α, IL-6, and IL-8 in the presence of human plasma. This may be due not to factors influenced directly by the meningococci but to a generally higher fitness of the DC in the presence of plasma (33). For the cytokine IL-1, we observed only insignificant secretion for infection with serogroups A and C, as was previously found for serogroup B infection (22). This is in contrast to data published by Dixon and colleagues (9), who demonstrated strong expression of IL-1 after treatment of DC with killed serogroup B strain H44/76 bacteria. However, in their study, Dixon et al. assessed the concentration of IL-1α, while we measured IL-1β. Since IL-1α is found preferentially as cell-associated protein and IL-1β is primarily a soluble protein, there may be different biological roles for the two types of IL-1 (4), which may explain the differences found for IL-1 induction in DC infected by meningococci.
It has previously been shown that the LOS produced by N. meningitidis serogroup B may be responsible for the production of the cytokines TNF-α, IL-1, IL-6, and IL-12 as well as the chemokine IL-8 by human DC (9, 22). Dixon and colleagues (9) employed an LOS-free lpxA mutant of N. meningitidis serogroup B strain H44/76. They treated DC with formalin-killed wild-type H44/76 bacteria and the H44/76 lpxA mutant strain and found a significantly weaker induction of TNF-α, IL-1, IL-6, and IL-12 with the killed lpxA mutant strain. Likewise, we demonstrated that purified neisserial LOS is a strong inducer of TNF-α, IL-6, and IL-8 in human DC (22). Recently, several groups independently demonstrated that H44/76 lpxA bacteria also elicited significantly lower levels of TNF-αand IL-1 than wild-type bacteria in human PBMCs and macrophages, regardless of whether viable or heat-killed meningococci were employed (19, 30, 37).
However, treatment of DC with killed bacteria or purified LOS may lead to an only partially adequate picture of DC induction by meningococcal LOS. Here, we analyzed the role of neisserial LOS in cytokine induction in human DC by infection with live meningococci of either the wild-type strain H44/76 or the isogenic H44/76 lpxA mutant strain. We observed a clearly higher production of TNF-α, IL-6, and IL-8 after infection with the wild-type bacteria, demonstrating that the LOS produced by live bacteria during infection is indeed the main mediator of cytokine induction. However, DC infected with the lpxA mutant produced more cytokines than untreated cells. Hence, neisserial LOS is an important mediator of the proinflammatory response, but other factors produced by N. meningitidis may also induce cytokine production in human DC. Similarly, gamma interferon and TNF-α are secreted by human PBMCs during incubation with viable or heat-killed N. meningitidis even in the absence of neisserial LOS (37), suggesting that non-LOS substances induced these cytokines. Other components of gram-negative bacteria which elicit proinflammatory responses include chaperones (20), membrane lipoproteins (1), and bacterial DNA (36). In this respect, the strong proinflammatory response induced in human PBMCs by the immunoglobulin A protease of pathogenic neisseriae may also play an important role (25).
Sialylation of neisserial LOS does not seem to have an impact on the proinflammatory response induced by meningococci. This is certainly due to the facts that the endotoxin effect is due to the lipid A portion of lipopolysaccharide and that the part of meningococcal LOS which is sialylated is the O-polysaccharide moiety. Sialylation of the O polysaccharide therefore does not seem to alter the release to the medium and/or the inflammatory capacity of meningococcal LOS. In line with our results, Svensson et al. (39) recently showed that different modifications of Salmonella lipid A do not affect the proinflammatory responses of DC induced by LPS. In contrast, the acylation pattern seems to be important for the proinflammatory capacity of meningococcal LOS. N. meningitidis strain H44/76 expressing penta- and tetra-acylated LOS species induced significantly lower levels of TNA in human macrophage cells than wild-type bacteria (41). Finally, the higher level of invasion found for the lst siaD mutant of strain MC58 in comparison to the MC58 siaD mutant does not seem to have a major impact on the cytokine induction in DC. This again emphasizes that the enormous amounts of LOS released to the extracellular medium by N. meningitidis in the form of membrane blebs (7) are the main reason for the inflammatory response seen during neisserial sepsis and meningitis.
Acknowledgments
A.U., U.K., and A.S. contributed equally to this paper.
We thank M. Dümig, S. Gross, and U. Panzner for expert technical assistance; A. Leimbach and T. Leimbach for help with cell culture assays; and H. Claus for electrophoretic typing of strains. We are grateful to I. Gentschev, W. Goebel, and U. Vogel for helpful discussions and to M. Dietrich, D. Felnerova, S. Kurz, and N. Smith for critical reading of the manuscript. We thank W. Zollinger for providing antibodies for immunotyping and E. R. Moxon, D. A. Caugant, and P. van der Ley for providing strains MC58, H44/76, and H44/76 lpxA, respectively.
This work was supported by a grant to M.F. within Sonderforschungsbereich 479 (Erregervariabilität und Wirtsreaktion bei infektiösen Krankheitsprozessen), project B2, and by grants from the Federal Ministry of Education and Research (01KS9603) and the Interdisciplinary Centre of Clinical Research Würzburg (IZKF) (A16) to U.K.
Editor: J. D. Clements
REFERENCES
- 1.Aliprantis, A. O., R. B. Yang, M. R. Mark, S. Suggett, B. Devaux, J. D. Radolf, G. R. Klimpel, P. Godowski, and A. Zychlinsky. 1999. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science 285:736-739. [DOI] [PubMed] [Google Scholar]
- 2.Austyn, J. M. 1996. New insights into mobilization and phagocytic activity of dendritic cells. J. Exp. Med. 183:1287-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 92:45-252. [DOI] [PubMed] [Google Scholar]
- 4.Boraschi, D., P. Bossu, G. Macchia, P. Ruggiero, and A. Tagliabue. 1996. Structure-function relationship in the IL-1 family. Front. Biosci. 1:270-308. [DOI] [PubMed] [Google Scholar]
- 5.Corinti, S., D. Medaglini, A. Cavani, M. Rescigno, G. Pozzi, P. Ricciardi-Castagnoli, and G. Girolomoni. 1999. Human dendritic cells very efficiently present a heterologous antigen expressed on the surface of recombinant gram-positive bacteria to CD4+ T lymphocytes. J. Immunol. 163:3029-3036. [PubMed] [Google Scholar]
- 6.Deghmane, A. E., S. Petit, A. Topilko, Y. Pereira, D. Giorgini, M. Larribe, and M. K. Taha. 2000. Intimate adhesion of Neisseria meningitidis to human epithelial cells is under the control of the crgA gene, a novel LysR-type transcriptional regulator. EMBO J. 19:1068-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Devoe, I. W., and J. E. Gilchrist. 1973. Release of endotoxin in the form of cell wall blebs during in vitro growth of Neisseria meningitidis. J. Exp. Med. 138:1156-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dietrich, G., A. Kolb-Mäurer, S. Spreng, M. Schartl, W. Goebel, and I. Gentschev. 2001. Gram-positive and Gram-negative bacteria as carrier systems for DNA vaccines. Vaccine 19:2506-2512. [DOI] [PubMed] [Google Scholar]
- 9.Dixon, G. L., P. J. Newton, B. M. Chain, D. Katz, S. R. Andersen, S. Wong, P. van der Ley, N. Klein, and R. E. Callard. 2001. Dendritic cell activation and cytokine production induced by group B Neisseria meningitidis: interleukin 12 production depends on lipopolysaccharide expression in intact bacteria. Infect. Immun. 69:4351-4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Filigueira, L., F. O. Nestle, M. Rittig, H. I. Joller, and P. Groscurth. 1996. Human dendritic cells phagocytose and process Borrelia burgdorferi. J. Immunol. 157:2998-3005. [PubMed] [Google Scholar]
- 11.Frosch, M., I. Gorgen, G. J. Boulnois, K. N. Timmis, and D. Bitter-Suermann. 1985. NZB mouse system for production of monoclonal antibodies to weak bacterial antigens: isolation of an IgG antibody to the polysaccharide capsules of Escherichia coli K1 and group B meningococci. Proc. Natl. Acad. Sci. USA 82:1194-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frosch, M., E. Schultz, E. Glenn-Calvo, and T. F. Meyer. 1990. Generation of capsule-deficient Neisseria meningitidis strains by homologous recombination. Mol. Microbiol. 4:1215-1218. [DOI] [PubMed] [Google Scholar]
- 13.Gentschev, I., G. Dietrich, S. Spreng, A. Kolb-Mäurer, V. Brinkmann, L. Grode, S. H. E. Kaufmann, J. Hess, and W. Goebel. 2001. Recombinant attenuated bacteria for the delivery of subunit vaccines. Vaccine 19:2621-2628. [DOI] [PubMed] [Google Scholar]
- 14.Gunn, J. S., and D. C. Stein. 1996. Use of a non-selective transformation technique to construct multiple restriction/modification-deficient Neisseria gonorrhoeae. Mol. Gen. Genet. 251:509-517. [DOI] [PubMed] [Google Scholar]
- 15.Hammerschmidt, S., R. Hilse, J. P. van Putten, R. Gerardy-Schahn, A. Unkmeir, and M. Frosch. 1996. Modulation of cell surface sialic acid expression in Neisseria meningitidis via a transposable genetic element. EMBO J. 15:192-198. [PMC free article] [PubMed] [Google Scholar]
- 16.Henderson, R. A., S. C. Watkins, and J. A. Flynn. 1997. Activation of human dendritic cells following infection with Mycobacterium tuberculosis. J. Immunol. 159:635-643. [PubMed] [Google Scholar]
- 17.Holten, E. 1979. Serotypes of Neisseria meningitidis isolated from patients in Norway during the first six months of 1978. J. Clin. Microbiol. 9:186-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inaba, K., M. Inaba, M. Naito, and R. M. Steinman. 1993. Dendritic cell progenitors phagocytose particulates, including bacillus Calmette-Guerin organisms, and sensitize mice to mycobacterial antigens in vivo. J. Exp. Med. 178:479-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ingalls, R. R., E. Lien, and D. T. Golenbock. 2001. Membrane-associated proteins of a lipopolysaccharide-deficient mutant of Neisseria meningitidis activate the inflammatory response through Toll-like receptor 2. Infect. Immun. 69:2230-2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kol, A., T. Bourcier, A. H. Lichtman, and P. Libby. 1999. Chlamydial and human heat shock protein 60s activate human vascular endothelium, smooth muscle cells, and macrophages. J. Clin. Investig. 103:571-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kolb-Mäurer, A., I. Gentschev, H.-W. Fries, F. Fiedler, E.-B. Bröcker, E. Kaempgen, and W. Goebel. 2000. Listeria monocytogenes-infected human dendritic cells: invasion and host cell response. Infect. Immun. 66:3680-3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kolb-Mäurer, A., A. Unkmeir, U. Kämmerer, C. Hübner, T. Leimbach, A. Stade, E. Kaempgen, M. Frosch, and G. Dietrich. 2001. Interaction of Neisseria meningitidis with human dendritic cells. Infect. Immun. 69:6912-6922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kroll, J. S., K. E. Wilks, J. L. Farrant, and P. R. Langford. 1998. Natural genetic exchange between Haemophilus and Neisseria: intergenic transfer of chromosomal genes between major human pathogens. Proc. Natl. Acad. Sci. USA 95:12831-12835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leib, S. L., and M. G. Tauber. 1999. Pathogenesis of bacterial meningitis. Infect. Dis. Clin. North. Am. 13:527-548. [DOI] [PubMed] [Google Scholar]
- 25.Lorenzen, D. R., F. Düx, U. Wölk, A. Tsirpouchtsidis, G. Haas, and T. F. Meyer. 1999. Immunoglobulin A1 protease, an exoenzyme of pathogenic Neisseriae, is a potent inducer of proinflammatory cytokines. J. Exp. Med. 190:1049-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Masurier, C., B. Salomon, N. Guettari, C. Pioche, F. Lachapelle, M. Guigon, and D. Klatzmann. 1998. Dendritic cells route human immunodeficiency virus to lymph nodes after vaginal or intravenous administration to mice. J. Virol. 72:7822-7829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McNeil, G., and M. Virji. 1997. Phenotypic variants of meningococci and their potential in phagocytic interactions: the influence of opacity proteins, pili, PilC and surface sialic acids. Microb. Pathog. 22:295-304. [DOI] [PubMed] [Google Scholar]
- 28.Moll, H., S. Flohe, and M. Roellinghoff. 1995. Dendritic cells in Leishmania major immune mice harbor persistent parasites and mediate an antigen-specific T cell response. Eur. J. Immunol. 25:693-699. [DOI] [PubMed] [Google Scholar]
- 29.Ohga, S., T. Aoki, K. Okada, H. Akeda, K. Fujioka, A. Ohshima, T. Mori, I. Minamishima, and K. Ueda. 1994. Cerebrospinal fluid concentrations of interleukin-1β, tumour necrosis factor-α, and interferon-γ in bacterial meningitis. Arch. Dis. Child. 70:123-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pridmore, A. C., D. H. Wyllie, F. Abdillahi, L. Steeghs, P. van der Ley, S. K. Dower, and R. C. Read. 2001. A lipopolysaccharide-deficient mutant of Neisseria meningitidis elicits attenuated cytokine release by human macrophages and signals via Toll-like receptor (TLR) 2 but not via TLR4/MD2. J. Infect. Dis. 183:89-96. [DOI] [PubMed] [Google Scholar]
- 31.Rescigno, M., M. Urbano, B. Valzasina, M. Francolini, G. Rotta, R. Bonasio, F. Granucci, J. P. Kraehenbuhl, and P. Ricciarda-Castagnoli. 2001. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat. Immunol. 2:361-367. [DOI] [PubMed] [Google Scholar]
- 32.Romani, N., S. Gruner, D. Brang, E. Kaempgen, A. Lenz, B. Trockenbacher, G. Konwalinka, P. O. Fritsch, R. M. Steinman, and G. Schuler. 1994. Proliferating dendritic cell progenitors in human blood. J. Exp. Med. 180:83-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Romani, N., D. Reider, M. Heuer, S. Ebner, E. Kaempgen, B. Eibl, D. Niederwieser, and G. Schuler. 1996. Generation of mature dendritic cells from human blood. An improved method with special regard to clinical applicability. J. Immunol. Methods 196:137-151. [DOI] [PubMed] [Google Scholar]
- 34.Sallusto, F., and A. Lanzavecchia. 1994. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor α. J. Exp. Med. 179:1109-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sallusto, F., M. Cella, C. Danieli, and A. Lanzavecchia. 1995. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J. Exp. Med. 182:389-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sparwasser, T., T. Miethke, G. Lipford, K. Borschert, H. Hacker, K. Heeg, and H. Wagner. 1997. Bacterial DNA causes septic shock. Nature 386:336-337. [DOI] [PubMed] [Google Scholar]
- 37.Sprong, T., N. Stikkelbroeck, P. van der Ley, L. Steeghs, L. van Alphen, N. Klein, M. G. Netea, J. W. van der Meer, and M. van Deuren. 2001. Contributions of Neisseria meningitidis LPS and non-LPS to proinflammatory cytokine response. J. Leukoc. Biol. 70:283-288. [PubMed] [Google Scholar]
- 38.Steeghs, L., R. den Hartog, A. den Boer, B. Zomer, P. Roholl, and P. van der Ley. 1998. Meningitis bacterium is viable without endotoxin. Nature 392:449-450. [DOI] [PubMed] [Google Scholar]
- 39.Svensson, M., C. Johansson, and M. J. Wick. 2000. Salmonella enterica serovar Typhimurium-induced maturation of bone-marrow-derived dendritic cells. Infect. Immun. 68:6311-6320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsai, C. M., and C. E. Frasch. 1982. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal. Biochem. 119:115-119. [DOI] [PubMed] [Google Scholar]
- 41.van der Ley, P., L. Steeghs, H. J. Hamstra, J. ten Hove, B. Zomer, and L. van Alphen. 2001. Modifications of lipid A biosynthesis in Neisseria meningitidis lpxL mutants: influence on lipopolysaccharide structure, toxicity, and adjuvant activity. Infect. Immun. 69:5981-5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Deuren, M., J. van der Ven-Jongekrijg, A. K. Bartelink, R. van Dalen, R. W. Sauerwein, and J. W. van der Meer. 1995. Correlation between proinflammatory cytokines and antiinflammatory mediators and the severity of disease in meningococcal infections. J. Infect. Dis. 172:433-439. [DOI] [PubMed] [Google Scholar]
- 43.Virji, M., H. Kayhty, D. J. P. Ferguson, J. E. Heckels, and E. R. Moxon. 1991. The role of pili in the interactions of pathogenic Neisseria with cultured human endothelial cells. Mol. Microbiol. 5:1831-1841. [DOI] [PubMed] [Google Scholar]
- 44.Vogel, U., H. Claus, G. Heinze, and M. Frosch. 1999. Role of lipopolysaccharide sialylation in serum resistance of serogroup B and C meningococcal disease isolates. Infect. Immun. 67:954-957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vogel, U., and M. Frosch. 1999. Mechanisms of neisserial serum resistance. Mol. Microbiol. 32:1133-1139. [DOI] [PubMed] [Google Scholar]
- 46.Waage, A., A. Halstensen, R. Shalaby, P. Brandtzaeg, P. Kierulf, and T. Espevik. 1989. Local production of tumor necrosis factor α, interleukin 1, and interleukin 6 in meningococcal meningitis. Relation to the inflammatory response. J. Exp. Med. 170:1859-1867. [DOI] [PMC free article] [PubMed] [Google Scholar]