Abstract
In renal bacterial infections granulocytes are of major importance in the primary immune defense against invading pathogens. However, the mechanisms of granulocytic activation in renal interstitial invasion have not been clarified. Renal tubular epithelial cell mechanisms inducing granulocytic activation and bacterial killing may include tubular cell expression of Tamm-Horsfall protein (THP), a urinary protein that is known to enhance cytokine expression in monocytes. We studied the role of THP in granulocytic activation. A strong binding of THP to human granulocytes was demonstrated by fluorescence-activated cell sorter analysis. Urinary THP and supernatants of THP-expressing cultured tubular epithelial cells (MDCK) enhanced interleukin-8 (IL-8) expression by human granulocytes. Renal tubular cells growing polarized on polycarbonate membranes were used to study apical versus basal THP expression. By electron microscopy THP immunoreactivity was exclusively found on the apical surfaces of tubular cells and was absent on the basolateral cell membrane. In the apical cell culture compartment we found significantly more stimulatory activity for granulocytic IL-8 expression. CD62L, a selectin less expressed in activated granulocytes, was decreased in granulocytes incubated with urinary THP and in supernatants of THP-producing renal tubular cells but not in supernatants from THP-negative cells. Again, the effect on CD62L expression was found only in apical culture media and was absent in the basal compartment. In summary our data give evidence that renal tubular cell THP expression may be relevant in kidney diseases since THP is a potent activator of human granulocytes. The regulation of apical versus basal THP expression and release in vivo may be crucial in the induction of the inflammatory response, e.g., in bacterial renal diseases.
Two virologists, Tamm and Horsfall, first described a glycoprotein (23, 24) that is expressed exclusively in the kidney and that is the most abundant protein in the normal urine. The mean urinary excretion of Tamm-Horsfall protein (THP) is 30 to 50 mg/24 h, but the physiological role of this protein is still unknown (11, 29). It is produced and secreted into the urine by cells of the thick limb of the loop of Henle and the distal convoluted tubules (15, 19). THP is normally expressed mainly at the apical surfaces of renal tubular epithelial cells (15, 19), but it has been detected at the basolateral surfaces and in interstitial infiltrates in inflammatory kidney diseases (16, 29). These immunohistochemical studies have shown an abnormal interstitial localization of THP in a variety of interstitial renal diseases (e.g., pyelonephritis and medullary cystic disease) and have demonstrated that THP deposits are surrounded by inflammatory infiltrates (29). It has been proposed that interstitial THP deposits result from severe tubular cell damage with release of THP from its normal intracellular and intraluminal location into the renal interstitium (16).
THP may inhibit the binding of microbial pathogens to epithelial cells within the urinary tract and may enhance their urinary clearance (12). There is evidence that THP may alter the immune response of the host in urinary tract infections since it was shown to induce tumor necrosis factor alpha expression in monocytes (22) and leukotriene B4 and superoxide and gelatinase release in human mononuclear phagocytes (7, 25). Therefore, the polarized expression of THP may have an impact on the interaction of tubular epithelial cells with monocytes and/or granulocytes in inflammatory kidney diseases.
Since we recently found an absence of interleukin-8 (IL-8) expression in polarized tubular epithelial cells following adhesion of Escherichia coli (10), we speculated that renal tubular cell proteins, e.g., THP, may be relevant for the initiation of granulocytic recruitment and activation in bacterial kidney infections. Therefore, we investigated the role of THP and polarized THP expression by renal tubular cells in the activation of human granulocytes.
MATERIALS AND METHODS
Reagents and cell culture of tubular epithelial cells
Lipopolysaccharides (LPS) from E. coli O26:B6, monensin, brefeldin A, mannosamine (2-amino-2-desoxy-d-mannose), and recombinant gamma interferon were obtained from Sigma Chemical Company (Munich, Germany). Recombinant IL-1α and human urinary THP were obtained from Tebu (Frankfurt, Germany). All cell culture media were from Seromed Biochrom (Berlin, Germany). Cell culture devices (flasks, plates, etc.) were from Sarstedt.
Preparation and culture of THP+ and THP− Madin-Darby canine kidney (MDCK) cells were performed as described previously (8, 12). The cells were transfected with plasmid pCMV-THP-neo, containing a cytomegalovirus promoter, THP cDNA, and the neomycin transferase gene. Stable transfectants were isolated and cloned to obtain a cell line expressing THP (MDCK:THP+). The expression of THP was confirmed by Northern blotting, immunohistochemistry, and Western blotting. As a control, an empty plasmid, pCMV-neo, containing a cytomegalovirus promoter and the neomycin phosphotransferase gene but no THP cDNA, was transferred into MDCK cells and stable transfectants were cloned to obtain a cell line that did not express THP (MDCK:THP−). For cell culture on polycarbonate membranes epithelial cells were grown overnight on polycarbonate membranes (Falcon; diameter, 20 mm; pore size, 0.4 μm). The lower and upper compartments had volumes of 800 μl. The cell culture media of both compartments were collected separately and stored at −80°C (10).
Immunohistochemistry.
Tissue samples of kidney cortex were obtained from the uninvolved poles of kidneys removed during surgery for renal carcinoma (9). Serial 6-μm-thick cryostat sections of that renal tissue were prepared for alkaline phosphatase and monoclonal anti-alkaline phosphatase complex staining as described earlier (10). A polyclonal rabbit anti-human THP antibody was used as the primary antibody (diluted 1:1,000; Tebu). Rabbit immunoglobulin G (IgG; Sigma) was used for isotypic controls.
Immunogold staining and electron microscopy of tubular epithelial cells
Immunolabeling was performed on ultrathin London resin (LR) white sections. MDCK:THP+ and MDCK:THP− cells grown on polycarbonate membrane filters were fixed with 2% paraformaldehyde-0.05% glutaraldehyde in phosphate-buffered saline (PBS), pH 7.4, for 1 h at 4°C. Then, the filters were cut into small pieces, and samples were contrasted with 2% uranyl acetate in 0.1 M maleate buffer, pH 6.5, containing 3.5% sucrose. Following dehydration in a graded acetone series, the samples were embedded in LR white (London Resin Co., Reading, United Kingdom). Ultrathin sections were mounted on 300 mesh nickel grids and were blocked for 30 min with 0.5% bovine serum albumin (BSA; Sigma) in Tris-buffered saline (TBS). The sections were incubated for 16 h with primary antibody rabbit anti-human THP (Tebu) diluted 1:1,000 with TBS containing 0.005% Tween 20. After being rinsed with TBS, sections were incubated for 2 h with donkey anti-rabbit IgG coupled to 12-nm gold particles (Jackson ImmunoResearch, West Grove, Pa.), diluted 1:100 with TBS. Sections were contrasted with uranyl acetate and lead citrate and were examined with a Phillips EM 400 electron microscope. In control incubations, a nonimmune rabbit serum was substituted for the primary antibody.
Preparation of human peripheral blood neutrophils.
Heparinized blood collected by venipuncture from healthy adult volunteers was layered on Histopaque 1119 (Sigma) and centrifuged for 20 min at 800 × g. The interphase, consisting mainly of lymphocytes and monocytes, was discarded. The granulocyte-rich layer of Histopaque 1119 was collected and washed twice in RPMI medium (Seromed-Biochrom, Berlin, Germany), and the cells were further fractionated on a discontinuous Percoll (Pharmacia, Uppsala, Sweden) gradient consisting of layers with densities of 1,105 (85%), 1,100 (80%), 1,093 (75%), 1,087 (70%), and 1,081 (65%) g/ml. After centrifugation for 20 min at 800 × g, the interface between 80 and 85% Percoll layers was collected and washed twice in RPMI 1640. All procedures were carried out at room temperature. The purity of granulocytes achieved by this isolation technique was always 99%, as determined microscopically after My-Gruenwald-Giemsa staining of cytocentrifuge (Shandon, Pittsburgh, Pa.) slides. The viability of cells was >98% as assessed by trypan blue dye exclusion.
ELISA for cell surface expression of THP by renal tubular epithelial cells.
For the detection of THP cell surface expression an enzyme-linked immunosorbent assay (ELISA) was established using polyclonal anti-THP (1:1,000; Tebu) as a primary antibody. Tubular epithelial cells (MDCK:THP+ and MDCK:THP−; 3 × 105/well) were cultured in flat-bottom 96-well cell culture plates in the presence or absence of conditioned medium for 24 h. The medium was removed, and the cellular monolayer was fixed with glutaraldehyde (1.25% in distilled water) for 1 h. After three washings with PBS the anti-THP antibody was added for 1 h at 37°C. Peroxidase-conjugated goat anti-rabbit IgG (1:1,000 in 0.1% BSA; 1 h, 37°C) was used as a developing antibody. After additional washings, ABTS (2,2′-azinobis[3-ethylbenzthiazolinesulfonic acid]; Sigma; 1 mg/ml)-hydrogen peroxide (0.28 μl of 30% H2O2 per ml dissolved in 0.1 M sodium acetate, pH 4.2) was used as a substrate (9). The color was allowed to develop for 30 min at room temperature, and the plates were read with a microreader (Behring EL 311; A405). In control experiments rabbit IgG (Sigma) was used instead of the rabbit anti-human THP antibody.
ELISA for IL-8 and FACS analysis of THP binding to granulocytes.
For the measurement of IL-8 in the cell culture supernatants, a sandwich ELISA was used (Dynatech) as described recently (9). For neutralization experiments THP (1 μg/ml) was incubated with anti-THP (1:1,000) at 37°C for 30 min.
To study the binding of THP to granulocytes, purified granulocytes (5 × 105) were incubated for 1 h at 4°C with either different concentrations of urine THP (100 μg, 10 μg, 1 μg, 100 ng, 10 ng, and 1 ng) or threefold-concentrated culture supernatant from MDCK:THP+ or MDCK:THP− cells. Subsequently, cells were washed with fluorescence-activated cells sorter (FACS) buffer (PBS-0.5% BSA-1.0% normal human serum-0.02% azide), and THP binding was assessed with goat anti-THP polyclonal IgG diluted in FACS buffer or FACS buffer alone. Following incubation for 1 h at 4°C, cells were washed with FACS buffer and incubated for 1 h with fluorescein isothiocyanate-conjugated rabbit anti-goat polyclonal IgG. After washings, cells were fixed with 1% paraformaldehyde in PBS and analyzed on a FACS-Calibur with CellQuest software.
Assessment of PMN activation by flow cytometry analysis.
The cell surface expression of CD62L was measured as a marker for PMN activation. CD62L is known to be downregulated on polymorphonuclear leukocytes (PMN) upon activation (3). PMN were stained with a phycoerythrin-conjugated monoclonal antibody to human CD62L (clone Dreg-56; BD/Pharmingen) or an isotype-matched control antibody and analyzed with a FACS-Calibur and CellQuest software (Becton Dickinson, San Diego, Calif.). The PMN isolation technique applied in our studies allowed us to work with nonpreactivated PMN as shown by the high CD62L expression of the cells used in the experiments (see Fig. 5A). This was achieved by using a method for the isolation of PMN which did not include a hypotonic step for lysing erythrocytes. As reported previously, membrane fragments of lysed erythrocytes stimulate PMN (6).
FIG. 5.
(A) Urinary THP reduces granulocytic CD62L expression. CD62L expression in human granulocytes was detected as described in Materials and Methods. In the presence of LPS (1 μg/ml) and in the presence of urinary THP (1 μg/ml) CD62L expression was reduced. Dotted-line histograms, conjugate controls; open histograms, CD62L expression; solid histograms, CD62L expression after sample incubation, as described in Materials and Methods. Out of three independent experiments one histogram is shown per condition. (B) Apical and basal culture media of MDCK:THP+ and MDCK:THP− cells differentially reduce CD62L expression in granulocytes. Apical and basal cell culture media were collected separately, and the effect on granulocytic CD62L expression was analyzed. Dotted-line histograms, conjugate controls; open histograms, CD62L expression; solid histograms, CD62L expression after sample incubation, as described in Materials and Methods. Out of three independent experiments one histogram is shown per condition.
Statistics.
In the figures the means and standard deviations are given. With the exception of neutralization experiments all experiments (n = 6/determination) were repeated at least three times. Statistical analyses of the THP ELISA experiments and IL-8 determinations were done by using the unpaired U test of Wilcoxon, Mann, and Whitney (18). Differences were considered significant if P values were <0.05. Results for immunohistochemistry, electron microscopy, and CD62L expression were not analyzed statistically.
RESULTS
Tissue expression of THP in normal kidneys: immunohistochemical detection.
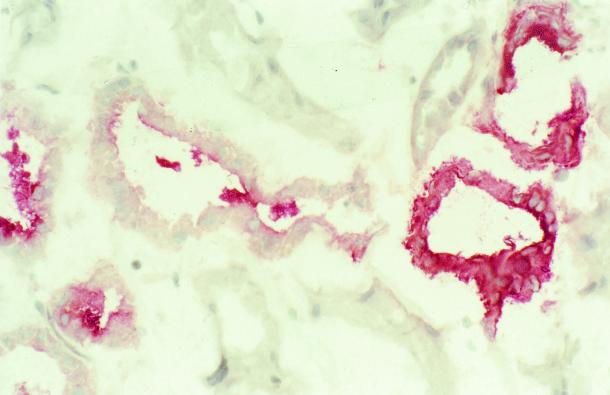
As shown in Fig. 1, in normal renal tissue THP expression was detected in renal tubules. The luminal expression tended to be more pronounced than the basolateral staining. By light-microscopic criteria (lack of a well-developed brush border, cuboidal cells, wide lumen) THP expression was located at segments representing the thick ascending limb of the loop of Henle. THP staining was absent in vessels and glomeruli.
FIG. 1.
Immunohistochemical detection of THP expression in a human kidney section. Normal human renal tissue was snap-frozen and stained for THP expression by immunohistochemistry as described in Materials and Methods. No staining was observed in glomeruli and vessels, but a fraction of tubular epithelial cells strongly stained for THP. Original magnification, ×100.
Cellular expression of THP in MDCK cells: detection by electron-microscopic immunogold labeling.
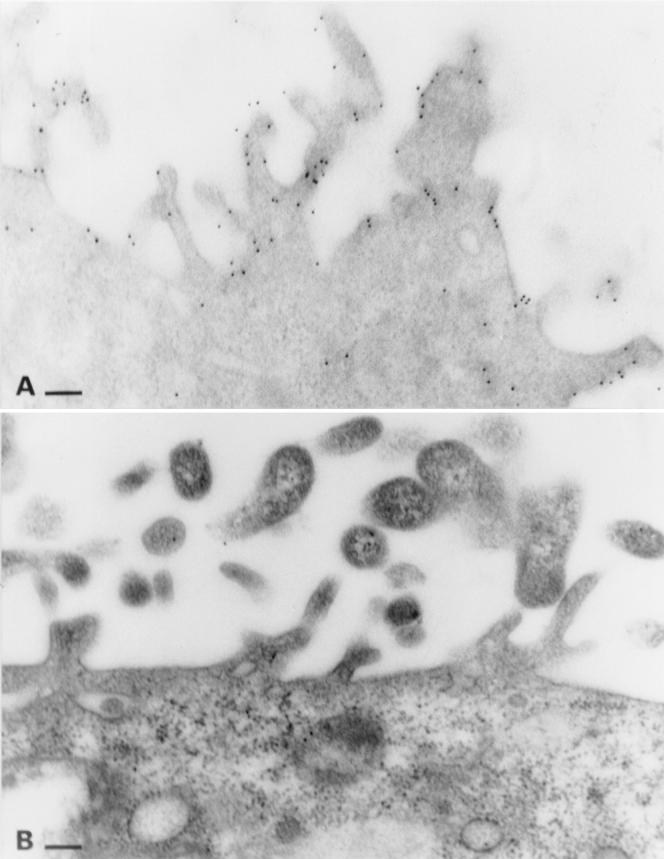
MDCK:THP+ and MDCK:THP− cells grown on polycarbonate membranes display a structural polarization, as demonstrated by the apical appearance of microvilli (Fig. 2). In MDCK:THP+ cells, the apical membrane and microvilli are immunolabled for THP, whereas label within the cytoplasm is negligible (Fig. 2A). This prevailing apical localization of THP is present in nearly all cells within a given culture of MDCK:THP+ cells. Immunolabeling performed on MDCK:THP− cells confirmed the specificity of the gold label (Fig. 2B).
FIG. 2.
Postembedding immunogold labeling for THP in MDCK:THP+ (A) and MDCK:THP− (B) cells. MDCK cells were grown on polycarbonate membranes and were embedded in LR white. Note the prevailing label on the apical membrane and microvilli in panel A. Bars: 150 nm.
Cell surface expression of THP: THP ELISA.
Cell surface expression of THP in MDCK cells was analyzed by ELISA (optical absorbance at 450 nm). THP was detected on the surfaces of MDCK:THP+ cells (optical density [OD], 1.1 ± 0.12; OD for isotype IgG control: 0.37 ± 0.01) but not on MDCK:THP− cells (OD: 0.38 ± 0.01). Pretreatment of epithelial cells with LPS (1 μg/ml) and IL-1α (1 ng/ml) and gamma interferon (100 U/ml) did not modify the cell surface expression of THP by MDCK:THP+ cells. THP cell surface expression was stable in numerous subcultures of MDCK:THP+ cells. Pretreatment for up to 24 h with monensin (1, 10, or 20 μg/ml) or brefeldin A (1, 10, or 20 μg/ml) did not reduce the cell surface expression of THP.
Binding of THP to human granulocytes.
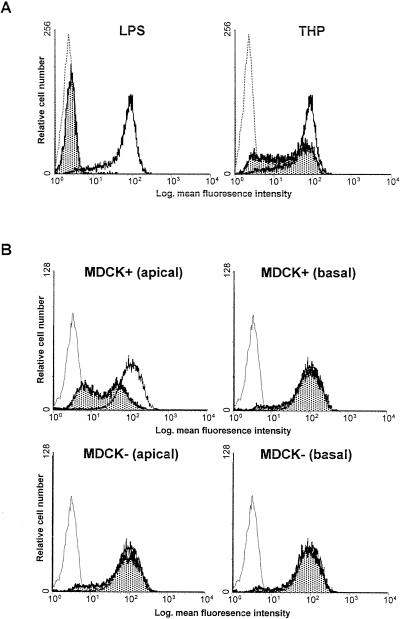
Following an incubation period of 1 h purified urinary THP demonstrated a dose-dependent binding to granulocytes (Fig. 3). When threefold-concentrated supernatant of MDCK:THP+ cells was used in this experiment, the binding of THP to human granulocytes was also evident. No THP binding was observed in granulocytes incubated with supernatants from MDCK:THP− cells.
FIG. 3.
FACS analysis of THP binding to granulocytes. THP binding to human granulocytes was detected by FACS analysis as described in Materials and Methods. Granulocytes were incubated with THP (1 ng/ml, 100 ng/ml, and 10 μg/ml) or in the presence of MDCK:THP+ or MDCK:THP− cell culture supernatants. Dotted-line histograms, controls for THP binding without addition of THP-containing samples; solid-line histograms, THP binding, as described in Materials and Methods. Out of three independent experiments one histogram is shown per condition.
Urinary THP and supernatants from MDCK:THP+ cells enhance IL-8 expression by human granulocytes.
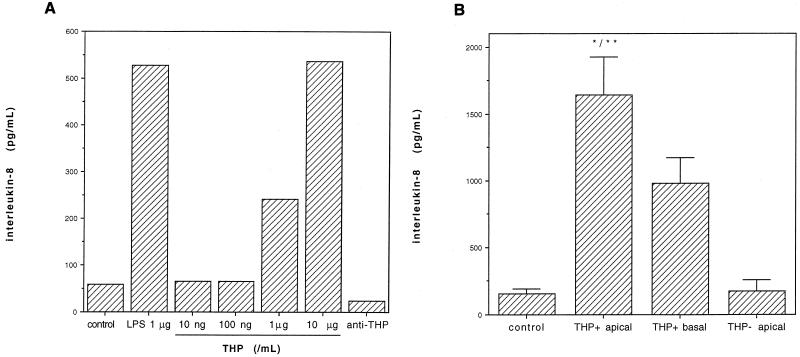
When urinary THP at 10 ng/ml up to 10 μg/ml was used, there was a clear effect of the dose on the IL-8 production of granulocytes (Fig. 4A). At a concentration of 10 μg/ml urinary THP enhanced IL-8 expression of granulocytes as strongly as LPS (1 μg/ml). Coincubation with THP and LPS did not further enhance the secretion of IL-8 (data not shown). The stimulatory effect of THP (1 μg/ml) on IL-8 expression was strongly reduced in the presence of anti-THP antibody (1:1,000) (Fig. 4A).
FIG. 4.
(A) Urinary THP stimulates IL-8 expression in granulocytes. Shown is the effect of THP dose (10 ng/ml to 10 μg/ml) on IL-8 expression by human granulocytes. Granulocytes were incubated with various concentrations of THP as indicated or LPS (1 μg/ml) for 24 h, and IL-8 expression was detected by ELISA (n = 3 determinations/bar). In the presence of anti-THP (right bar) the effect of THP (1 μg/ml) was reduced to control levels. (B) Stimulation of IL-8 by apical versus basal supernatants of MDCK:THP+ and MDCK:THP− cells. MDCK:THP+ and MDCK:THP− cells were cultured on polycarbonate microtiter plates, and the apical and basal cell culture media were harvested separately. IL-8 expression by human granulocytes was measured by ELISA as described in Materials and Methods. Control granulocytes were incubated with cell culture medium only. Bars show means of six determinations/column plus standard deviations. ∗, P < 0.05 versus value for THP+ basal medium; ∗∗, P < 0.01 versus value for controls or MDCK:THP− apical cell supernatants (U test).
Apical versus basal culture medium from MDCK:THP+ cells.
To demonstrate the effects of a polarized THP expression, MDCK cells were cultured on polycarbonate membranes and the apical and basal culture media were analyzed separately for IL-8 expression of granulocytes. As shown in Fig. 4B, we found significantly more activity in apical than in basal cell culture medium with respect to IL-8 release of granulocytes. Supernatants from MDCK:THP− cells were without stimulatory effects on granulocytic IL-8 expression. When LPS was used in controls, an enhanced expression of IL-8 was also detected in all experiments (data not shown).
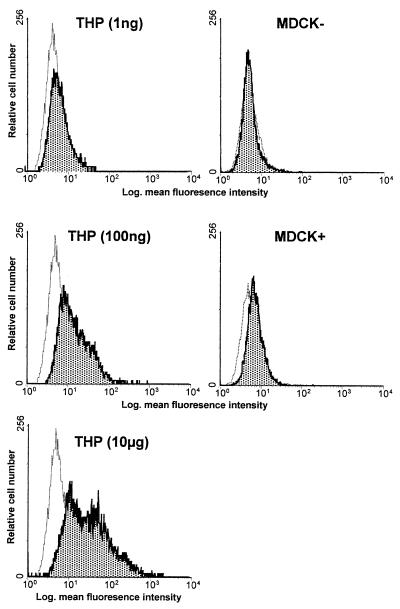
THP and supernatants from MDCK:THP+ cells reduce granulocytic CD62L expression.
As shown in Fig. 5A, urinary THP reduced CD62L expression by human granulocytes. This effect depended on the concentration of THP used and was most strong at 1 μg/ml. LPS was used as a positive control and reduced CD62L expression significantly.
Effects of apical versus basal cell culture medium from MDCK:THP+ cells on granulocytic CD62L expression.
To demonstrate a polarized THP expression, THP supernatants were collected as described above and used to stimulate granulocytes. We found that only apical MDCK:THP+ supernatants reduced CD62L expression, whereas basal MDCK:THP+ supernatants and both apical and basal supernatants from MDCK:THP− cells had no effect (Fig. 5B). In contrast to apical MDCK:THP+ cell medium, culture media of MDCK:THP− cells and basal medium of MDCK:THP+ cells did not reduce CD62L expression.
DISCUSSION
The role of THP in renal inflammatory diseases is not completely understood. Numerous reports have documented an abnormal interstitial localization of this protein in nephritis (16, 29), and the role of THP as an autoantigen has been discussed (2). These observations lead to the hypothesis that THP may have inflammatory potential. Accordingly, effects of particulate THP on mononuclear cells have been investigated. Purified THP enhances the expression and secretion of cytokines by monocytic cells (22) and can induce the respiratory burst in granulocytes (25). These studies have focused on effects of THP isolated and purified from human urine (22, 25). In the urine, however, THP forms large aggregates with a molecular mass of 7 × 106 kDa, while the monomeric molecular mass of THP is 90 to 95 kDa (10). In addition, intrarenal in vivo stimulation of granulocytes by THP presumably requires that the expression of that protein not be restricted to the apical (luminal) tubular surface; basolateral expression adjacent to the peritubullary vessels should also occur. Using a renal tubular cell line stably transfected with the cDNA for THP and therefore producing THP in a cell culture system (8, 12) we were able to analyze apical versus basolateral THP expression and its impact on granulocytic stimulation. MDCK cells have been widely used in studies, e.g., on the nephrotoxocity of immunosuppressive drugs (1), because these cells retain the vectorial ion transport and certain functions and structural properties characteristic of distal nephron and collecting tubule cells (17, 28). MDCK cells are also responsive to aldosterone (14, 21). In addition MDCK cells have been proposed as an appropriate model for studies on the intracellular sorting of THP by renal cells (13).
We have shown by FACS analysis that THP binds to human granulocytes. The binding of THP was shown for purified THP from the human urine and for the soluble cell culture (MDCK)-derived form of THP. Cavallone et al. demonstrated that human granulocytes do not bind to cell surface-anchored THP (4). Binding and activation of granulocytes were demonstrated only after opsonization of THP-expressing cells with anti-THP antibodies. In light of data showing binding of urinary THP to granulocytes (4, 27), the authors concluded that polymeric urinary THP rather than monomeric membrane-bound THP favors adhesion of granulocytes (4). We did not analyze the binding of granulocytes to THP+ or THP− cells but demonstrate a prominent binding of urinary and cell culture-produced THP to human granulocytes. By Western blotting we have shown that the anti-THP antibody we used in our studies reacts with both the urinary and the cell membrane-bound THP protein (data not shown). THP had been shown to activate monocytes but not endothelial cells, and it had been speculated that different binding receptors for THP are present on these cells (22). The THP receptor on human granulocytes is a single class of sialic acid surface receptor (26) with a density of 26.218/cell and a Kd of 4.2 × 10−9. THP binding was restricted to the plasma membrane and absent in cytosolic and nuclear membranes of granulocytes (26). Thus, a single class of cell surface receptors of particular importance for the terminal sialic acid residues on the polybranched oligosaccharide chains of the glycoprotein has been demonstrated (26).
Our results demonstrate that purified THPs from the human urine induce IL-8 expression in human granulocytes and reduce CD62L expression in these cells. The activation of IL-8 expression was reduced in the presence of a neutralizing antibody to THP. In addition, we found that cell culture-derived THP produced in MDCK:THP+ cells is capable of inducing IL-8 production and reducing granulocytic CD62L expression, thus causing neutrophil activation. The decrease of granulocytic CD62L cell surface expression is indicative of activation, as demonstrated by the decrease of CD62L expression of these cells following LPS exposure. Apical supernatants from THP-producing MDCK cells enhanced granulocytic IL-8 production and reduced the membrane expression of CD62L, but no effect on CD62L expression was evident when basal supernatants from MDCK:THP+ cells or apical and basal supernatants from MDCK:THP− cells were studied.
Intracellular THP is, at least in HeLa cells, mainly located at the Golgi apparatus before being exposed at the cell surface (13, 20). Monensin, a carboxylic ionophore affecting the function of the Golgi apparatus, has been shown to interfere with the intracellular maturation of THP and its delivery to the cell surface (13) but did not decrease the cell surface expression of THP by MDCK:THP+ cells in our ELISA experiments. This result is explained by the occurrence after monensin treatment of an intermediate form of THP in the cell culture, which is not exposed at the surface (13). For the urinary excretion of THP, which is inserted into luminal cell surface membranes by the glycosy-phosphatidylinositol anchor, proteolytic cleavage of a juxtamembrane ectodomain is responsible (5). Thus, conditions altering the intracellular transport of THP and reducing the delivery of THP to the luminal surface may lead to basolateral expression of this protein and to the activation of interstitial granulocytes.
The binding of THP to granulocytes is sialic acid dependent (26). This is of particular interest since we have previously shown that THP reduces the binding of S-fimbriae of E. coli, which recognize sialic acid-containing receptor molecules on, e.g., tubular epithelial cells (12). Therefore, one might speculate that THP modulates inflammatory processes in bacterial renal infections not only by reducing bacterial adhesion to epithelial cells but also by activation of invading granulocytes. Whether or not this activation is restricted to the apical plasma membrane of tubular epithelial cells and thus to the urinary space of the tubules or occurs basolaterally in vivo remains to be determined.
The use of polarized epithelial cell cultures allows studies on the role of proteins expressed or secreted on the apical or basolateral surface. Since the polarized expression of different molecules may be crucial in epithelial infections, the epithelial immune response studies of the apical versus basolateral expression of these molecules, e.g., THP, may be helpful in further understanding molecular mechanisms in epithelial infections.
Acknowledgments
The study was supported by The Medical University of Lübeck, FUL 800/A1 and N13, 16/98.
We appreciate the expert technical assistance of Annette Willemsen, Heidi Schnorfeil, and Maren Behrensen.
Editor: A. D. O'Brien
REFERENCES
- 1.Aker, S., P. Heering, E. Kinne-Saffran, C. Deppe, B. Grabensee, and R. K. H. Kinne. 2001. Different effects of cyclosporin A and FK506 on potassium transport systems in MDCK cells. Exp. Nephrol. 9:332-340. [DOI] [PubMed] [Google Scholar]
- 2.Bade, J. J., J. Marrink, A. Karrenbeld, L. van der Weele, and H. J. A. Mensink. 1996. Increased urinary levels of Tamm-Horsfall glycoprotein suggest a systemic etiology of interstitial cystitis. J. Urol. 156:943-946. [PubMed] [Google Scholar]
- 3.Berg, M., and S. P. James. 1990. Human neutrophils release the Leu-8 lymph node homing receptor during cell activation. Blood 76:2381-2388. [PubMed] [Google Scholar]
- 4.Cavallone, D., N. Malagolini, and F. Serafini-Cessi. 1999. Binding of human neutrophils to cell-surface anchored Tamm-Horsfall glycoprotein in tubulo-interstitial nephritis. Kidney Int. 55:1787-1799. [DOI] [PubMed] [Google Scholar]
- 5.Cavallone, D., N. Malagolini, and F. Serafini-Cessi. 2001. Mechanism of release of urinary Tamm-Horsfall glycoprotein from the kidney GPI-anchored counterpart. Biochem. Biophys. Res. Commun. 280:110-114. [DOI] [PubMed] [Google Scholar]
- 6.Dominguez, M., and A. Torano. 1999. Immune adherence-mediated opsonophagocytosis: the mechanism of Leishmania infection. J. Exp. Med. 189:25-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horton, J. K., M. Davies, N. Topley, D. Thomas, and J. D. Williams. 1990. Activation of the inflammatory response of neutrophils by Tamm-Horsfall glycoprotein. Kidney Int. 37:717-726. [DOI] [PubMed] [Google Scholar]
- 8.Kaji, D. M., J. Bates, J. D. Goyzueta, K. Prasadan, H. Yu, and S. Kumar. 1996. Transfection alters ion transport in MDCK cells. J. Membr. Biol. 149:49-55. [DOI] [PubMed] [Google Scholar]
- 9.Kreft, B., M. Placzek, C. Doehn, J. Hacker, G. Schmidt, G. Wasenauer, M. R. Daha, F. J. van der Woude, and K. Sack. 1995. S fimbriae of uropathogenic Escherichia coli bind to primary human renal proximal tubular epithelial cells but do not induce expression of intercellular adhesion molecule 1. Infect. Immun. 63:3235-3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krüger, S., E. Brandt, M. Klinger, S. Krüger, and B. Kreft. 2000. Interleukin-8 secretion of cortical tubular epithelial cells is directed to the basolateral environment and is not enhanced by apical exposure to Escherichia coli. Infect. Immun. 68:328-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar, S., and A. Muchmore. 1990. Tamm-Horsfall protein-uromodulin (1950-1990). Kidney Int. 37:1395-1401. [DOI] [PubMed] [Google Scholar]
- 12.Leeker, A., B. Kreft, J. Sandmann, J. Bates, G. Wasenauer, H. Müller, K. Sack, and S. Kumar. 1997. Tamm-Horsfall protein inhibits binding of S- and P-fimbriated Escherichia coli to human renal tubular epithelial cells. Exp. Nephrol. 5:38-46. [PubMed] [Google Scholar]
- 13.Malagolini, N., D. Cavallone, and F. Serafini-Cessi. 1997. Intracellular transport, cell-surface exposure and release of recombinant Tamm-Horsfall glycoprotein. Kidney Int. 52:1340-1350. [DOI] [PubMed] [Google Scholar]
- 14.Oberleithner, H., U. Vogel, U. Kersting, and W. Steigner. 1990. Madin-Darby canine kidney cells. II. Aldosterone stimulates Na+/H+ and Cl −HCO3-exchange. Pfluegers Arch. 416:533-539. [DOI] [PubMed] [Google Scholar]
- 15.Pollak, V. E., and C. Arbel. 1969. The distribution of Tamm-Horsfall mucoprotein (uromucoid) in the human nephron. Nephron 6:667-672. [DOI] [PubMed] [Google Scholar]
- 16.Resnick, J. S., S. Sisson, and R. L. Vernier. 1978. Tamm-Horsfall protein. Abnormal localization in renal disease. Lab. Investig. 38:550-555. [PubMed] [Google Scholar]
- 17.Rindler, M. J., L. M. Chuman, L. Shaffer, and M. H. Saier. 1979. Retention of differentiated properties in an established dog kidney epithelial cell line (MDCK). J. Cell Biol. 81:635-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sachs, L. 1992. Angewandte Statistik. Statistische Methoden und ihre Auswertung. Springer Verlag, Berlin, Germany.
- 19.Schenk, E. A., Schwartz, R. H., and R. A. Levis. 1971. Tamm-Horsfall mucoprotein. I. Localization in the kidney. Lab. Investig. 25:92-95. [PubMed] [Google Scholar]
- 20.Serafini-Cessi, F., N. Malagolini, T. C. Hoops, and M. J. Rindler. 1993. Biosynthesis and oligosaccharide processing of human Tamm-Horsfall glycoprotein permanently expressed in HeLa cells. Biochem. Biophys. Res. Commun. 194:784-790. [DOI] [PubMed] [Google Scholar]
- 21.Shahedi, M., K. Laborde, L. Bussieres, and C. Sachs. 1993. Acute and early effects of aldosterone on Na-K-ATPase activity in Madin-Darby canine kidney epithelial cells. Am. J. Physiol. 264:F1021-F1026. [DOI] [PubMed] [Google Scholar]
- 22.Su, S. J., K. L. Chang, T. M. Lin, Y. H. Huang, and T. M. Yeh. 1997. Uromodulin and Tamm-Horsfall protein induce human monocytes to secrete TNF and express tissue factor. J. Immunol. 158:3449-3456. [PubMed] [Google Scholar]
- 23.Tamm, I., and F. L. Horsfall. 1950. Characterization and separation of an inhibitor of viral hemagglutination present in urine. Proc. Soc. Exp. Biol. Med. 74:108-114. [PubMed] [Google Scholar]
- 24.Tamm, I., and F. L. Horsfall. 1952. A mucoprotein derived from human urine which reacted with influenza, mumps, and Newcastle disease virus. J. Exp. Med. 95:71-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas, D. B. L., M. Davies, and J. D. Williams. 1993. Release of gelatinase and superoxide from human mononuclear phagocytes in response to particulate Tamm Horsfall protein. Am. J. Pathol. 142:249-260. [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas, D. B. L., M. Davies, J. R. Peters, and J. D. Williams. 1993. Tamm Horsfall protein binds to a single class of carbohydrate specific receptors on human neutrophils. Kidney Int. 44:423-429. [DOI] [PubMed] [Google Scholar]
- 27.Toma, G., J. M. Bates, Jr., and S. Kumar. 1994. Uromodulin (Tamm-Horsfall protein) is a leucocyte adhesion molecule. Biochem. Biophys. Res. Commun. 200:275-282. [DOI] [PubMed] [Google Scholar]
- 28.Valentich, J. D. 1981. Morphological similarities between the dog kidney cell line MDCK and the mammalian cortical collecting tubule. Ann. N. Y. Acad. Sci. 372:394-405. [DOI] [PubMed] [Google Scholar]
- 29.Zager, R. A., R. S. Cotran, and J. R. Hoyer. 1978. Pathologic localization of Tamm-Horsfall protein in interstitial deposits in renal disease. Lab. Investig. 38:52-57. [PubMed] [Google Scholar]