Abstract
The study reported here investigated the immunogenicity and protective potential of a lipid core peptide (LCP) construct containing a conserved region determinant of M protein, defined as peptide J8. Parenteral immunization of mice with LCP-J8 led to the induction of high-titer serum immunoglobulin G J8-specific antibodies when the construct was coadministered with complete Freund's adjuvant (CFA) or administered alone. LCP-J8 in CFA had significantly enhanced immunogenicity compared with the monomeric peptide J8 given in CFA. Moreover, LCP-J8/CFA and LCP-J8 antisera opsonized four different group A streptococcal (GAS) strains, and the antisera did not cross-react with human heart tissue proteins. These data indicate the potential of an LCP-based M protein conserved region GAS vaccine in the induction of broadly protective immune responses in the absence of a conventional adjuvant.
The development of a vaccine against group A streptococci (GAS)—the etiologic agents of rheumatic heart disease—has focused largely on the bacterial surface M protein (10, 21). The M protein is a major virulence factor in GAS infection and consists of a variable amino-terminal region which defines the GAS serotype (over 100 serotypes are known) and a highly conserved carboxy-terminal C-repeat region (10). Protective immunity to GAS infection has been associated with type-specific opsonic antibodies against M protein (10, 21), although the presence of opsonic antibodies specific to the C-region has been demonstrated in humans (17) and in mice immunized with C-region peptides (18) and is also important in the elicitation of protective immunity to GAS (4). The variability in M proteins and the potential for the induction of autoimmunity due to antigenic molecular mimicry between the GAS M protein and heart antigens (6, 11, 13, 19) represent significant hurdles in the development of a vaccine covering a wide range of strains. Multivalent M protein constructs containing epitopes from several type-specific regions of different M proteins (4, 7, 8) and those based on the conserved C-region (2-5) have shown promising results in animal trials. However, the GAS vaccine constructs require for their efficacy the use of adjuvants that are not suitable for use in humans due to their toxicity. The development of novel synthetic adjuvants offers the possibility of vaccine delivery without the need for additional toxic adjuvants. Lipopeptide compounds utilizing a synthetic analog of the N-terminal moiety of bacterial lipoprotein from Escherichia coli (Pam3cys [tripalmitoyl-S-glyceryl cysteine]) as a lipidic anchor moiety (26) were found to be potent immunogens with self-adjuvanting properties, eliciting humoral and cellular responses irrespective of the route of administration (9, 15, 16, 27). The lipidic polylysine core peptide (LCP) system (24) has also been described as using a lipidic anchor moiety in conjunction with the multiple antigenic peptide system (23). Furthermore, lipopeptide compounds are potentially safe options for vaccine delivery in humans (1). The study reported here investigated the LCP system as a self-adjuvanting GAS vaccine delivery approach. An LCP construct (LCP-J8) was synthesized by incorporating multiple copies of a GAS M protein C-region peptide, referred to as J8, which contains a conformational B-cell epitope and lacks potentially deleterious T-cell autoepitopes (14). The J8 peptide (QAEDKVKQSREAKKQVEKALKQLEDKVQ, consisting of residues 344 to 355 of the GAS M1 protein) is a chimeric peptide that contains 12 amino acids from the C-region (shown in bold) and is flanked by GCN4 DNA-binding protein sequences, which are required to maintain the correct helical folding and conformational structure of the peptide (20). J8 was synthesized by manual solid-phase peptide synthesis using Boc (tert-butoxycarbonyl) chemistry (22), and peptide purification was carried out on a Waters HPLC system (model 600 controller, 490 E UV detector, 60 F pump).
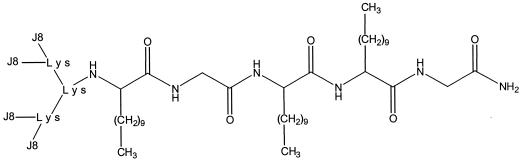
Boc-l-amino acids and MBHA (4-methylbenzhydrylamine) resin (Novabiochem, Läufelfingen, Switzerland) were used to synthesize the LCP-J8 construct (Fig. 1). Racemic lipoamino acids were synthesized with Boc protection according to the procedures of Gibbons et al. (12). Preactivated Boc-Gly-OH was coupled to the MBHA resin. The next two cycles were carried out with Boc-lipoamino acids containing 12 carbon atoms (C12: HN-CH[(CH2)9CH3]-OH); this was followed sequentially by the coupling of Boc-Gly-OH, Boc-C12-OH, and Boc-Lys(Boc)-OH. After deprotection of the lysine α- and ɛ-amino groups, a four-branch system was formed by the coupling of Boc-Lys(Boc)-OH to the free amino groups. After deprotection, four identical peptide J8 sequences were synthesized directly onto each of the α- and ɛ-amino groups of each lysine of the branched lysine core, with the appropriate protecting groups applied on the side chains of the amino acids. Thus, the lipophilic anchor of the LCP-J8 construct contains three 2-amino-dodecanoic lipoamino acids attached to the polylysine core, with glycine spacers employed—one between the resin and the first lipoamino acid and another between the second and third lipoamino acids. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis resulted in a band for LCP-J8 of the expected size. Determination of the mass spectrum of LCP-J8 resulted in a calculated molecular weight of 14,158.45.
FIG. 1.
Chemical structure of the LCP-J8 construct. Four identical peptide J8 sequences were synthesized directly onto the two amino groups of each lysine (Lys) of a branched polylysine core. The lipophilic anchor of the LCP-J8 construct contains three 2-amino-dodecanoic lipoamino acids (shown as branched alkyl side chains) attached to the polylysine core, with one glycine amino acid spacer between the resin and the first lipoamino acid and a second between the second and third lipoamino acids.
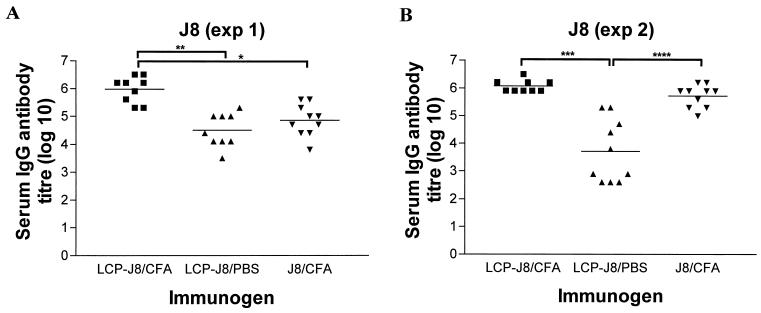
To assess the immunogenicity of the LCP-J8 construct, immunoglobulin G (IgG) antibody responses to the peptide J8 were measured in sera from 4- to 6-week-old female B10.BR mice (Animal Resource Centre, Perth, Australia) In two separate experiments, the mice (n = 10 per group) were immunized subcutaneously at the tail base with 30 μg of LCP-J8 construct, which was either emulsified 1:1 (vol/vol) with complete Freund’s adjuvant (CFA) (Sigma, Castle Hill, Australia) or given alone in a total volume of 50 μl of sterile-filtered phosphate-buffered saline (PBS) (Fig. 2). Three weeks after the primary immunization, the mice received at weekly intervals a further four (experiment 1 [exp 1]) or five (experiment 2 [exp 2]) booster injections of doses of 3 μg of LCP-J8 construct in PBS prior to the collection of blood via the tail artery. Mice in the control group received 30 μg of J8 in CFA or 20 μg of pepM1 (the amino-terminal half of the M protein) in CFA, with booster injections of 3 μg each. Antibody titers were determined by enzyme-linked immunosorbent assay (18) and defined as the lowest dilutions that gave optical density (OD) readings at 450 nm more than three standard deviations above the mean OD of control wells containing normal mouse sera (obtained from mice immunized with CFA in PBS). In the first experiment (Fig. 2A), in which mice received a primary immunization and four booster injections each of the same immunogen, J8-specific antibodies were detected in all mice 3 weeks after the primary immunizations with LCP-J8 in CFA and J8 in CFA, with final average antibody titers after four booster injections of 1.5 × 106 and 1.4 × 105, respectively (exp 1) (Fig. 2A). J8-specific antibodies were not detected at 3 weeks postimmunization in the mice immunized with LCP-J8 without adjuvant. However, after one booster injection of immunogen, six of the nine mice had J8-specific antibodies, and after the third booster injection, antibodies to J8 were detected in all mice. After the final booster injection (booster injection number 4), the average J8 antibody titer in serum samples from mice immunized with the LCP construct without adjuvant was 6.4 × 104 (exp 1). In the second experiment (Fig. 2B), in which mice received a primary immunization and five booster injections each of the same immunogen, the final average J8-specific serum IgG antibody titers were 1.4 × 106, 5.0 × 104, and 7.5 × 105 for mice immunized with LCP-J8/CFA, LCP-J8/PBS, and J8/CFA, respectively. IgG isotyping demonstrated strong J8-specific IgG1, IgG2b, and IgG2a antibody responses with lower titers or no J8-specific antibodies detected for IgG3 in LCP-J8/CFA- and J8/CFA-immunized mice. In mice immunized with LCP-J8 alone, there was a strong IgG1 response, and IgG3 was detected in only one mouse. A few of the mice (50%) demonstrated an IgG2a response (exp 2), and IgG2b was barely detected in either experiment. No IgG2a was detected in exp 1. Our data show that the LCP-J8 construct was more immunogenic in CFA than was monomeric J8 peptide given in CFA and was also immunogenic when delivered in the absence of adjuvant. LCP-based vaccine candidates incorporating differing domains of Chlamydia trachomatis outer membrane protein have also been shown in previous studies to significantly enhance peptide immunogenicity (28). In addition, it has been shown that an LCP construct incorporating a foot-and-mouth disease viral peptide was immunogenic, resulting in the induction of antipeptide antibodies in the absence of additional adjuvant (25).
FIG. 2.
Serum IgG antibody response in B10.BR mice immunized parenterally with the LCP-J8 construct in the presence and absence of CFA for exp 1 (A) and exp 2 (B). Antibody titers to the J8 peptide for individual mice are shown, with the average titers (geometric means) represented as bars. Antibody titers to J8 are shown for individual mice from the control group that had been immunized with J8 in CFA. The two experiments were performed separately and differ in that mice received four booster injections of immunogen in exp 1 and five booster injections in exp 2. Using analysis of variance (with Tukey's post hoc method for multiple comparisons), antibody titers were significantly greater in mice immunized with LCP-J8/CFA than in those immunized with J8 in CFA alone (exp 1; ∗, P < 0.001). Significant differences in antibody titers were also observed as indicated (∗∗, P < 0.001; ∗∗∗, P < 0.001; ∗∗∗∗, P < 0.001).
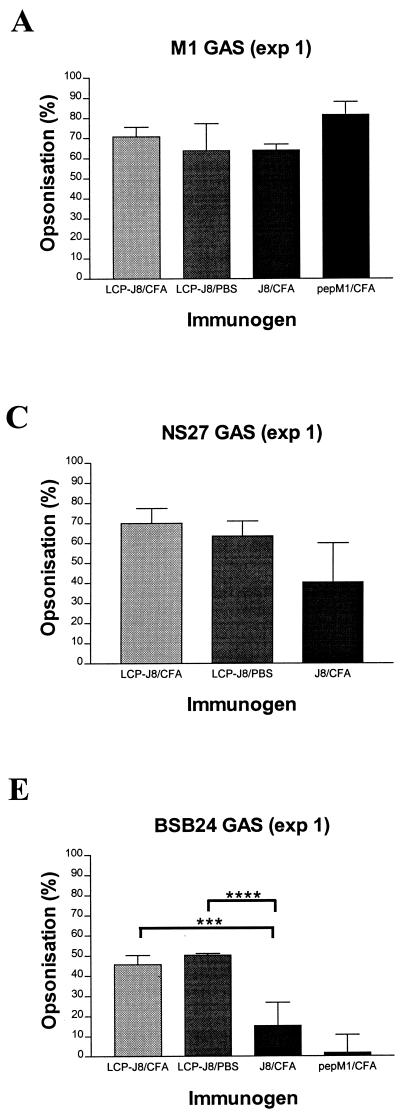
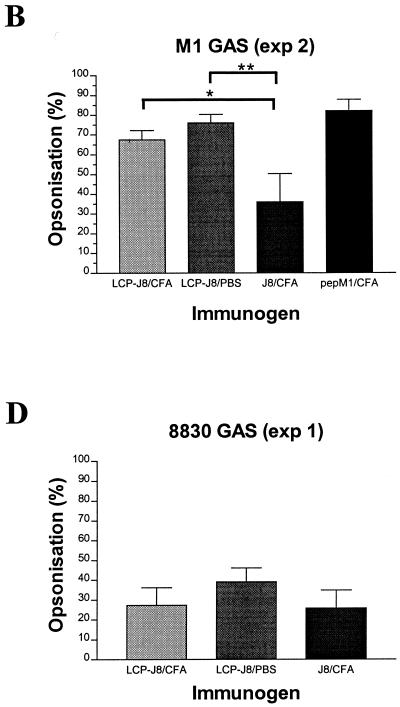
Using an in vitro indirect bactericidal assay (17, 18) which compares the growth of bacterial population (in CFU) following incubation with immune sera to that with control normal mouse sera obtained from mice immunized with adjuvant alone, we assessed the opsonic activity (measured as the percentage of reduction in bacterial population [in CFU]) of serum IgG antibodies elicited after parenteral delivery of LCP-J8 to the homologous M1 GAS strain (Table 1 and Fig. 3). The average levels of opsonization of M1 GAS by J8/CFA antisera generated in exp 1 and exp 2 were 64% and 36%, respectively, and the average level of opsonization of the pepM1 positive control was 82% for each experiment. The average levels of opsonization for LCP-J8/CFA antisera against M1 were 71% (exp 1) and 68% (exp 2) and for LCP-J8 antisera against M1 were 64% (exp 1) and 76% (exp 2). Thus, results from the opsonization of M1 GAS by LCP-J8/CFA and LCP-J8/PBS antisera indicated that serum opsonic antibodies were induced in mice following immunization with the LCP-J8 construct. Moreover, the induction of opsonic antibodies was not dependent on the presence of a conventional adjuvant, supporting expectations of high efficacy of the LCP system as a self-adjuvanting vaccine delivery modality. To confirm the specificity of the opsonic antibodies induced in LCP-J8-immunized mice, a peptide inhibition bactericidal assay was performed which involved the preincubation of immune sera with either 100 μg of J8 peptide, nonspecific peptide from Schistosoma (EGKVSTLPLDIQIIAATMSK), or no peptide prior to the assessment of opsonization. The levels of opsonization of M1 GAS by pooled LCP-J8/CFA and LCP-J8 antisera in the absence of peptide were 37% and 55%, respectively. Preincubation of LCP-J8/CFA antisera with J8, however, led to the complete inhibition of opsonization. There was a 91% inhibition of opsonization for LCP-J8 antisera preincubated with J8. The nonspecific peptide was shown to inhibit opsonization of LCP-J8/CFA and LCP-J8 antisera by 12% and 27%, respectively. Incubation of pepM1 antisera with J8 also had little effect on opsonization (23% inhibition of opsonization), consistent with the fact that pepM1 represents the amino-terminal half of M protein only. Together, these data indicate that LCP-J8 induced serum opsonic antibodies specifically directed against the M protein conserved C-region J8 peptide epitope on GAS and that these antibodies may potentially be important in protective immunity against GAS.
TABLE 1.
Opsonization of GAS by LCP-J8 and control antiseraa
| GAS strain | No. of bacteria in GAS inoculum (CFU) | No. of bacteria in normal mouse serum (mean CFU) | Immunogen | No. of bacteria in immune serum (mean CFU) | % Average population reduction (CFU) |
|---|---|---|---|---|---|
| M1 (exp 1) | 110 | 2,393 | LCP-J8/CFA | 694 | 71 |
| LCP-J8/PBS | 863 | 64 | |||
| J8/CFA | 862 | 64 | |||
| PepM1/CFA | 435 | 82 | |||
| M1 (exp 2) | 17 | 956 | LCP-J8/CFA | 310 | 68 |
| LCP-J8/PBS | 229 | 76 | |||
| J8/CFA | 611 | 36 | |||
| PepM1/CFA | 169 | 82 | |||
| NS27 (exp 1) | 20 | 6,496 | LCP-J8/CFA | 1,959 | 70 |
| LCP-J8/PBS | 2,347 | 64 | |||
| J8/CFA | 3,880 | 40 | |||
| 88/30 (exp 1) | 40 | 5,496 | LCP-J8/CFA | 3,990 | 27 |
| LCP-J8/PBS | 3,350 | 39 | |||
| J8/CFA | 4,072 | 26 | |||
| BSB24 (exp 1) | 33 | 32,254 | LCP-J8/CFA | 17,519 | 46 |
| LCP-J8/PBS | 16,002 | 50 | |||
| J8/CFA | 27,342 | 15 | |||
| PepM1/CFA | 31,697 | 2 |
By using an indirect bactericidal assay and four different GAS strains, the means of bacterial populations (in CFU) from individual serum samples plated out in duplicate were determined following incubation of GAS of a known inoculum size in the presence of immune sera (LCP-J8/CFA, LCP-J8/PBS, J8/CFA, and pepM1/CFA) and control normal mouse sera obtained from mice immunized with CFA in PBS alone.
The average percentages of bacterial population reduction (in CFU) were determined by comparing the mean CFU of immune sera to those of control sera.
FIG. 3.
Opsonization of GAS by LCP-J8/CFA, LCP-J8/PBS, and control (J8/CFA and pepM1/CFA) B10.BR mouse antisera for exp 1 (panels A and C to E) and exp 2 (B). The average percentage of opsonization (measured as the percentage of reduction in bacterial population [in CFU]) for each individual group is shown with the standard error of the mean represented as a bar. Opsonization was determined by an indirect bactericidal assay which compares the growth of bacterial population (in CFU) following incubation in the presence of immune sera to that of control normal mouse sera obtained from mice immunized with adjuvant in PBS alone. Using analysis of variance (with Tukey's post hoc method for multiple comparisons), opsonization against M1 and BSB24 GAS was significantly greater in mice immunized with LCP-J8/CFA and LCP-J8/PBS than in those immunized with J8 in CFA alone. ∗, P = 0.051; ∗∗, P = 0.008; ∗∗∗, P = 0.031; ∗∗∗∗, P = 0.013.
This finding prompted us to determine whether the serum antibodies induced by LCP-J8 could opsonize other GAS strains and might possibly be involved in mediating broadly protective immune responses and, secondly, whether heart cross-reactive antibodies were elicited. We therefore assessed the opsonic ability of the LCP-J8 antisera against three other GAS strains—NS27, 8830, and BSB24 (Table 1 and Fig. 3). Both NS27 and 8830 contain sequences identical to that of J8 in the C region, whereas BSB24 has disparities in three amino acids in the sequence SREAKKKVEADL (the three amino acids are indicated by boldface). LCP-J8/CFA and LCP-J8 antisera opsonized NS27 GAS with average levels of opsonization of 70% and 64%, respectively. Less opsonization was observed against 8830 GAS (27% for LCP-J8/CFA antisera and 39% for LCP-J8 antisera). In the case of BSB24, LCP-J8/CFA and LCP-J8 antisera opsonized GAS with averages of 46% and 50% opsonization, respectively. J8/CFA antisera opsonized NS27, 8830, and BSB24 GAS with average levels of opsonization of 40%, 26%, and 15%, respectively. Antiserum to pepM1 was shown not to opsonize the heterologous GAS strain, BSB24. The induction of serum opsonic antibodies against different GAS strains following immunization of mice with LCP-J8 supports expectations of the efficacy of a broadly protective GAS vaccine based on the conserved region of the GAS M protein. Moreover, using standard sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blot analysis (18), no cross-reactivity of LCP-J8 antisera to heart proteins in human heart and mitral valve extracts was observed.
This report has demonstrated that a GAS vaccine construct, incorporating multiple copies of a nonhost cross-reactive conserved region determinant of M protein, is highly immunogenic when administered parenterally to mice. Moreover, immunization with the construct led to the induction of heterologous opsonic antibodies, even when the construct was delivered in the absence of additional adjuvant. These findings indicate the potential utility of the LCP system in the delivery of a synthetic GAS vaccine with self-adjuvanting properties with a view to the development of a mucosa-based vaccine for human application.
Acknowledgments
This work was supported by the National Health and Medical Research Council of Australia, the National Heart Foundation of Australia, and the Cooperative Research Centre for Vaccine Technology, to which we express our thanks.
We also thank Ben Tsang for his help in the synthesis of LCP-J8, David Purdie (QIMR) for statistical analysis of the data, and Kadaba Sriprakash (QIMR) for critically reviewing the manuscript.
Editor: J. T. Barbieri
REFERENCES
- 1.BenMohamed, L., H. Gras-Masse, A. Tartar, P. Daubersies, K. Brahimi, M. Bossus, A. Thomas, and P. Druilhe. 1997. Lipopeptide immunization without adjuvant induces potent and long-lasting B, T helper, and cytotoxic T lymphocyte responses against a malaria liver stage antigen in mice and chimpanzees. Eur. J. Immunol. 27:1242-1253. [DOI] [PubMed] [Google Scholar]
- 2.Bessen, D., and V. A. Fischetti. 1988. Influence of intranasal immunization with synthetic peptides corresponding to conserved epitopes of M protein on mucosal colonization by group A streptococci. Infect. Immun. 56:2666-2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bessen, D., and V. A. Fischetti. 1990. Synthetic peptide vaccine against mucosal colonization by group A streptococci. I. Protection against a heterologous M serotype with shared C repeat region epitopes. J. Immunol. 145:1251-1256. [PubMed] [Google Scholar]
- 4.Brandt, E. R., K. S. Sriprakash, R. I. Hobb, W. A. Hayman, W. Zeng, M. R. Batzloff, D. C. Jackson, and M. F. Good. 2000. Novel multi-epitope strategy for a group A streptococcal vaccine designed for the Australian Aboriginal population. Nat. Med. 6:455-459. [DOI] [PubMed] [Google Scholar]
- 5.Bronze, M. S., H. S. Courtney, and J. B. Dale. 1992. Epitopes of group A streptococcal M protein that evoke cross-protective local immune responses. J. Immunol. 148:888-893. [PubMed] [Google Scholar]
- 6.Dale, J. B., and E. H. Beachey. 1985. Multiple, heart-cross-reactive epitopes of streptococcal M proteins. J. Exp. Med. 161:113-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dale, J. B., E. Y. Chiang, and W. Lederer. 1993. Recombinant tetravalent group A streptococcal M protein vaccine. J. Immunol. 151:2188-2194. [PubMed] [Google Scholar]
- 8.Dale, J. B., M. Simmons, E. C. Chiang, and E. Y. Chiang. 1996. Recombinant, octavalent group A streptococcal M protein vaccine. Vaccine 14:944-948. [DOI] [PubMed] [Google Scholar]
- 9.Deres, K., H. Schild, K. H. Wiesmuller, G. Jung, and H. G. Rammensee. 1989. In vivo priming of virus-specific cytotoxic T lymphocytes with synthetic lipopeptide vaccine. Nature 342:561-564. [DOI] [PubMed] [Google Scholar]
- 10.Fischetti, V. A. 1989. Streptococcal M protein: molecular design and biological behavior. Clin. Microbiol. Rev. 2:285-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Froude, J., A. Gibofsky, D. R. Buskirk, A. Khanna, and J. B. Zabriskie. 1989. Cross-reactivity between streptococcus and human tissue: a model of molecular mimicry and autoimmunity. Curr. Top. Microbiol. Immunol. 145:5-26. [DOI] [PubMed] [Google Scholar]
- 12.Gibbons, A. W., R. A. Hughes, A. Szeto, M. Charalambous, A. Aulabaugh, P. Mascagni, and I. Toth. 1990. Lipidic peptides I. Synthesis resolution and structural elucidation of fatty amino acids and their homo- and hetero-oligomers. Liebigs Ann. Chem. 1990:1175-1183. [Google Scholar]
- 13.Guilherme, L., S. E. Oshiro, K. C. Fae, E. Cunha-Neto, G. Renesto, A. C. Goldberg, A. C. Tanaka, P. M. A. Pomerantzeff, M. H. Kiss, C. Silva, F. Guzman, M. E. Patarroyo, S. Southwood, A. Sette, and J. Kalil. 2001. T-cell reactivity against streptococcal antigens in the periphery mirrors reactivity of heart-infiltrating T lymphocytes in rheumatic heart disease patients. Infect. Immun. 69:5345-5351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayman, W. A., E. R. Brandt, W. A. Relf, J. Cooper, A. Saul, and M. F. Good. 1997. Mapping the minimal murine T cell and B cell epitopes within a peptide vaccine candidate from the conserved region of the M protein of group A streptococcus. Int. Immunol. 9:1723-1733. [DOI] [PubMed] [Google Scholar]
- 15.Mora, A. L., and J. P. Tam. 1998. Controlled lipidation and encapsulation of peptides as a useful approach to mucosal immunizations. J. Immunol. 161:3616-3623. [PubMed] [Google Scholar]
- 16.Nardelli, B., P. B. Haser, and J. P. Tam. 1994. Oral administration of an antigenic synthetic lipopeptide (MAP-P3C) evokes salivary antibodies and systemic humoral and cellular responses. Vaccine 12:1335-1339. [DOI] [PubMed] [Google Scholar]
- 17.Pruksakorn, S., B. Currie, E. Brandt, D. Martin, A. Galbraith, C. Phornphutkul, S. Hunsakunachai, A. Manmontri, and M. F. Good. 1994. Towards a vaccine for rheumatic fever: identification of a conserved target epitope on M protein of group A streptococci. Lancet 344:639-642. [DOI] [PubMed] [Google Scholar]
- 18.Pruksakorn, S., A. Galbraith, R. A. Houghten, and M. F. Good. 1992. Conserved T and B cell epitopes on the M protein of group A streptococci: induction of bactericidal antibodies. J. Immunol. 149:2729-2735. [PubMed] [Google Scholar]
- 19.Quinn, A., S. Kosanke, V. A. Fischetti, S. M. Factor, and M. W. Cunningham. 2001. Induction of autoimmune valvular heart disease by recombinant streptococcal M protein. Infect. Immun. 69:4072-4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Relf, W. A., J. Cooper, E. R. Brandt, W. A. Hayman, R. F. Anders, S. Pruksakorn, B. Currie, A. Saul, and M. F. Good. 1996. Mapping a conserved conformational epitope from the M protein of group A streptococci. Pept. Res. 9:12-20. [PubMed] [Google Scholar]
- 21.Robinson, J. H., and A. Kehoe. 1992. Group A streptococcal M protein: virulence factors and protective antigens. Immunol. Today 13:362-367. [DOI] [PubMed] [Google Scholar]
- 22.Schnölzer, M., P. Alewood, A. Jones, D. Alewood, and S. B. H. Kent. 1992. In situ neutralization in Boc-chemistry solid phase peptide synthesis. Rapid, high yield assembly of difficult sequences. Int. J. Pept. Protein Res. 40: 180-193. [DOI] [PubMed] [Google Scholar]
- 23.Tam, J. P., and Y.-A. Lu. 1989. Enhancement of immunogenicity of synthetic peptide vaccines related to hepatitis in chemically defined models consisting of T- and B-cell epitopes. Proc. Natl. Acad. Sci. USA 86:9084-9088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toth, I., M. Danton, N. Flinn, and W. A. Gibbons. 1993. A combined adjuvant and carrier system for enhancing synthetic peptides immunogenicity utilizing lipidic amino acids. Tetrahedron Lett. 34:3925-3928. [Google Scholar]
- 25.Toth, I., N. Flinn, W. A. Gibbons, M. Good, W. Hayman, and F. Brown. 1996. Immunological evaluation of the lipid-core-peptide (LCP) adjuvant/carrier system, p. 810-811. In T. Pravin, P. Kaumaya, and R. S. Hodges (ed.), Peptides: chemistry, structure and biology. Mayflower Scientific Ltd., Kingswinford, United Kingdom.
- 26.Wiesmuller, K. H., W. Bessler, and G. Jung. 1983. Synthesis of the mitogenic S-[2,3-bis(palmitoyloxy)propyl]-N-palmitoylpentapeptide from Escherichia coli lipoprotein. Hoppe-Seyler's Z. Physiol. Chem. 364:593-606. [DOI] [PubMed] [Google Scholar]
- 27.Zeng, W., D. C. Jackson, J. Murray, K. Rose, and L. E. Brown. 2000. Totally synthetic lipid-containing polyoxime peptide constructs are potent immunogens. Vaccine 18:1031-1039. [DOI] [PubMed] [Google Scholar]
- 28.Zong, G., I. Toth, R. Reid, and R. C. Brunham. 1993. Immunogenicity evaluation of a lipidic amino acid-based synthetic peptide vaccine for Chlamydia trachomatis. J. Immunol. 151:3728-3736. [PubMed] [Google Scholar]