Abstract
Cattle are important reservoirs of enterohemorrhagic Escherichia coli (EHEC) O157:H7 that cause disease in humans. Both dairy and beef cattle are asymptomatically and sporadically infected with EHEC. Our long-term goal is to develop an effective vaccine to prevent cattle from becoming infected and transmitting EHEC O157:H7 to humans. We used passive immunization of neonatal piglets (as a surrogate model) to determine if antibodies against EHEC O157 adhesin (intiminO157) inhibit EHEC colonization. Pregnant swine (dams) with serum anti-intimin titers of ≤100 were vaccinated twice with purified intiminO157 or sham-vaccinated with sterile buffer. IntiminO157-specific antibody titers in colostrum and serum of dams were increased after parenteral vaccination with intiminO157. Neonatal piglets were allowed to suckle vaccinated or sham-vaccinated dams for up to 8 h before they were inoculated with 106 CFU of a Shiga toxin-negative (for humane reasons) strain of EHEC O157:H7. Piglets were necropsied at 2 to 10 days after inoculation, and intestinal samples were collected for determination of bacteriological counts and histopathological analysis. Piglets that ingested colostrum containing intiminO157-specific antibodies from vaccinated dams, but not those nursing sham-vaccinated dams, were protected from EHEC O157:H7 colonization and intestinal damage. These results establish intiminO157 as a viable candidate for an EHEC O157:H7 antitransmission vaccine.
Enterohemorrhagic Escherichia coli (EHEC) O157:H7 is a common cause of bloody diarrhea and the most common cause of hemolytic-uremic syndrome in humans in the United States (1). EHEC strains can be foodborne pathogens, and cattle are important reservoirs of EHEC O157:H7 strains. All EHEC strains produce cytotoxins called Shiga toxins (Stx1 and Stx2), previously called Shiga-like toxins (SLT-1 and SLT-2) or verotoxins (VT1 and VT2). Many EHEC strains, including EHEC O157:H7, can attach intimately to host cell membranes and efface microvilli and cytoplasm in a pattern referred to as an attaching-and-effacing (A/E) lesion (18). EHEC intimin is an outer membrane protein adhesin encoded by the eae gene of EHEC (16) that is required for intestinal colonization and for A/E activity of EHEC O157:H7 in piglets (6, 8, 9, 19) and neonatal calves (6). Antibodies against intiminO157 significantly reduce the adherence of E. coli O157:H7 strains, as well as certain related strains, to HEp-2 cells (11). Based on this observation, we hypothesize that a vaccine that contains intiminO157 may induce an immune response in cattle that will prevent or decrease the level of intestinal colonization by E. coli O157:H7. We speculate that the resultant diminution in fecal shedding of EHEC O157:H7 by these animals could, in turn, lead to a decrease in the transmission of EHEC O157:H7 and other A/E E. coli strains to humans.
In the study reported here, we tested the concept that anti-intimin antibodies can protect animals from colonization with EHEC O157:H7. For that purpose, we selected neonatal piglets as the bacterial challenge model. Although pigs have not been identified as a common reservoir of EHEC O157:H7 strains, the neonatal piglet EHEC infection model has been used to demonstrate the critical role of intimin in EHEC O157:H7 infections (6, 19). Furthermore, neonatal suckling piglets are a good surrogate model for determining if antibodies against intimin prevent EHEC colonization and disease.
The objectives of this investigation were twofold. First, we sought to determine whether parenteral vaccination of pregnant swine (first-litter dams) with intiminO157 elicits anti-intiminO157 antibodies in their serum and colostrum. Second, we wanted to assess whether piglets that ingest colostrum (first milk) containing anti-intiminO157 antibodies are passively immunized and protected from EHEC O157:H7 infection and disease. To prevent the development of Stx-mediated systemic disease in the neonatal animals, we challenged them with an Stx-negative E. coli O157:H7 strain. We felt confident that substitution of this Stx− strain for wild-type EHEC O157:H7 (which was done for humane purposes) would not compromise our results because both Stx+ and Stx− E. coli O157:H7 strains similarly colonize and cause A/E lesions in the large intestines of suckling piglets (3).
MATERIALS AND METHODS
IntiminO157 purification.
The histidine-tagged intiminO157 protein RIHisEae was purified as described previously (11, 20) and used for vaccination of pregnant dams and for anti-intiminO157 dot blot assays. RIHisEae is a histidine-tagged version of the entire intimin protein from EHEC O157:H7 strain 86-24 minus the N-terminal 35 amino acids, which are thought to be part of a cleaved N-terminal signal sequence (13, 20). For Western blot analysis of colostrum samples, a histidine-tagged N-terminal two-thirds fragment of intimin and a histidine-tagged C-terminal one-third fragment of intimin were purified as described previously (11).
Animals and vaccinations.
Three crossbred pregnant dams (first-litter dams; dams A, C, and E) were vaccinated intramuscularly with intiminO157 mixed with TiterMax Gold adjuvant (500 μg of intimin/dose) at 2 and 4 weeks prior to farrowing (giving birth). Three pregnant dams (dams B, D, and F) were sham vaccinated with Tris-buffered saline (TBS) mixed with adjuvant. Piglets naturally farrowed at the National Animal Disease Center by these dams were allowed to suckle colostrum before inoculation with E. coli O157:H7. One intiminO157-vaccinated dam (dam C) had complications at farrowing and may not have allowed all of the piglets to nurse freely prior to inoculation.
Colostrum and blood samples.
Colostrum was collected from each dam at parturition. Blood samples were collected from the dams prior to the first vaccination (prevaccination) and at 7 to 11 days after farrowing when dams were euthanatized (postvaccination). Blood samples were collected from all of the piglets in the last two litters tested, one vaccinated litter (dam E) and one sham-vaccinated litter (dam F), immediately before they were inoculated with E. coli O157:H7. Colostral whey and sera were prepared as previously described and stored at −80°C until tested (4).
Inoculation with E. coli O157:H7.
All piglets were inoculated with 106 CFU of Stx-negative E. coli O157:H7 strain 87-23 (17, 25) administered via stomach tube after all of the piglets had nursed and before the piglets were 8 h old. Inocula were prepared and stored as previously described (5). Piglets were returned to the dam immediately after inoculation and observed clinically every 4 to 8 h.
Necropsy.
Piglets were euthanatized with sodium pentobarbital and necropsied at 2 to 10 days postinoculation (p.i.). Piglets were randomly assigned to each of the necropsy groups. At necropsy, sections of the cecum were collected aseptically for determination of bacterial counts and sections of the distal colon, spiral colon, cecum, and ileum were collected for histopathologic examination and immunoperoxidase (horseradish peroxidase [HRPO]) staining with anti-O157:H7 antibody (see below).
Bacteriologic examination.
Sorbitol-negative E. coli O157:H7 bacteria were quantitated on sorbitol-MacConkey agar. Selected sorbitol-negative colonies were tested for O157:H7 antigens by latex agglutination assay (5). Bacterial colonization was defined as ≥106 CFU of inoculum bacteria/g of cecal tissue. Because dilutions made in counting did not permit recovery of <104 CFU of E. coli per g, tissues from which the inoculum strain was not recovered were recorded as having <104 CFU/g of tissue.
Histologic studies.
Tissues were fixed in neutral buffered 10% formalin, processed by routine methods, sectioned, and stained with hematoxylin and eosin (H&E). Stained tissue slides were coded and examined by light microscopy. A/E lesions were scored from 0 (negative) to 4+ (≥50% of villi or surface epithelium affected) as previously described (5).
Immunoperoxidase staining with anti-O157:H7.
O157:H7 bacteria were identified in paraffin-embedded, formalin-fixed tissues by indirect immunoperoxidase staining with anti-O157:H7 as previously described (5).
Anti-intiminO157 dot blot assay.
Anti-intiminO157 titers in swine serum and colostrum were determined with a dot blot enzyme-linked immunosorbent assay. Purified intiminO157 (100 μl/dot; 12 μg of intimin/ml in 0.1 M Tris-154 mM NaCl, pH 7.4 [TBS]) was applied to nitrocellulose filters (BA85; Schleicher & Schuell, Inc., Keene, N.H.) with a 96-well Milliblot apparatus (Millipore Corp., Bedford, Mass.). Unbound intimin was removed and additional binding sites on the filters were blocked by three washes with 0.05% Tween 20 (Sigma, St. Louis, Mo.) in TBS (TBS-T). All remaining incubations were for 1 h at 22°C. Diluted serum or colostrum (100 μl of serial 10-fold dilutions ranging from 10−2 to 10−4 or 10−5 per well) in TBS-T was added, and the mixture was incubated. After three washes with TBS-T, filters were removed from the Millipore apparatus and washed for an additional 10 min in TBS-T. Total bound immunoglobulin was detected by sequential incubations with peroxidase-labeled goat anti-swine immunoglobulin G (heavy and light chains; Kirkegaard & Perry Laboratories, Gaithersburg, Md.) and substrate (0.5 mg of 4-chloro-1-naphthol per ml, 0.015% H2O2, and 16.7% methanol in TBS). The anti-intiminO157 titer is the reciprocal of the highest dilution of serum or colostrum that yielded a purple (peroxidase-positive) precipitate on a filter.
Adherence assays.
The ability of porcine colostrum from intimin-vaccinated dam A and sham-vaccinated dam B to inhibit the adherence of E. coli strain 87-23 to HEp-2 (human laryngeal epithelial) cells was assessed as described previously (11). Briefly, colostrum samples were diluted 1:27 into 0.3 ml of adherence medium (Eagle minimum essential medium, 1% mannose, 0.4% sodium bicarbonate), mixed with 4 × 106 bacteria of E. coli strain 87-23, and incubated for 35 min at 37°C. The bacterium-colostrum mixtures were added to monolayers of HEp-2 cells that were incubated for 3 h at 37°C and washed twice. Fresh adherence medium with colostrum was added for a second 3-h incubation, after which the cells were washed and stained as described previously. Adherence was quantitated by determining the number of bacteria per HEp-2 cell, with at least 250 HEp-2 cells and at least 600 bacteria counted per sample. The adherence inhibition assays of these colostrum samples were done three times on different days.
Western blot analysis.
Colostrum samples from dams A and B were subjected to Western blot analysis for reactivity with whole-cell lysates of EHEC and for reactivity with purified intimin protein. Whole-cell bacterial lysates of wild-type EHEC O157:H7 strain 86-24 (14) and its isogenic eae− mutant derivative 86-24 eaeΔ10 (19) were prepared and loaded onto sodium dodecyl sulfate-polyacrylamide gels as described previously (11). Proteins separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis were blotted onto nitrocellulose and processed for immunoblot analysis with visualization by chemiluminescence as described previously (11). A 1:7,500 dilution of each colostrum sample was used, followed by an anti-swine secondary antibody (Sigma). Western blots were scanned, and images of the blots were arranged and labeled with Adobe Photoshop version 5.0.2 for Macintosh.
RESULTS
Anti-intiminO157 titers.
As shown in Table 1, the serum anti-intiminO157 titers of all of the pregnant dams were ≤100 before they were vaccinated. At the time of farrowing, all of the intiminO157-vaccinated dams had colostral anti-intiminO157 titers of ≥100,000 and all of the sham-vaccinated dams had titers of ≤100. The serum anti-intiminO157 titers of the vaccinated dams after they had farrowed were >10,000, but those of the sham-vaccinated dams were ≤100. All seven of the piglets that nursed a vaccinated dam (dam E) and were at least 2 h old when they were inoculated with E. coli O157:H7 had serum anti-intiminO157 titers of ≥10,000 at the time they were challenged. Two piglets in this same litter that were inoculated before they were 2 h old had a titer of 100. All eight of the piglets nursing sham-vaccinated dam F had titers of <100.
TABLE 1.
Anti-intiminO157 titers in serum and colostrum of pregnant dams after vaccination with intiminO157
| Vaccination and dam | Anti-intiminO157 titer
|
||
|---|---|---|---|
| Serum
|
Colostrum | ||
| Prevaccination | Postvaccination | ||
| IntiminO157 | |||
| A | ≤100 | ≥10,000 | ≥100,000 |
| C | ≤100 | ≥10,000 | ≥100,000 |
| E | ≤100 | ≥10,000 | ≥100,000 |
| Sham (TBS) | |||
| B | ≤100 | ≤100 | ≤100 |
| D | ≤100 | ≤100 | ≤100 |
| F | ≤100 | ≤100 | ≤100 |
Adherence of E. coli strain 87-23 to HEp-2 cells in vitro in the presence of colostrum.
A small but reproducible difference in the number of adherent bacteria was observed in the presence of colostrum from the intiminO157-immunized dam compared to colostrum from the sham-vaccinated dam (35% fewer adherent bacteria in the presence of colostrum from the intiminO157-immunized dam, as averaged from the three replicate assays; P < 0.05; data not shown). Neither colostrum sample agglutinated the bacteria, as assessed by slide agglutination assays. Further, the growth of strain 87-23 in the presence of colostrum from the intiminO157-immunized dam was comparable to that in the presence of colostrum from the sham-vaccinated dam, as determined from colony counts after growth in adherence medium.
Western blot analysis.
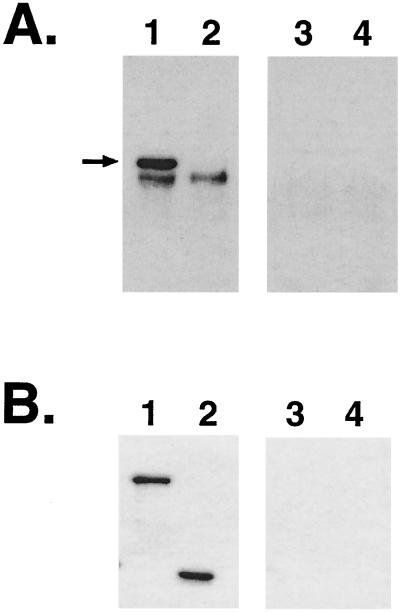
Colostrum samples from one intimin-vaccinated and one sham-vaccinated dam (dams A and B) were also analyzed by Western blotting. The colostrum from the intiminO157-vaccinated dam, but not that from the sham-vaccinated dam, reacted with intimin in a whole-cell lysate of EHEC O157:H7 (Fig. 1A). The immune colostrum contained antibodies that recognized N- and C-terminal portions of intiminO157, as demonstrated by reactivity against purified intimin fragments (Fig. 1B).
FIG. 1.
Western blot analysis of colostrum. Colostrum samples from an intimin-vaccinated dam (lanes 1 and 2) and a sham (TBS)-vaccinated dam (lanes 3 and 4) were assayed by Western blotting. (A) Reactivity of colostrum samples against whole-cell lysates of EHEC O157: H7 strain 86-24 (lanes 1 and 3) and intimin mutant strain 86-24 eaeΔ10 (lanes 2 and 4). The region of the blot containing intimin (arrow) is shown; the colostrum also reacted with lower-molecular-weight bands in the lysates. The band migrating just below intimin may be a cross-reacting band. (B) Reactivity of colostrum samples with a purified N-terminal two-thirds fragment of intimin (lanes 1 and 3) and a purified C-terminal one-third fragment of intimin (lanes 2 and 4) from EHEC strain 86-24.
Clinical and gross pathological observations.
All but one of the piglets remained clinically healthy following inoculation with Stx-negative E. coli O157:H7 strain 87-23. One weak and depressed piglet in a vaccinated litter (litter A) had mild diarrhea between 8 and 24 h after inoculation but not between 24 and 48 h when it was necropsied. This pig and two other piglets (one of which was lame) from the same litter had moderate colonic edema and hyperemia when necropsied at 2 or 3 days after inoculation (but none of these three pigs were colonized with the inoculum strain or had A/E lesions).
Bacterial colonization.
Table 2 shows the numbers of inoculum bacteria that were recovered from the suckling piglets. Most (25 of 30) of the piglets nursing sham-vaccinated dams were colonized by E. coli O157:H7 (i.e., had ≥106 CFU of inoculum bacteria/g of cecal tissue) at 2 to 10 days after inoculation. The five piglets that were not colonized were necropsied at either 2 or 3 days after inoculation. In contrast, only 6 of the 25 piglets nursing intiminO157-vaccinated dams were colonized or had A/E lesions at 2 to 10 days after inoculation. Five of these six colonized piglets were from litter C, the litter that might not have had free access to colostrum before challenge (see Materials and Methods). No E. coli O157:H7 bacteria (i.e., <104 CFU of inoculum bacteria/g of cecal tissue) were recovered from 15 (including the 2 piglets in litter E that had low serum anti-intiminO157 titers) of the 19 piglets suckling vaccinated dams that were not colonized.
TABLE 2.
Effect of vaccination of pregnant dams with intiminO157 on experimental E. coli O157:H7 infection in suckling piglets
| Vaccination and litter(s) | No. of piglets positive/no. tested
|
|||||
|---|---|---|---|---|---|---|
| Colonizeda
|
A/E lesions in large intestineb
|
|||||
| Total | 2-4 days pi | 6-10 days pi | Total | 3-4 days pi | 6-10 days pi | |
| IntiminO157 | ||||||
| A + C + E | 6/25c | 5/20 | 1/5 | 8/25c, d | 6/20d | 2/5 |
| A | 1/8 | 1/8 | NDe | 1/8d | 1/8d | ND |
| C | 5/8 | 4/6 | 1/2 | 7/8d | 5/6d | 2/2 |
| E | 0/9 | 0/6 | 0/3 | 0/9 | 0/6 | 0/3 |
| Sham (TBS) | ||||||
| B + D + F | 25/30 | 17/22 | 8/8 | 26/30 | 18/22 | 8/8 |
| B | 8/11 | 6/9 | 2/2 | 9/11 | 7/9 | 2/2 |
| D | 9/9 | 6/6 | 3/3 | 9/9 | 6/6 | 3/3 |
| F | 8/10 | 5/7 | 3/3 | 8/10 | 5/7 | 3/3 |
≥106 CFU of inoculum bacteria/g of cecum.
A/E lesions identified as O157:H7+ by immunostaining.
Significantly different from sham-vaccinated piglets (P < 0.05 [chi-square analysis]).
A/E lesions were detected only by HRPO, and not by H&E staining, in one piglet in litter A and two in litter C.
ND, not done.
A/E lesions.
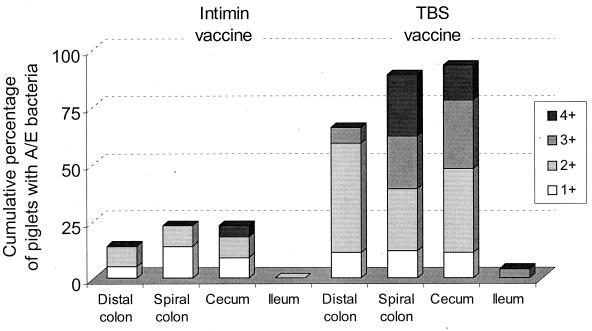
Most (25 of 30) piglets nursing the three sham-vaccinated control dams had A/E lesions in the large intestines at 2 to 10 days after inoculation with E. coli O157:H7 (Table 2 and Fig. 2). All sham-vaccinated piglets that had ≥106 CFU of O157:H7/g of cecal tissue had A/E lesions. In contrast, only 8 of the 25 piglets nursing intiminO157-vaccinated dams had A/E lesions. Three of these piglets had rare O157:H7-positive A/E lesions that were detected only by HRPO staining. No A/E lesions were seen in the two piglets in litter E that had low serum anti-intiminO157 titers at the time of inoculation. A/E lesions were found in all cecal tissues that had at least 106 CFU of inoculum bacteria/g of cecal tissue. O157:H7-positive A/E lesions were identified by HRPO in all tissue sections in which A/E lesions were detected by H&E staining. As shown in Fig. 2, the A/E scores of vaccinated piglets with A/E lesions were considerably lower than those of the sham-vaccinated piglets. Five of the piglets that nursed vaccinated dam C, but none of the piglets that nursed the other two vaccinated dams, had A/E lesions that were detected by H&E staining. Two additional piglets from litter C and one piglet from litter A (clinically normal and without gross lesions) had rare O157:H7-positive A/E lesions that were detected only by HRPO staining.
FIG. 2.
Effect of intiminO157 vaccination of pregnant gilts on the distribution and intensity of A/E lesions in suckling piglets at 3 to 10 days p.i. A/E scores reflect the extent of villi or surface epithelium affected, as estimated by examination of H&E-stained sections. Scoring: 1+, detected only in HRPO-stained sections; 2+, <10% of the villi or surface epithelium affected; 3+, 10 to 50% of the villi or surface epithelium affected; 4+, ≥50% of the villi or surface epithelium affected.
DISCUSSION
A vaccine preparation consisting of intiminO157 mixed with TiterMax Gold adjuvant and administered parenterally at 2 and 4 weeks prior to farrowing was immunogenic in pregnant swine. Most suckling piglets that ingested colostrum containing anti-intiminO157 antibodies from intiminO157-vaccinated dams were not colonized and did not develop intestinal lesions when they were challenged with E. coli O157:H7. The two piglets from litter E that were inoculated before they were 2 h old may have had a serum anti-intiminO157 titer of only 100 at the time of inoculation because they had not yet had time to absorb antibody from colostrum into the serum. They might have had higher serum titers if sampled later. This is supported by the observation that they were protected from colonization after challenge. The protective antibodies were most likely transmitted via colostrum because immunoglobulins are not transmitted in utero to the fetuses of healthy sows.
Colostrum from the one intiminO157-vaccinated dam that we tested, but not that from a sham-vaccinated dam, inhibited the adherence of E. coli O157:H7 strain 87-23 to HEp-2 cells. This colostrum contained antibodies against the C-terminal third of intimin, the region that is known to be critical for interaction with mammalian cells (7, 10). The inhibitory effect on adherence did not appear to be due to agglutination or inhibition of bacterial growth by the colostrum. These observations support the hypothesis that the presence of antibodies against intiminO157 in the intestines of suckling piglets inhibits bacterial adherence. The effects of antibodies on adherence in vitro (35% inhibition) were weaker than those observed in vivo (seven of eight piglets were protected from adherence and colonization).
None of the pregnant dams in this study had preexisting anti-intiminO157 titers of >100, but all of the intiminO157-vaccinated dams had colostral anti-intiminO157 titers of ≥100,000 at farrowing. However, one pregnant dam was excluded from an earlier pilot study because it had an anti-intiminO157 titer of 1:10,000 prior to sham vaccination (with TBS and adjuvant) and a colostral anti-intiminO157 titer of 100,000 at farrowing. The presence of anti-intiminO157 antibodies in a nonvaccinated pig is consistent with previous reports that some porcine E. coli isolates have A/E activity and/or contain the eae gene, which encodes intimin (15, 21, 22, 26), and could be reservoirs of EHEC associated with human infections (2, 23, 24).
In this study, as in previous studies, the presence of A/E lesions containing O157: H7-positive bacteria correlated with the number of inoculum bacteria recovered from the ceca of piglets inoculated with E. coli O157:H7 bacteria. A/E lesions were seen in most piglets that had ≥106 CFU of O157:H7/g of cecum but rarely in ones with fewer bacteria.
Consistent with our earlier observations (3), most of the sham-vaccinated suckling piglets were colonized and had intestinal lesions by 3 days p.i. but showed no signs of disease. It was somewhat surprising that all of the sham-vaccinated suckling piglets examined between 6 and 10 days p.i. were colonized with O157:H7 bacteria and had A/E lesions. Apparently, E. coli O157:H7 was not cleared from the large intestines of neonatal piglets (based on high cecal counts) as rapidly as it is thought to be cleared from some EHEC-infected humans (as estimated by negative fecal cultures). The inability to recover EHEC O157:H7 from the feces of some human patients with bloody diarrhea or hemolytic-uremic syndrome has been attributed to clearance of the causative EHEC bacteria by the time the patients seek medical care and samples are collected for culture. It was encouraging to see that piglets nursing the vaccinated dams were still not colonized at 9 to 10 days after inoculation.
The long-term goal of our research is to develop a means for reducing the transmission of E. coli O157:H7 from cattle to humans. The concept that antibodies to intiminO157 may block colonization of animals with O157:H7 was strongly supported by the passive immunization studies with suckling piglets described here. Indeed, the results of these experiments clearly demonstrated that piglets that ingested maternal antibodies against intiminO157 were protected from colonization and intestinal damage following experimental inoculation with an intimin-producing, nontoxigenic E. coli O157:H7 strain. These results establish intiminO157 as a viable candidate for an EHEC O157:H7 antitransmission vaccine. Recent evidence showing that active immunization of mice with intimin prevents colonization by the intimin-positive mouse pathogen Citrobacter rodentium (12) further bolsters the idea of using intimin in vaccines against EHEC pathogens. For humane reasons, we have not tested the vaccine against Stx-positive strains in neonatal piglets because they are highly sensitive to Stx-mediated neurological disease (3). However, since cattle appear to be resistant to Stx-mediated disease, we will use Stx-positive EHEC strains in future studies to determine if anti-intiminO157 antibodies can also prevent EHEC colonization and transmission in cattle.
Acknowledgments
This work was partly supported by grant AI20148-18 from the National Institutes of Health, by grant 97-35201-4578 from the U.S. Department of Agriculture to A.D.O., and by grant R01A141328 from the National Institutes of Health to H.W.M.
We thank G. Buck, M. Church, J. Donald, R. Ezarski, M. I. Inbody, N. C. Lyon, R. W. Morgan, R. A. Schneider, W. C. Stoffregen, E. M. Twiddy, and B. K. Wheeler for technical assistance; S. L. Johnson for preparation of the manuscript; and S. H. Hurd and J. A. Roth for critical review of the manuscript.
Editor: J. D. Clements
REFERENCES
- 1.Boyce, T. G., D. L. Swerdlow, and P. M. Griffin. 1995. Escherichia coli O157:H7 and the hemolytic-uremic syndrome. N. Engl. J. Med. 333:364-368. [DOI] [PubMed] [Google Scholar]
- 2.Chapman, P. A., J. Cornell, and C. Green. 2000. Infection with verocytotoxin-producing Escherichia coli O157 during a visit to an inner city open farm. Epidemiol. Infect. 125:531-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dean-Nystrom, E. A., J. Pohlenz, H. W. Moon, and A. D. O'Brien. 2000. Escherichia coli O157:H7 causes more severe systemic disease in suckling piglets than in colostrum-deprived neonatal piglets. Infect. Immun. 68:2356-2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dean-Nystrom, E. A., J. I. Sarmiento, and P. L. Runnels. 1992. Effect of intestinal receptors for K88 fimbriae on the immune responses of weaned pigs to K88 fimbriae of enterotoxigenic Escherichia coli. Immunol. Infect. Dis. 2:263-267. [Google Scholar]
- 5.Dean-Nystrom, E. A., B. T. Bosworth, W. C. Cray, Jr., and H. W. Moon. 1997. Pathogenicity of Escherichia coli O157:H7 in the intestines of neonatal calves. Infect. Immun. 65:1842-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dean-Nystrom, E. A., B. T. Bosworth, H. W. Moon, and A. D. O'Brien. 1998. Escherichia coli O157:H7 requires intimin for enteropathogenicity in calves. Infect. Immun. 66:4560-4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeVinney, R., M. Stein, D. Reinscheid, A. Abe, S. Ruschkowski, and B. B. Finlay. 1999. Enterohemorrhagic Escherichia coli O157:H7 produces Tir, which is translocated to the host cell membrane but is not tyrosine phosphorylated. Infect. Immun. 67:2389-2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donnenberg, M. S., S. Tzipori, M. L. McKee, A. D. O'Brien, J. Alroy, and J. B. Kaper. 1993. The role of the eae gene of enterohemorrhagic Escherichia coli in intimate attachment in vitro and in a porcine model. J. Clin. Investig. 92:1418-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Francis, D. H., R. A. Moxley, and C. Y. Andraos. 1989. Edema disease-like brain lesions in gnotobiotic piglets infected with Escherichia coli serotype O157:H7. Infect. Immun. 57:1339-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frankel, G., D. D. Candy, P. Everest, and G. Dougan. 1994. Characterization of a C-terminal domain of intimin-like proteins of enteropathogenic and enterohemorrhagic Escherichia coli, Citrobacter freundii, and Hafnia alvei. Infect. Immun. 62:1835-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gansheroff, L. J., M. R. Wachtel, and A. D. O'Brien. 1999. Decreased adherence of enterohemorrhagic Escherichia coli to HEp-2 cells in the presence of antibodies that recognize the C-terminal region of intimin. Infect. Immun. 6:6409-6417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghaem-Maghami, M., C. P. Simmons, S. Daniell, M. Pizza, D. Lewis, G. Frankel, and G. Dougan. 2001. Intimin-specific immune responses prevent bacterial colonization by the attaching-effacing pathogen Citrobacter rodentium. Infect. Immun. 69:5597-5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomez-Duarte, O. G., and J. B. Kaper. 1995. A plasmid-encoded regulatory region activates chromosomal eaeA expression in enteropathogenic Escherichia coli. Infect. Immun. 63:1767-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Griffin, P. M., S. M. Ostroff, R. V. Tauxe, K. D. Greene, J. G. Wells, J. H. Lewis, and P. A. Blake. 1988. Illnesses associated with Escherichia coli O157:H7 infections. A broad clinical spectrum. Ann. Intern. Med. 109:705-712. [DOI] [PubMed] [Google Scholar]
- 15.Janke, B. H., D. H. Francis, J. E. Collins, M. C. Libal, D. H. Zeman, and D. D. Johnson. 1989. Attaching and effacing Escherichia coli infections in calves, pigs, lambs, and dogs. J. Vet. Diagn. Investig. 1:6-11. [DOI] [PubMed] [Google Scholar]
- 16.Kaper, J. B., L. J. Gansheroff, M. R. Wachtel, and A. D. O'Brien. 1998. Intimin-mediated adherence of Shiga toxin-producing Escherichia coli and attaching-and-effacing pathogens, p. 148-156. In J. B. Kaper and A. D. O'Brien (ed.), Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. ASM Press, Washington, D.C.
- 17.Karpman, D., H. Connell, M. Svensson, F. Scheutz, P. Alm, and C. Svandborg. 1997. The role of lipopolysaccharide and Shiga-like toxin in a mouse model of Escherichia coli O157:H7 infection. J. Infect. Dis. 175:611-620. [DOI] [PubMed] [Google Scholar]
- 18.Knutton, S. 1994. Attaching and effacing Escherichia coli, p. 567-591. In C. L. Gyles (ed.), Escherichia coli in domestic animals and humans. CAB International, Wallingford, United Kingdom.
- 19.McKee, M. L., A. R. Melton-Celsa, R. A. Moxley, D. H. Francis, and A. D. O'Brien. 1995. Enterohemorrhagic Escherichia coli O157:H7 requires intimin to colonize the gnotobiotic pig intestine and to adhere to HEp-2 cells. Infect. Immun. 63:3739-3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKee, M. L., and A. D. O'Brien. 1996. Truncated enterohemorrhagic Escherichia coli (EHEC) O157:H7 intimin (EaeA) fusion proteins promote adherence of EHEC strains to HEp-2 cells. Infect. Immun. 64:2225-2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moon, H. W., L. J. Hoffman, N. A. Cornick, S. L. Booher, and B. T. Bosworth. 1999. Prevalences of some virulence genes among Escherichia coli isolates from swine presented to a diagnostic laboratory in Iowa. J. Vet. Diagn. Investig. 11:557-560. [DOI] [PubMed] [Google Scholar]
- 22.Neef, N. A., S. Mc Orist, R. J. Lysons, A. P. Bland, and B. G. Miller. 1994. Development of large intestinal attaching and effacing lesions in pigs in association with the feeding of a particular diet. Infect. Immun. 62:4325-4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rios, M., V. Prado, M. Trucksis, C. Arellano, C. Borie, M. Alexandre, A. Fica, and M. M. Levine. 1999. Clonal diversity of Chilean isolates of enterohemorrhagic Escherichia coli from patients with hemolytic-uremic syndrome, asymptomatic subjects, animal reservoirs, and food products. J. Clin. Microbiol. 37:778-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Synge, B., and G. Paiba. 2000. Verocytotoxin-producing E. coli O157. Vet. Rec. 147:27.. [PubMed] [Google Scholar]
- 25.Tarr, P. I., M. A. Neill, C. R. Clausen, J. W. Newland, R. J. Neill, and S. L. Moseley. 1989. Genotypic variation in pathogenic Escherichia coli O157:H7 isolated from patients in Washington, 1984-1987. J. Infect. Dis. 159:344-347. [DOI] [PubMed] [Google Scholar]
- 26.Zhu, C., J. Harel, M. Jacques, C. Desautels, M. S. Donnenberg, M. Beaudry, and J. M. Fairbrother. 1994. Virulence properties and attaching-effacing activity of Escherichia coli O45 from swine postweaning diarrhea. Infect. Immun. 62:4153-4159. [DOI] [PMC free article] [PubMed] [Google Scholar]