Abstract
Enterohemorrhagic Escherichia coli (EHEC) infections are associated with hemorrhagic colitis and the hemolytic-uremic syndrome (HUS). In vivo, elevated plasma levels of the proinflammatory cytokine interleukin-8 (IL-8) in EHEC-infected children are correlated with a high risk of developing HUS. As IL-8 gene transcription is regulated by the transcription factors NF-κB and AP-1, we analyzed the role of these factors in the regulation of IL-8 production after infection of the epithelial intestinal T84 cell line by EHEC. By 6 h of infection, EHEC had induced significant secretion of IL-8 (35.84 ± 6.76 ng/ml versus 0.44 ± 0.04 ng/ml in control cells). EHEC induced AP-1 and NF-κB activation by 3 h of infection. Moreover, the three mitogen-activated protein kinases (MAPK) (ERK1/2, p38, and JNK) were phosphorylated in EHEC-infected T84 cells concomitant with induction of AP-1 DNA binding activity, and IκB-α was phosphorylated and then degraded concomitant with induction of NF-κB DNA binding activity. Pretreatment of cells with the highly specific MEK1/2 inhibitor U0126, the p38 inhibitor SB203580, and/or the proteasome inhibitor ALLN led to inhibition of the IL-8 secretion induced in EHEC-infected T84 cells. These findings demonstrate that (i) EHEC can induce in vitro a potent proinflammatory response by secretion of IL-8 and (ii) the secretion of IL-8 is due to the involvement of MAPK, AP-1, and NF-κB signaling pathways.
Enterohemorrhagic Escherichia coli (EHEC) is a pathogenic bacterium that causes acute gastroenteritis and hemorrhagic colitis which may lead to severe complications, including the hemolytic-uremic syndrome (HUS) (24). The pathogenic mechanisms of diarrheal disease in response to EHEC remain to be elucidated. Upon bacterial attachment, a dedicated protein secretion system termed the type III system is activated in EHEC. This protein secretion system directs the secretion and subsequent translocation into the host cell of a number of proteins that have the capacity to elicit host cell signaling pathways, leading to a variety of responses (15, 19, 20, 25). EHEC is known to produce verotoxins (VT) 1 and 2 that bind globotriaosylceramide (Gb3) on the surface of cells and, once internalized, inhibit protein synthesis, ultimately causing cell death (29). The sensitivity of kidneys to the cytotoxic effects of VT is proportional to the Gb3 content of the different renal cell types (2, 28, 48), but the human intestine has not been found to express Gb3 (1). The human colonic epithelial cell line T84, which does not express detectable amounts of Gb3, is an appropriate model for studying EHEC-induced changes in enterocyte function (35). In vitro assays have shown that toxin-positive or -negative strains of EHEC eliminate the barrier function of T84 monolayers, while purified VT do not alter transepithelial resistance (35). Thus, these toxins do not appear to play a role in the diarrheal illness induced by EHEC. Moreover, in vivo, VT-negative strains of EHEC still cause diarrhea (27, 46, 49).
A recent study correlated inflammatory serum parameters with a high risk of developing typical HUS during the prodromal phase of diarrhea caused by EHEC; low neopterin and interleukin-10 (IL-10) levels and high IL-8 levels are indicators of a high risk for developing HUS in EHEC-infected children (51). In particular, IL-8 appears to be one of the major products secreted by infected epithelial cells (12). This proinflammatory cytokine is a potent chemoattractant for polymorphonuclear cells; it can recruit these cells into the infected site and promote their infiltration of the epithelial layer infected by invasive or noninvasive bacteria (30, 38).
IL-8 gene expression is regulated by several pathways. The IL-8 gene promoter region contains binding sequences for various transcription factors, including NF-IL-6, NF-κB, and AP-1 (32). Elewaut et al. (13) found that NF-κB is a central regulator of the epithelial cell innate immune response to infection with enteroinvasive bacteria. In most cell types, NF-κB is inactive in cytoplasm through its binding to an inhibitory protein, called IκB, that masks the nuclear localization signal on NF-κB and thus prevents its nuclear translocation. The translocation of NF-κB requires phosphorylation of IκB-α; once phosphorylated, IκB-α is ubiquitinilated and then degraded by the 26S subunit of the proteasome (3, 22, 44). AP-1 activation is dependent on mitogen-activated protein kinases (MAPK) that are central in many host responses, including the regulation of cytokine responses, stress responses, and cytoskeletal reorganization (8, 9). The MAPK form a group of three pathways, including extracellular signal-regulated protein kinases (ERK1/2) and two stress-activated protein kinases designated p38 (also known as the hyperosmolarity glycerol kinase) and JNK (c-jun N-terminal kinase). Most eukaryotic surface receptors use at least one of these highly conserved MAPK cascades for signaling inside the cell (37).
Recently, we demonstrated the role of MAPK activation in the host cell response to enteropathogenic E. coli (EPEC) infection. Activation of ERK1/2 is required for EPEC internalization in T84 cells (6), while activation of ERK1/2 and p38 is required for EPEC-induced IL-8 production (7).
The aim of this study was to investigate the molecular mechanisms implicated in the stimulation of IL-8 production by intestinal epithelial cells in response to EHEC infection.
The data presented in this paper show that strain EDL 931, a VT-producing EHEC strain, induces IL-8 secretion in T84 cells. EHEC infection activates the MAPK pathway as well as the transcription factors NF-κB and AP-1. Preincubation of T84 cells with the MEK1/2 MAPK kinase (MAPKK) inhibitor U0126 (11), the p38 MAPK inhibitor SB203580 (5), or the NF-κB inhibitor ALLN (31) significantly lowers EHEC-induced IL-8 production.
MATERIALS AND METHODS
Cell lines, media, and bacterial strains.
The T84 human colonic cell line was obtained from the European Collection of Animal Cell Cultures (Salisbury, England). The T84 culture medium contained a 1:1 mixture of Dulbecco-Vogt modified Eagle medium and Ham's F12 medium (DMEM/F12) supplemented with 50 μg of penicillin per ml, 50 μg of streptomycin (Sigma, Saint Quentin Fallavier, France) per ml, and 4% fetal bovine serum (HyClone, Bezons, France). The wild-type EHEC strain EDL 931, which produces Stx1/2 (21) and was used in this study, was kindly provided by Institut Pasteur (Paris, France). Bacteria were stored in Luria-Bertani medium containing 15% glycerol at −80°C and were grown in Luria broth overnight at 37°C without shaking.
Inhibitors.
The MEK1/2 inhibitor (U0126) (11), the p38 inhibitor (SB203580) (5), and the calpain inhibitor I (ALLN) (31) (Calbiochem, Meudon, France) were stored in dimethyl sulfoxide (DMSO) at −20°C. Cells were preincubated for 90 min with U0126 (10 μM), SB203580 (10 μM), and ALLN (100 μM), alone or in combination. The inhibitors were maintained during the infection period.
Infection procedure.
Bacteria, grown overnight in Luria broth medium, were pelleted by centrifugation, resuspended in sterile phosphate-buffered saline (PBS), and added to T84 cells (100 bacteria/cell).
IL-8 assay.
IL-8 assays were performed at 70 to 90% confluence of T84 monolayers grown in 60-mm petri dishes. Prior to infection, cells were washed and incubated for at least 2 h in serum- and antibiotic-free DMEM/F12. Cells were infected with EHEC for 6 h in 2.5 ml of this medium. The culture supernatants were then centrifuged for 10 min at 10,000 × g to pellet the residual bacteria. The IL-8 concentration was determined by the Quantikine human IL-8 immunoassay (R&D System, Abington, United Kingdom). Where indicated, the inhibitors U0126 (10 μM), SB203580 (10 μM), and ALLN (100 μM) were added 90 min prior to infection and maintained throughout the infection period. In the assay performed with inhibitors, an EHEC control was pretreated with DMSO, and DMSO was maintained during the infection.
Electrophoretic mobility shift assay (EMSA).
T84 cells were seeded into six-well plates. At 70 to 90% confluence, the cells were washed and infected with EHEC in serum- and antibiotic-free DMEM/F12. At the times indicated below, the infected cells were washed with PBS. AP-1 and NF-κB DNA binding activities were analyzed in total cleared cellular extracts prepared in totex buffer (20 mM HEPES [pH 7.9], 350 mM NaCl, 20% glycerol, 1% NP-40, 1 mM MgCl2, 0.5 mM EDTA, 0.1 mM EGTA, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, 2 μg of aprotinin per ml). Samples (10 μg) were incubated for 25 min at 25°C with radiolabeled double-stranded oligonucleotide containing the AP-1 site (5′-TTCGTGACTCAGCGG-3′) or the κB site (5′-GATCCAAGGGGACTTTCCATG-3′). The specificity of the complexes was analyzed by incubation with an excess of unlabeled AP-1 or κB oligonucleotides. Complexes were separated by electrophoresis on a 6% nondenaturing polyacrylamide gel in 0.5× Tris-borate-EDTA buffer. The dried gels were autoradiographed (Amersham Hyperfilms).
Western blotting.
T84 cells were seeded into 100-mm petri tissue culture dishes. At 70 to 90% confluence, cells were depleted overnight in serum- and antibiotic-free DMEM/F12 supplemented with 0.1% bovine serum albumin (Sigma). Infections were carried out in this medium. At the times indicated below, the infected cells were washed with PBS and scraped at 4°C into lysis buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1% NP-40, 2 mM Na3VO4, 1 mM EDTA, 1 μM aprotinin, 25 μM leupeptin, 1 μM pepstatin, 1 mM 4-(2-aminoethyl)-benzene sulfonyl fluoride, 10 mM NaF, 5 mM NaPPi, 10 mM β-glycerophosphate). The lysate was sonicated and solubilized for 30 min at 4°C, and then it was centrifuged at 14,000 × g for 20 min at 4°C. The protein concentration of the supernatant was determined by using Bio-Rad DC reagents.
Equal amounts (50 μg) of whole-cell lysates were subjected to sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis (SDS-PAGE). The proteins were transferred onto a polyvinylidene fluoride membrane (Hybond-P; Amersham, Orsay, France) and incubated overnight at 4°C with anti-phospho-ERK1/2, anti-phospho-p38, anti-phospho-JNK, anti-phospho-IκB-α, and anti-IκB-α rabbit antibodies (New England Biolabs) or with anti-ERK2, anti-p38, anti-JNK (Santa Cruz Biotechnology, Santa Cruz, Calif.), and horseradish peroxidase-conjugated anti-rabbit antibodies (New England Biolabs). The presence of antibodies was revealed with the enhanced chemiluminescence detection system (ECL; Amersham).
Statistical analysis.
Results are presented below as means ± standard errors of the means. Statistical significance was determined by analysis of variance with the StatView program for MacIntosh, followed by post hoc comparison with Bonferroni-Dunn tests.
Densitometric analysis.
The changes in signal or band intensity were quantified by densitometric analysis by using the NIH Image program for MacIntosh. All experiments were repeated at least four times, and a representative result is shown for each experiment.
RESULTS
EHEC infection increases IL-8 release by T84-infected cells.
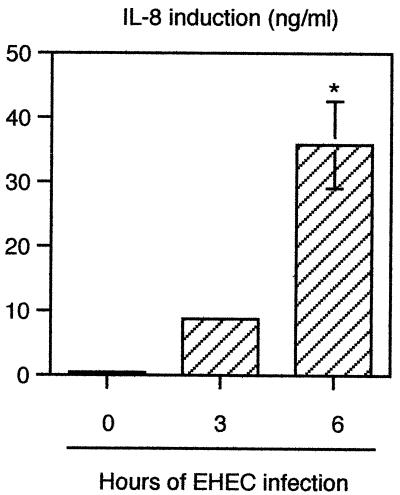
A previous study, performed in vivo, demonstrated that EHEC infection induces an inflammatory response by secretion of IL-8 (51). This observation prompted us to establish a relationship between the physiopathology of EHEC infection in vivo and the physiopathology of EHEC infection in vitro. For this purpose, T84 cells were infected for various times (3 or 6 h), and the IL-8 concentrations were determined in culture supernatants by an enzyme-linked immunosorbent assay (ELISA). Kinetic studies showed that low levels of IL-8 (<20 ng/ml) were detectable after 3 h of infection. By 6 h after infection, EHEC induced significant secretion of IL-8 (35.84 ± 6.76 ng/ml versus 0.44 ± 0.04 ng/ml in control cells) (Fig. 1).
FIG. 1.
EHEC infection increases IL-8 release by T84 infected cells. Il-8 content was estimated in the supernatant of T84 cells by ELISA after 3 and 6 h of infection with EHEC strain EDL 931. Errors bars indicate standard deviations. The asterisk indicates that the value is significantly different from the value for uninfected control cells (n = 5, P < 0.01), as determined by the Bonferroni-Dunn tests.
In agreement with the observation made in vivo by Westerholt et al. (51), these results demonstrated that in vitro, EHEC infection can lead to production of IL-8 proinflammatory cytokines.
EHEC activates AP-1 and NF-κB in T84 cells.
The data described above suggest that EHEC infection of intestinal cells regulates expression of the IL-8 gene. The promoter region of the human IL-8 gene carries putative NF-κB and AP-1 binding sites. As these transcription factors are prime regulatory elements for IL-8 gene expression (32), we performed EMSA to investigate the ability of EHEC to activate AP-1 and/or NF-κB in T84 cells. As shown in Fig. 2A, uninfected cells displayed only weak AP-1 DNA binding activity. Infection with EHEC induced AP-1 after 3 h. The densitometric analysis showed that EHEC infection led to activation of AP-1 equivalent to that observed after phorbol ester (phorbol myristate acetate) stimulation of T84 cells for 30 min. To confirm the specificity of this signal, competition studies were performed with increasing amounts of unlabeled probe. As shown in Fig, 2A, AP-1 binding of the 32P-labeled oligonucleotide probe was inhibited by a 200-fold excess of unlabeled probe.
FIG. 2.
EHEC infection induces AP-1 and NF-κB DNA binding activity in T84 cells. (A) Kinetic study of AP-1 activation by EHEC infection. The control lane contained uninfected T84 cells. Phorbol myristate acetate (PMA) (20 ng/ml) was used as the positive control. AP-1 DNA binding activity was examined by EMSA using a 32P-labeled probe corresponding to the AP-1 site. The specificity of the complex was analyzed by incubation with an excess of unlabeled AP-1 oligonucleotide. (B) Kinetic study of NF-κB complex activation by EHEC infection. The control lane contained uninfected T84 cells. TNF-α (10 ng/ml) was used as the positive control. NF-κB DNA binding activity was examined by EMSA using a 32P-labeled probe corresponding to the κB site. The specificity of the complex was analyzed by incubation with an excess of unlabeled NF-κB oligonucleotide. The changes in signal intensity were quantified by densitometric analysis.
As shown in Fig. 2B, NF-κB activation was studied in similar conditions. While no NF-κB activation was detectable in nontreated cells, infection with EHEC led to gradual stimulation of NF-κB DNA binding activity that was strong after 1 h and maximal after 3 h. The effect of EHEC infection on NF-κB activation was just as great as the effect after tumor necrosis factor alpha (TNF-α) stimulation for 1 h (as shown by densitometric analysis). To confirm the specificity of this signal, competition studies were performed with increasing amounts of unlabeled probe. As shown in Fig. 2B, NF-κB binding of the 32P-labeled oligonucleotide probe was blocked by a 50-fold excess of unlabeled probe.
Figure 2 shows that the AP-1 and NF-κB transcription factors are activated concomitantly 3 h following EHEC infection of T84 cells.
EHEC induces activation of MAPK (ERK1/2, p38, and JNK).
AP-1 activity can occur through transcriptional and posttranscriptional mechanisms (23). Regulation of AP-1 activity depends on phosphorylations by members of the MAPK family, including ERK1/2, p38, and JNK (9, 52). To gain insight into the signaling mechanisms induced by EHEC that lead to AP-1 activation, we examined activation of the various MAPK modules after infection of T84 cells. The kinetics of MAPK activation after infection were assessed by Western blotting using antibodies that specifically recognize the phosphorylated forms of ERK1/2 (Fig. 3A), p38 (Fig. 3B), and JNK (Fig. 3C). Blots were probed with antibodies recognizing the nonphosphorylated form of MAPK to rule out a difference between the levels of whole-cell lysates and the amounts of a specific protein during the infection procedure. The active forms of ERK1/2 (p42 and p44), p38, and JNK (p46 and p54) were nearly undetectable in control cells. Activation of p38 was detectable after 1 h of EHEC infection and increased further after 3 h. ERK1/2 activation and JNK activation were not detectable until after 3 h of EHEC infection. As a control, epidermal growth factor stimulation of T84 cells for 15 min led to activation of all three MAPK.
FIG. 3.
Activation of MAPK in EHEC-infected T84 cells. T84 cells were lysed at different times after infection. Samples were resolved by SDS-PAGE and analyzed by immunoblotting using anti-phospho-ERK1/2 and anti-ERK2 antibodies (A), anti-phospho-p38 and anti-p38 antibodies (B), and anti-phospho-JNK and anti-JNK antibodies (C). The control lane contained uninfected T84 cells. Epidermal growth factor (EGF) (10 nM) was used as the positive control. The increase in MAPK activity was quantified after densitometric analysis.
These results show that activation of all three MAPK pathways occurred at the same time as AP-1 activation.
EHEC induces phosphorylation and degradation of IκB-α.
In most cell types, NF-κB remains latent in the cytoplasm owing to its binding to inhibitory proteins, called IκB, that mask the nuclear localization signal on NF-κB and thus prevent its nuclear translocation. Translocation of NF-κB requires phosphorylation on serine residues 32 and 36 of IκB-α and subsequent degradation of IκB-α by the 26S proteasome (3, 22, 44). Phosphorylation of IκB-α was analyzed by Western blotting with antibodies that recognize the phosphorylated form of IκB-α. As shown in Fig. 4A, phosphorylation of IκB-α was detectable after 3 h of infection. We next studied the degradation of IκB-α by Western blotting using antibodies against the whole form (Fig. 4B). By 1 h, EHEC infection had induced the disappearance of around 50% of the total IκB-α; degradation was quasi-complete after 3 h. As a control, 1 h of stimulation of T84 cells with TNF-α induced both phosphorylation and degradation of IκB-α.
FIG. 4.
Phosphorylation and degradation of IκB-α by EHEC infection of T84 cells. T84 cells were lysed at different times after infection. Samples were resolved by SDS-PAGE and analyzed by immunoblotting using an anti-phospho-IκB-α antibody (A) and an anti-IκB-α antibody (B). The control lane contained uninfected T84 cells. TNF-α (10 ng/ml) was used as the positive control. The increase in IκB-α phosphorylation and the percentage of IκB-α degradation were quantified by densitometric analysis.
These results show that IκB-α was phosphorylated and then degraded at the same time as NF-κB activation in EHEC-infected T84 cells.
Inhibition of MAPK and NF-κB pathways prevents EHEC-induced IL-8 production.
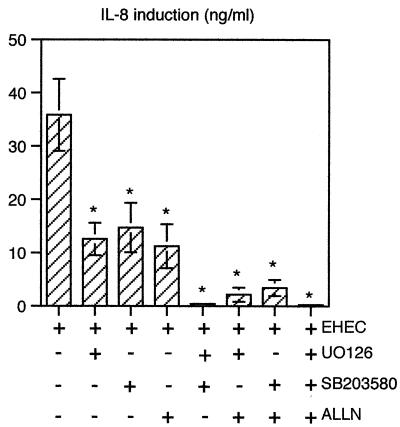
To test the direct implication of MAPK and NF-κB pathways in EHEC-induced production of IL-8, we used a pharmacological approach to block these pathways. For this purpose, T84 cells were pretreated before infection for 90 min with various inhibitors of the pathways; U0126 (10 μM) was used to specifically inhibit ERK1/2 activation by MEK1/2 (11), SB203580 (10 μM) was used to specifically inhibit p38 (5), and ALLN (100 μM) was used to specifically inhibit IκB-α degradation and, as a consequence, NF-κB activation (31). The inhibitors were maintained during the 6 h of EHEC infection. The control infected cells were pretreated with DMSO, and DMSO was maintained during infection. The IL-8 concentrations in culture supernatants were determined by ELISA. Figures 1 and 5 show that the presence of DMSO did not significantly modify IL-8 secretion in infected cells (P < 0.01). As shown in Fig. 5, each inhibitor alone significantly reduced (by 60 to 70%) IL-8 production. A combination of two or three inhibitors nearly completely inhibited EHEC-induced IL-8 secretion.
FIG. 5.
Inhibition of MAPK and/or NF-κB pathway activation prevents IL-8 secretion induced by EHEC infection of T84 cells. Il-8 content was estimated by ELISA in the supernatant of T84 cells after 6 h of infection with EHEC strain EDL 931. Where indicated, the inhibitors U0126 (10 μM), SB203580 (10 μM), and ALLN (100 μM) were added 90 min prior to infection and maintained throughout the infection period. To rule out an effect of the vehicle on IL-8 secretion, data for EHEC-induced IL-8 secretion (control lane) were obtained with DMSO. Error bars indicate standard deviations. An asterisk indicates that a value is significantly different from the value for EHEC-infected cells (n = 5, P < 0.01), as determined by the Bonferroni-Dunn tests.
These results show that the MAPK and NF-κB pathways are both required for production of IL-8 proinflammatory cytokines.
DISCUSSION
In this study, we showed that EHEC infection of T84 cells induces NF-κB activation. We also demonstrated that the inhibitory subunit of NF-κB, IκB-α, is phosphorylated and then degraded in EHEC-infected T84 cells. All of these cellular events follow the same time course (i.e., 3 h of EHEC infection). When ALLN was used to block IκB-α degradation (31), a 65% decrease in EHEC-induced IL-8 secretion was observed. These results demonstrate that activation of the NF-κB pathway in EHEC-infected T84 cells is involved in the regulation of IL-8 production.
We also observed increased AP-1 DNA binding activity after 3 h of EHEC infection. Our findings reveal that the three main groups of the MAPK family, ERK1/2, p38, and JNK, are phosphorylated at the same time in EHEC-infected T84 cells. Furthermore, use of the specific inhibitor of MEK1/2, U0136 (11), and/or the specific inhibitor of p38, SB203580 (5), prevented the IL-8 secretion induced by EHEC infection. These results demonstrate that MAPK regulate IL-8 production induced by EHEC in T84 cells.
Activation of MAPK has been reported mainly in response to infection by invasive bacteria, such as Salmonella enterica serovar Typhimurium or Listeria monocytogenes, in epithelial cells (18, 43) or in macrophages (40). In these enteroinvasive infections, activation of MAPK is due to the ability of the bacteria to invade the cellular target. Recently, we demonstrated the involvement of the MAPK pathway in response to infection by EPEC, an enteroadherent bacterium (6, 7). This allowed us to establish a direct relationship among MAPK activation, EPEC internalization, and EPEC-induced IL-8 secretion by T84 cells (6, 7). Moreover, activation of NF-κB in epithelial cells has been reported in response to infection by S. enterica serovar Typhimurium, Shigella flexneri, Helicobacter pylori, or EPEC (16, 36, 39, 41). A relationship between NF-κB activation and increased IL-8 production has been established in all of these bacterial infections but not in EHEC infections.
Various attempts have been made to identify the EHEC factor(s) that directs the cytokine response. Thorpe et al. (45) implicated VT as the mediator of IL-8 secretion by intestinal cells. In their study, these authors used intestinal cell line Hct-8, which expresses the VT receptor. To date, the only characterized VT receptor is Gb3. The T84 cell line has been reported to express undetectable levels of Gb3 (35), but we cannot rule out the possible participation of another, as-yet-unidentified VT receptor. However, in a previous study (7), we demonstrated that a VT-negative EPEC strain induces IL-8 secretion in T84 cells; these data suggest that even if VT participate in IL-8 secretion, other factors, such as bacterial attachment or secreted proteins, might be implicated in cytokine responses to infection by enteroadherent bacteria.
Lipopolysaccharide (LPS), a component of the cell walls of gram-negative bacteria, is a potential candidate for stimulating the cell responses. LPS has been shown to activate MAPK pathways in macrophages (50) and monocytes (47). This activation involves the macrophage receptor CD14, which is not expressed in epithelial cells. However, Cario et al. (4) reported that Toll-like receptors present on the surfaces of T84 intestinal epithelial cells appear to mediate LPS stimulation of ERK1/2 and JNK. As LPS failed to stimulate the p38 pathway in T84 cells that is activated by EHEC infection, bacterial factors other than LPS may be implicated in this cellular response. This is in agreement with our previous observations (7) made with EPEC mutants. These strains, which had mutations in the bacterial adhesion or type III secretion system but were not affected in LPS expression, failed to stimulate MAPK.
Cario et al. (4) also reported that LPS activates NF-κB, probably through Toll-like receptors. However, LPS-induced activation of the NF-κB factor in intestinal cells depends on the presence of serum. Since our experiments were conducted in serum-free media, we speculate that LPS does not participate in the NF-κB signaling responses to EHEC infection described in this report. Our experimental procedures were in accordance with those of Savkovic et al. (39), who did not observe any NF-κB activation by LPS-stimulated T84 cells in serum-free media. It thus appears that bacterial factors other than LPS are necessary to trigger host cell responses. It remains to be determined if the same factor(s) mobilizes both AP-1 and NF-κB pathways.
Even if LPS is a key activator of subepithelial macrophages, a protein called flagellin, a component of bacterial flagella, now appears to play a major role in triggering intestinal mucosal stimulation of an intestinal response, such as IL-8 secretion (14). Recently, Steiner et al. generated a novel paradigm of E. coli enteric pathogenesis (42). Flagellin has been implicated in the proinflammatory response of enteroaggregative E. coli-infected Caco-2 cells. More recently, Gewirtz et al. found that translocation of flagellin across epithelia, subsequent to an apical epithelium-S. enterica serovar Typhimurium interaction, was likely a major means of activating a mucosal inflammation response (17).
The type III protein secretion system has been involved in activation of the MAPK modules and induction of the nuclear responses in enteroinvasive S. enterica serovar Typhimurium infections (18) and enteradherent EPEC infections (7). This type III protein secretion system is encoded in the chromosome known as the locus of enterocyte effacement (LEE). A comparison of the LEE of EPEC and the LEE of EHEC shows that they are highly conserved, particularly the type III secretion system. This type III system allows the translocation of the intimin receptor (Tir) in EPEC and EHEC infections. Whereas in EPEC infections Tir is implicated in several host cell responses (Ca2+, inositol triphosphate), in EHEC infections Tir is involved only in structural cellular rearrangements (10, 14, 26, 33, 34). In order to understand the mechanism of EHEC infection, identification of secreted proteins required for initiating the signaling events in host cells is currently being investigated in our laboratory.
In this report, we show that EHEC infection of the colonocyte T84 cell line results in activation of the transcription factors AP-1 and NF-κB and subsequent production of the proinflammatory cytokine IL-8.
Acknowledgments
This study was supported by Laboratoires BIOCODEX, the Region Provence-Alpes Côte d'Azur, the Conseil General des Alpes Maritimes, the Faculte de Medecine de l'Universite de Nice-Sophia Antipolis, and the Centre Hospitalier Regional de Nice.
We thank Naofumi Mukaida for assistance with EMSA and Michel Warmy and J. Thomas LaMont for critical reading of the manuscript.
Editor: A. D. O'Brien
REFERENCES
- 1.Bjork, S., M. E. Breimer, G. C. Hansson, K. A. Karlsson, and H. Leffler. 1987. Structures of blood group glycosphingolipids of human small intestine. A relation between the expression of fucolipids of epithelial cells and the ABO, Le and Se phenotype of the donor. J. Biol. Chem. 262:6758-6765. [PubMed] [Google Scholar]
- 2.Boyd, B., and C. Lingwood. 1989. Verotoxin receptor glycolipid in human renal tissue. Nephron 51:207-210. [DOI] [PubMed] [Google Scholar]
- 3.Brown, K., L. Gerstberger, L. Carlson, G. Franzoso, and U. Siebenlist. 1995. Control of I-κB-α proteolysis by site specific, signal-induction phosphorylation. Science 267:1485-1488. [DOI] [PubMed] [Google Scholar]
- 4.Cario, E., I. M. Rosenberg, S. L. Brandwein, P. L. Beck, H.-C. Reinecker, and D. K. Podolsky. 2000. LPS activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J. Immunol. 164:966-972. [DOI] [PubMed] [Google Scholar]
- 5.Cuenda, A., J. Rouse, Y. N. Doza, R. Meier, P. Cohen, T. F. Gallagher, P. R. Young, and J. C. Lee. 1995. SB203580 is a specific inhibitor of MAP kinase homologue which is stimulated by cellular stress and IL-1. FEBS Lett. 364:229-233. [DOI] [PubMed] [Google Scholar]
- 6.Czerucka, D., S. Dahan, B. Mograbi, B. Rossi, and P. Rampal. 2000. Saccharomyces boulardii preserves barrier function and modulates the signal transduction pathway induced in enteropathogenic Escherichia coli-infected T84 cells. Infect. Immun. 68:5998-6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Czerucka, D., S. Dahan, B. Mograbi, B. Rossi, and P. Rampal. 2001. Implication of mitogen-activated protein kinases in T84 cell responses to enteropathogenic Escherichia coli infection. Infect. Immun. 69:1298-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis, R. J. 1993. The mitogen-activated protein kinase signal transduction pathway. J. Biol. Chem. 268:14553-14556. [PubMed] [Google Scholar]
- 9.Davis, R. J. 2000. Signal transduction by the JNK group of MAP kinases. Cell 103:239-252. [DOI] [PubMed] [Google Scholar]
- 10.Deibel, C., S. Kramer, T. Chakraborty, and F. Ebel. 1998. EspE, a novel secreted protein of attaching and effacing bacteria, is directly translocated into infected host cells, where it appears as a tyrosine-phosphorylated 90kDa protein. Mol. Microbiol. 28:463-474. [DOI] [PubMed] [Google Scholar]
- 11.Duncia, J. V., J. B. Santella 3rd, C. A. Higley, W. J. Pitts, J. Wityak, W. E. Frietze, F. W. Rankin, J. H. Sun, R. A. Earl, A. C. Tabaka, C. A. Teleha, K. F. Blom, M. F. Favata, E. J. Manos, A. J. Daulerio, D. A. Stradley, K. Horiuchi, R. A. Copeland, P. A. Scherle, J. M. Trzaskos, R. L. Magolda, G. L. Trainor, R. R. Wexler, F. W. Hobbs, and R. E. Olson. 1998. MEK inhibitors: the chemistry and biological activity of U0126, its analogs, and cyclisation products. Bioorg. Med. Chem. Lett. 8:2839-2844. [DOI] [PubMed] [Google Scholar]
- 12.Eckmann, L., M. F. Kagnoff, and J. Fierer. 1993. Epithelial cells secrete the chemokine interleukin-8 in response to bacterial entry. Infect. Immun. 61:4569-4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elewaut, D., J. A. Didonato, J. M. Kim, F. Truong, L. Eckmann, and M. F. Kagnoff. 1999. NF-kB is a central regulator of innate immune response induced by infection with enteroinvasive bacteria. J. Immunol. 163:1457-1466. [PubMed] [Google Scholar]
- 14.Fleckenstein, J. M., and D. J. Kopecko. 2001. Breaching the mucosal barrier by stealth: an emerging pathogenic mechanism for enteroadherent bacterial pathogens. J. Clin. Investig. 107:27-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frankel, G., A. D. Phillips, I. Rosenshine, G. Dougan, J. B. Kaper, and S. Knutton. 1998. Enteropathogenic and enterohaemorrhagic Escherichia coli: more subversive elements. Mol. Microbiol. 30:911-921. [DOI] [PubMed] [Google Scholar]
- 16.Gewirtz, A. T., A. S. Rao, P. O. Simon, Jr., D. Merlin, D. Carnes, J. L. Madara, and A. S. Neish. 2000. Salmonella typhimurium induces epithelial IL-8 expression via Ca2+-mediated activation of the NF-κB pathway. J. Clin. Investig. 105:79-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gewirtz, A. T., P. O. Simon, Jr., C. K. Schmitt, L. J. Taylor, C. H. Hagedorn, A. D. O'Brien, A. S. Neish, and J. L. Madara. 2001. Salmonella typhimurium translocates flagellin across intestinal epithelia, inducing a proinflammatory response. J. Clin. Investig. 107:99-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hobbie, S., L. M. Chen, R. J. Davis, and J. E. Galan. 1997. Involvement of mitogen-activated protein kinase pathways in the nuclear responses and cytokine production induced by Salmonella typhimurium in cultured intestinal epithelial cells. J. Immunol. 159:5550-5559. [PubMed] [Google Scholar]
- 19.Ismaili, A., D. J. Philpott, M. T. Dytoc, and P. M. Sherman. 1995. Signal transduction responses following adhesion of verocytotoxin-producing Escherichia coli. Infect. Immun. 63:3316-3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ismaili, A., E. McWhirter, M. Y. C. Handelsman, J. L. Brunton, and P. M. Sherman. 1998. Divergent signal transduction responses to infection with attaching and effacing Escherichia coli. Infect. Immun. 66:1688-1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Isogai, E., H. Isogai, K. Kimura, S. Hayashi, T. Kubota, N. Fujii, and K. Takeshi. 1998. Role of tumor necrosis factor alpha in gnotobiotic mice infected with an Escherichia coli O157:H7 strain. Infect. Immun. 66:197-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Israel, A. 2000. The IKK complex: an integrator of all signals that activate NF-κB? Trends Cell Biol. 10:129-133. [DOI] [PubMed] [Google Scholar]
- 23.Karin, M. 1995. The regulation of AP-1 activity by mitogen-activated protein kinases. J. Biol. Chem. 270:16483-16486. [DOI] [PubMed] [Google Scholar]
- 24.Karmali, M. A. 1989. Infection by verocytotoxin-producing Escherichia coli. Clin. Microbiol. Rev. 2:15-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knutton, S., T. Baldwin, P. H. Williams, and A. S. McNeish. 1989. Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic and enterohemorrhagic Escherichia coli. Infect. Immun. 57:1290-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kresse, A. U., M. Rohde, and C. A. Guzman. 1999. The EspD protein of enterohemorrhagic Escherichia coli is required for the formation of bacterial surface appendages and is incorporated in the cytoplasmic membranes of target cells. Infect. Immun. 67:4834-4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li, Z., C. Bell, A. Buret, R. Robins-Browne, D. Stiel, and E. O'Loughlin. 1993. The effect of enterohemorrhagic Escherichia coli O157:H7 on intestinal structure and solute transport in rabbits. Gastroenterology 104:467-474. [DOI] [PubMed] [Google Scholar]
- 28.Lingwood, C. A. 1994. Verotoxin-binding in human renal sections. Nephron 66:21-28. [DOI] [PubMed] [Google Scholar]
- 29.Lingwood, C. A. 1996. Role of verotoxin receptors in pathogenesis. Trends Microbiol. 4:147-153. [DOI] [PubMed] [Google Scholar]
- 30.McCormick, B. A., S. I. Miller, D. Carnes, and J. L. Madara. 1993. Transepithelial signaling to neutrophils by salmonellae: a novel virulence mechanism for gastroenteritis. Infect. Immun. 63:2302-2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Milligan, S. A., M. W. Owens, and M. B. Grisham. 1996. Inhibition of IkappaB-alpha and IkappaB-beta proteolysis by calpain inhibitor I blocks nitric oxide synthesis. Arch. Biochem. Biophys. 335:388-395. [DOI] [PubMed] [Google Scholar]
- 32.Mukaida, N., S.-I. Okamoto, Y. Ishikawa, and K. Matsushima. 1994. Molecular mechanism of interleukin-8 gene expression. J. Leukoc. Biol. 56:554-558. [PubMed] [Google Scholar]
- 33.Nicholls, L., T. H. Grant, and R. M. Robins-Browne. 2000. Identification of a novel genetic locus that is required for in vitro adhesion of a clinical isolate of enterohaemorrhagic Escherichia coli to epithelial cells. Mol. Microbiol. 35:275-288. [DOI] [PubMed] [Google Scholar]
- 34.Perna, N. T., G. F. Mayhew, G. Posfai, S. Elliot, M. S. Donnenberg, J. B. Kaper, and F. R. Blattner. 1998. Molecular evolution of a pathogenicity island from enterohemorrhagic Escherichia coli O157:H7. Infect. Immun. 66:3810-3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Philpott, D. J., C. A. Ackerley, A. J. Kiliaan, M. A. Karmali, M. H. Perdue, and P. M. Sherman. 1997. Translocation of verotoxin-1 across T84 monolayers: mechanism of bacterial toxin penetration of epithelium. Am. J. Physiol. 273:G1349-G1358. [DOI] [PubMed] [Google Scholar]
- 36.Philpott, D. J., S. Yamaoka, A. Israel, and P. J. Sansonetti. 2000. Invasive Shigella flexneri activates NF-κB through a LPS-dependent innate intracellular response and leads to IL-8 expression in epithelial cells. J. Immunol. 165:903-914. [DOI] [PubMed] [Google Scholar]
- 37.Robinson, M. J., and M. H. Cobb. 1997. Mitogen-activated protein kinase pathways. Curr. Opin. Cell Biol. 9:180-186. [DOI] [PubMed] [Google Scholar]
- 38.Savkovic, S. D., A. Koutsouris, and G. Hecht. 1996. Attachment of a noninvasive enteric pathogen, enteropathogenic Escherichia coli, to cultured human intestinal epithelial monolayers induces transmigration of neutrophils. Infect. Immun. 64:4480-4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Savkovic, S. D., A. Koutsouris, and G. Hecht. 1997. Activation of NF-κB in intestinal epithelial cells by enteropathogenic Escherichia coli. Am. J. Physiol. 273:C1160-C1167. [DOI] [PubMed] [Google Scholar]
- 40.Schwan, W. R., S. Kugler, S. Schuller, D. J. Kopecko, and W. Goebel. 1996. Detection and characterization by differential PCR of host eukaryotic cell genes differentially transcribed following uptake of intracellular bacteria. Infect. Immun. 64:91-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sharma, S. A., M. K. R. Tummuru, M. J. Blaser, and L. D. Kerr. 1998. Activation of IL-8 gene expression by Helicobacter pylori is regulated by transcription factor NF-κB in gastric epithelial cells. J. Immunol. 160:2401-2407. [PubMed] [Google Scholar]
- 42.Steiner, T. S., J. P. Nataro, C. E. Poteet-Smith, J. A. Smith, and R. L. Guerrant. 2000. Enteroaggregative Escherichia coli expresses a novel flagellin that causes IL-8 release from intestinal epithelial cells. J. Clin. Investig. 105:1769-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang, P., C. L. Sutherland, M. R. Gold, and B. B. Finlay. 1998. Listeria monocytogenes invasion of epithelial cells requires the MEK-1/ERK-2 mitogen-activated protein kinase pathway. Infect. Immun. 66:1106-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thanos, D., and T. Maniatis. 1995. NF-κB: a lesson in family values. Cell 80:529-532. [DOI] [PubMed] [Google Scholar]
- 45.Thorpe, C. M., B. P. Hurley, L. L. Lincicome, M. S. Jacewicz, G. T. Keusch, and D. W. K. Acheson. 1999. Shiga toxins stimulate secretion of interleukin-8 from intestinal epithelial cells. Infect. Immun. 67:5985-5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tzipori, S., H. Karch, K. I. Wachsmuth, R. M. Robins-Browne, A. D. O'Brien, H. Lior, M. L. Cohen, J. Smithers, and M. M. Levine. 1987. Role of a 60-megadalton plasmid and Shiga-like toxin in the pathogenesis of infection caused by enterohemorrhagic Escherichia coli O157:H7 in gnotobiotic piglets. Infect. Immun. 55:3117-3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van der Bruggen, T., S. Nijenhuis, E. van Raaij, J. Verhoef, and B. Sweder van Asbeck. 1999. Lipopolysaccharide-induced tumor necrosis factor alpha production by human monocytes involves the Raf-1/MEK1-MEK2/ERK1-ERK2 pathway. Infect. Immun. 67:3824-3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van Setten, P. A., V. W. M. Van Hinsbergh, T. J. A. N. Van Der Velden, N. C. A. J. Van De Kar, M. Vermeer, J. D. Mahan, K. J. M. Assmann, L. P. W. J. Van Den Heuvel, and L. A. H. Monnens. 1997. Effects of TNFα on verocytotoxin cytotoxicity in purified human glomerular microvascular endothelial cells. Kidney Int. 51:1245-1256. [DOI] [PubMed] [Google Scholar]
- 49.Waddell, T. E., C. A. Lingwood, and C. L. Gyles. 1996. Interaction of verotoxin 2e with pig intestine. Infect. Immun. 64:1714-1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weinsten, S. L., J. S. Sanghera, K. Lemke, A. L. DeFranco, and S. L. Pelech. 1992. Bacterial LPS induces tyrosine phosphorylation and activation of mitogen-activated protein kinases in macrophages. J. Biol. Chem. 267:14955-14962. [PubMed] [Google Scholar]
- 51.Westerholt, S., T. Hartung, M. Tollens, A. Gustrau, M. Oberhoffer, H. Karch, B. Klare, K. Pfeffer, P. Emmrich, and R. Oberhoffer. 2000. Inflammatory and immunological parameters in children with haemolytic uremic syndrome (HUS) and gastroenteritis-pathophysiological and diagnostic clues. Cytokines 12:822-827. [DOI] [PubMed] [Google Scholar]
- 52.Whitmarsh, A. J., and R. J. Davis. 1996. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J. Mol. Med. 74:589-607. [DOI] [PubMed] [Google Scholar]