Abstract
We compared the magnitude and duration of fecal shedding of wild-type Escherichia coli O157:H7 to that of an isogenic intimin mutant in young adult cattle and sheep. In both ruminant species, wild-type E. coli O157:H7 was shed in greater numbers and for a longer duration than was the intimin mutant.
Cattle are a major reservoir of Escherichia coli O157:H7, and estimates of the prevalence in the United States cattle population range from 2 to 28% (9-11, 13, 21, 22). E. coli O157:H7 can persist for more than 2 months in clinically normal adult cattle and sheep (3-5, 14, 16). It is not known if the organism actually colonizes the alimentary tracts of adult ruminants or if persistence is the result of continuous reintroduction from the environment (17). The bacterial outer membrane protein intimin mediates intimate attachment of E. coli O157:H7 to intestinal epithelial cells in vitro and is thought to facilitate intestinal colonization by the bacterium in humans (19, 20). Although attaching and effacing (AE) lesions are extensive in the intestines of neonatal calves with clinical E. coli O157:H7 infections (6), they either do not occur during the carrier state in adult cattle or affect such small areas of the intestine as to be below the level of detection (3, 5). However, AE lesions are occasionally detected in older calves with asymptomatic E. coli O157:H7 infections (7). The latter observation, plus the ubiquity of intimin among E. coli O157:H7 strains, suggests that intimin provides some ecological advantage to the organism in its natural environment, which would putatively include the adult ruminant gastrointestinal tract. We hypothesized that intimin facilitates colonization by E. coli O157:H7 in mature ruminants.
A preliminary report of this work was presented at the Verocytotoxigenic E. coli in Europe Pathogenicity and Virulence meeting, Liege, Belgium, 1999.
The strains of E. coli O157:H7 used in this study are listed in Table 1. Strain 86-24 Δeae10 contains an in-frame deletion in the eae gene for intimin, and strain 86-24Δeae10(pEB310) is the deletion mutant complemented with eae on a plasmid (18). Spontaneous mutants resistant to nalidixic acid and streptomycin were selected from the wild-type strain 86-24 and the intimin mutant.
TABLE 1.
Strains of E. coli O157:H7 used in this study
Trial 1.
Sheep (6 to 12 months old) were obtained from the Iowa State University herd, housed 2/room in BL-2 isolation facilities, and acclimated to a maintenance diet of concentrate and alfalfa and grass hay for at least 2 weeks as previously described (4). Groups of 8 sheep were given 1010 CFU of either wild-type E. coli O157:H7, the intimin mutant, or the complemented intimin mutant. Individual fecal samples were collected from each sheep on 2, 3, 4, 14, 15, 16, 58, 59, and 60 days postinoculation (p.i.). Samples (5 g) were immediately processed in a Stomacher, serially diluted in phosphate-buffered saline, and plated in triplicate onto MacConkey agar with antibiotics (Table 1). The sensitivity of the direct plating was ≥50 CFU/g. A 10-g fecal sample was also added to enrichment broth (Tryptic soy broth with 0.15% bile salts), incubated overnight, and plated onto selective medium (4, 5). Colonies isolated on selective medium were confirmed as E. coli O157 with a latex agglutination kit. Bacterial counts (CFU/gram) were converted to log10 and averaged over days 2, 3, 4 (initial period); 14, 15, 16 (2 weeks); 28, 29, 30 (1 month, trial 2 only); and 58, 59, 60 (2 months). The differences in the magnitude of fecal shedding (CFU/gram) between strains inoculated individually into sheep were compared with repeated measures analysis of variance (ANOVA).
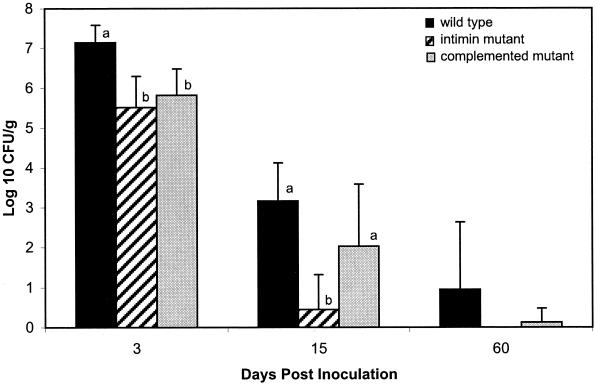
The average magnitude of fecal shedding of E. coli O157:H7 by sheep inoculated with either the wild type, the intimin mutant, or the complemented mutant is shown in Fig 1. All of the sheep shed the organism during the initial period (2 to 4 days p.i.). The magnitude of shedding by sheep inoculated with the wild-type strain was significantly greater (P < 0.001) than that of the sheep inoculated with either the intimin mutant or the complemented mutant. The wild type was recovered from 8 out of 8 sheep, the mutant from 2 out of 7 sheep, and the complemented mutant from 6 out of 8 sheep at 2 weeks p.i. At this time, the magnitude of shedding was significantly greater by sheep inoculated with either the wild-type strain (P < 0.001) or the complemented mutant (P < 0.05) compared to that by sheep inoculated with the intimin mutant (Fig. 1). At 2 months p.i. the intimin mutant was not recovered from any sheep. In contrast, the wild-type strain was isolated from 3 out of 8 sheep, and the complemented mutant was recovered from 1 out of 8 sheep.
FIG. 1.
Average magnitude of fecal shedding of E. coli O157:H7 by sheep inoculated with one of three isogenic strains of the organism (n = 8 sheep/group). A bar is one standard deviation. Bars marked with a or b are significantly different from one another (P < 0.05).
Trial 2.
To further define the role of intimin and to compare the magnitude and duration of fecal shedding by sheep with that of cattle, experiments involving dual inoculation with the wild-type strain and the intimin mutant were conducted. Yearling cattle (n = 8) were obtained from the Iowa State University beef herd, housed 1/pen in BL-2 isolation facilities, and acclimated to a maintenance diet of grain and alfalfa and grass hay for at least 2 weeks. Sheep (n = 8) were obtained and acclimated as described for trial 1. Each animal was orally inoculated with both the wild-type parent E. coli O157:H7 and the intimin mutant at a dose of 1010 CFU/strain/animal. Fecal samples were collected and plated as described for trial 1. Fecal samples cultured in enrichment broth overnight were also concentrated with anti-O157 immunomagnetic beads and were plated onto selective media. Differences in the magnitude of shedding between strains were evaluated with the paired t test. Differences in shedding between cattle and sheep were measured with repeated measures ANOVA.
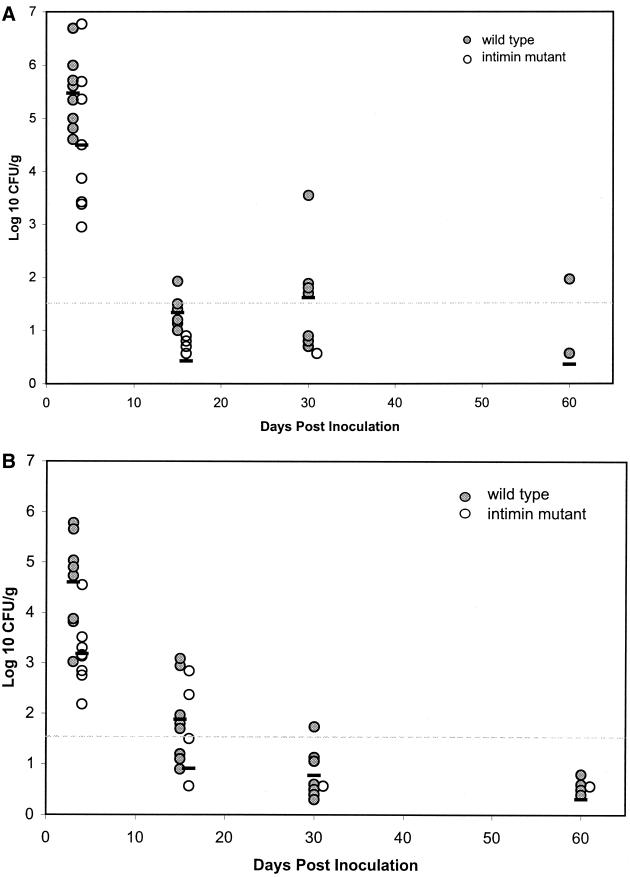
The magnitude and duration of shedding of the dually inoculated animals is shown in Fig. 2. All of the animals shed both strains during the initial period p.i. (days 2 to 4). The mean fecal count of E. coli O157:H7 detected in sheep was 3.2 × 105 CFU/g for the wild-type strain and 3.2 × 104 CFU/g for the intimin mutant. The mean shedding of the wild-type strain by cattle during the initial p.i. period was 4.0 × 104 CFU/g and was 1.6 × 103 CFU/g for the intimin mutant. The difference in shedding between the wild-type strain and the intimin mutant during the initial period was significant for both sheep (P < 0.05) and cattle (P < 0.01).
FIG. 2.
Average magnitude of fecal shedding of E. coli O157:H7 by sheep (A) and cattle (B) inoculated with two isogenic strains of the organism (n = 8 animals/group). Circles are values for individual animals, and a bar is the mean for each strain. Values below the dotted line were positive by enrichment culture only.
The differences in shedding between the wild-type strain and the intimin mutant were again significant in both sheep and cattle (P < 0.01) at 2 weeks p.i. and at 1 month (P < 0.05) p.i. At 2 months p.i. 2 out of 7 sheep and 4 out of 8 cattle continued to shed the wild-type strain. The intimin mutant was recovered from none of the sheep and only one of the cattle. There were no consistent significant differences between cattle and sheep in the magnitude or duration of shedding of either strain.
The differences in both the magnitude and duration of colonization between the intimin mutant and the wild-type strain (Fig. 1 and 2) indicate that intimin is active in the alimentary tract of adult ruminants and facilitates colonization by E. coli O157:H7 in this setting. The partial restoration of persistence at 2 weeks p.i. by the complemented mutant carrying eae on a plasmid (Fig. 1) provides additional evidence for the role of intimin in the persistence of the organism in mature ruminants. Unless intimin is acting by some unknown mechanism (for which we have no evidence), it seems likely that AE lesions probably occur in the intestinal tracts of asymptomatic cattle and sheep carrying E. coli O157:H7. AE lesions are formed on bovine mucosal explants of Peyer's patch (20), colonic and rectal tissue (1) collected from mature cattle inoculated with E. coli O157:H7. The lack of reports of detectable AE lesions in mature ruminants combined with the data presented here suggests that some such lesions probably occur but are difficult to detect, since histological methods require approximately 106 CFU/cm of tissue in order for patches of colonized bacteria to be detected by microscopy (2, 7). The data presented here provides rationale in support of intimin as a potential antigen in vaccines or in alternative anti-intimin strategies to reduce the colonization of ruminants by E. coli O157:H7 (8, 15). These data also provide evidence that experimentally infected adult sheep are an appropriate model for E. coli O157:H7 colonization of adult cattle.
Acknowledgments
We thank Pedro Navarro, Megan Black, Amy Helgerson, and Carisa Ralph for technical assistance and Ilze Matise, Dianna Jordan, and Nathan Eslick for help with the animal work. We also thank Richard Evans for statistical advise and Alison O'Brien for bacterial strains.
This work was supported in part by the U.S. Department of Agriculture National Research Initiative Competitive Grants Program 00-02515, National Institutes of Health grant AI-41328, and the Frank K. Ramsey endowment.
Editor: J. D. Clements
REFERENCES
- 1.Baehler, A. A., and R. A. Moxley. 2000. Escherichia coli O157:H7 induces attaching-effacing lesions in large intestinal mucosal explants from adult cattle. FEMS Microbiol. Lett. 185:239-242. [DOI] [PubMed] [Google Scholar]
- 2.Bertschinger, H. U., H. W. Moon, and S. C. Whipp. 1972. Association of Escherichia coli with the small intestinal epithelium. Infect. Immun. 5:595-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown, C. A., B. G. Harmon, T. Zhao, and M. P. Doyle. 1997. Experimental Escherichia coli O157:H7 carriage in calves. Appl. Environ. Microbiol. 63:27-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cornick, N. A., S. L. Booher, T. A. Casey, and H. W. Moon. 2000. Persistent colonization of sheep by E. coli O157:H7 and other pathotypes of E. coli. Appl. Environ. Microbiol. 66:4926-4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cray, W. C. J., and H. W. Moon. 1995. Experimental infection of calves and adult cattle with Escherichia coli O157:H7. Appl. Environ. Microbiol. 61:1586-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dean-Nystrom, E. A., B. T. Bosworth, W. C. J. Cray, and H. W. Moon. 1997. Pathogenicity of Escherichia coli O157:H7 in the intestines of neonatal calves. Infect. Immun. 65:1842-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dean-Nystrom, E. A., B. T. Bosworth, H. W. Moon, and A. D. O'Brien. 1998. Bovine infection with Shiga toxin-producing Escherichia coli, p. 261-267. In J. B. Kaper and A. D. O'Brien (ed.), Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. American Society for Microbiology, Washington, D.C.
- 8.Dean-Nystrom, E. A., J. F. L. Pohlenz, H. W. Moon, and A. D. O'Brien. 2000. Escherichia coli O157:H7 causes more severe systemic disease in suckling piglets than in colostrum-deprived neonatal piglets. Infect. Immun. 68:2356-2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elder, R. O., J. E. Keen, G. R. Siragusa, G. A. Barkocy-Gallagher, M. Koohmaraie, and W. W. Laegreid. 2000. Correlation of enterohemorrhagic Escherichia coli O157 prevalence in feces, hides, and carcasses of beef cattle during processing. Proc. Natl. Acad. Sci. USA 97:2999-3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faith, N. G., J. A. Shere, R. Brosch, K. W. Arnold, S. E. Ansay, M. S. Lee, J. B. Luchansky, and C. W. Kaspar. 1996. Prevalence and clonal nature of Escherichia coli O157:H7 on dairy farms in Wisconsin. Appl. Environ. Microbiol. 62:1519-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garber, L. P., S. J. Wells, D. D. Hancock, M. P. Doyle, J. Tuttle, J. A. Shere, and T. Zhao. 1995. Risk factors for fecal shedding of Escherichia coli O157:H7 in dairy calves. J. Am. Vet. Med. Assoc. 207:46-49. [PubMed] [Google Scholar]
- 12.Griffin, P. M., S. M. Ostroff, R. V. Tauxe, K. D. Greene, J. G. Wells, J. H. Lewis, and P. A. Blake. 1988. Illnesses associated with Escherichia coli O157:H7 infections. Ann. Intern. Med. 109:705-712. [DOI] [PubMed] [Google Scholar]
- 13.Hancock, D. D., T. E. Besser, M. L. Kinsel, P. I. Tarr, D. H. Rice, and M. G. Paros. 1994. The prevalence of Escherichia coli O157:H7 in dairy and beef cattle in Washington state. Epidemiol. Infect. 113:199-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hovde, C. J., P. R. Austin, K. A. Cloud, C. J. Williams, and C. W. Hunt. 1999. Effect of cattle diet on Escherichia coli O157:H7 acid resistance. Appl. Environ. Microbiol. 65:3233-3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaper, J. B., L. J. Gansheroff, M. R. Wachtel, and A. D. O'Brien. 1998. Intimin-mediated adherence of Shiga toxin-producing Escherichia coli and attaching-and-effacing pathogens, p. 148-156. In J. B. Kaper and A. D. O'Brien (ed.), Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. American Society for Microbiology, Washington, D.C.
- 16.Kudva, I. T., P. G. Hatfield, and C. J. Hovde. 1995. Effect of diet on the shedding of Escherichia coli O157:H7 in a sheep model. Appl. Environ. Microbiol. 61:1363-1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LeJeune, J. T., T. E. Besser, and D. D. Hancock. 2001. Cattle water troughs as reservoirs of Escherichia coli O157. Appl. Environ. Microbiol. 67:3053-3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKee, M. L., A. R. Melton-Celsa, R. A. Moxley, D. H. Francis, and A. D. O'Brien. 1995. Enterohemorrhagic Escherichia coli O157:H7 requires intimin to colonize the gnotobiotic pig intestine and to adhere to HEp-2 cells. Infect. Immun. 63:3739-3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phillips, A. D., S. Navabpour, S. Hicks, G. Dougan, T. Wallis, and G. Frankel. 2000. Enterohemorrhagic Escherichia coli O157:H7 target Peyer's patches in humans and cause attaching/effacing lesions in both human and bovine intestine. Gut 47:377-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rahn, K., S. A. Renwick, R. P. Johnson, J. B. Wilson, R. C. Clarke, D. Alves, S. McEwen, H. Lior, and J. Spika. 1997. Persistence of Escherichia coli O157:H7 in dairy cattle and the dairy farm environment. Epidemiol. Infect. 119:251-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao, T., M. P. Doyle, J. Shere, and L. Garber. 1995. Prevalence of enterohemorrhagic Escherichia coli O157:H7 in a survey of dairy herds. Appl. Environ. Microbiol. 61:1290-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]