Abstract
Cell extracts from Yersinia pseudotuberculosis induced multinucleation in HEp-2 cells in a manner similar to the effect caused by Escherichia coli cytotoxic necrotizing factor (CNF). The activity was not dependent on the Yersinia 70-kb virulence plasmid, and the activity was not inhibited by antibodies capable of neutralizing E. coli CNF type 1. The nucleotide sequence of the Yersinia cnf gene was 65.1% identical to the E. coli cnf gene.
Cytotoxic necrotizing factor (CNF) is a protein toxin (110 to 115 kDa) that is prevalent among Escherichia coli isolates from humans and domesticated animals with extraintestinal infections (8; reviewed in references 16 and 27). Caprioli and coworkers (11) identified the prototypical E. coli CNF type 1 (CNF1), and CNF1 is the best-characterized member of an emergent class of dermonecrotic bacterial toxins that constitutively activate eukaryotic small GTP-binding proteins (GTPases) (21, 31, 42, 46). However, there are independent descriptions of a dermonecrotic protein exotoxin in sterile broth culture supernatants from Yersinia pseudotuberculosis (9, 24, 29, 41). This report describes the identification of a CNF from Y. pseudotuberculosis. For clarity, the CNF and the gene encoding the toxin from Yersinia pseudotuberculosis will be referred to, respectively, as CNFY and cnfY. The gene and gene product from E. coli will be referred to, respectively, as cnfE and CNFE.
The following Yersinia species were examined: Y. pseudotuberculosis (33 strains from serogroups IA, IB, II, III, and V), Y. enterocolitica (9 strains from serogroups O3; O4,32; O5,27; O6; O8; O9; O20; O21; and O27), Y. bercovieri (3 strains), Y. frederiksenii (4 strains), Y. intermedia (3 strains), Y. kristensenii (3 strains), Y. mollaretii (2 strains), and Y. rohdei (1 strain). E. coli strain J96 (CNF1+) was the source of E. coli CNFE as previously described (39). E. coli strain DH5α (Life Technologies, Inc., Gaithersburg, Md.) was the host for cloned cnf genes. pHLK102 is a clone of the E. coli cnfE gene (39), and pHLK602 expresses the cnfY gene cloned from Y. pseudotuberculosis strain YPIII as part of the present work. Bacteria were grown for 18 to 24 h at 26 or 37°C in L broth (30) or tryptic soy broth (Difco Laboratories, Inc., Detroit, Mich.), and on L agar (30). Broth cultures were aerated on a shaker (200 rpm) or on a roller drum. Strains containing recombinant plasmids were cultured in media containing ampicillin (100 μg/ml) or chloramphenicol (30 μg/ml).
CNF biological activity and specific activity were assayed with HEp-2 cells as previously described (34, 39). Tryptic soy broth cultures were centrifuged at 4°C to separate the bacteria from the culture broth supernatants, and broth culture supernatants were filter sterilized through 0.2-μm-pore-size low protein-binding membranes (Millipore Corp., Bedford, Mass.). Bacterial cells were concentrated 10-fold in phosphate-buffered saline containing gentamicin (100 μg/ml) and lysed by sonication or by three cycles of freezing and thawing (35). Bacterial lysates were clarified by centrifugation at 4°C and filter sterilized. Cell-free lysates and sterile supernatants were diluted in 0.1 ml of Eagle minimum essential medium with Earle's balanced salt solution (BioWhittaker, Inc., Walkersville, Md.) containing 10% fetal bovine serum (BioWhittaker, Inc.), gentamicin (100 μg/ml), penicillin (100 U/ml), and streptomycin (100 μg/ml) and added to HEp-2 cells cultured in the same medium. The cultures were incubated for 72 h at 37°C in an atmosphere containing 5% CO2. Culture fluid was then removed, and the cells were fixed and stained with a modified Wright's stain (Hema 3; Fisher Scientific Co.). Stained cells were examined by bright-field microscopy and digitally photographed with a Kodak DC120 camera. The 50% cytopathic dose (CD50) was determined by the dilution of a sample that produced multinucleation in 50% of the cells in a well of the tissue culture plate. Specific activity was defined as the number of CD50s per microgram of protein in a sample. The amount of protein in bacterial cell-free lysates and sterile supernatants was quantitated spectrophotometrically (BCA Protein Assay Kit; Pierce Chemical Co., Rockford, Ill.), and bovine serum albumin was used as a standard. Neutralization of biological activity by specific antibodies was assayed in vitro with HEp-2 cells as previously described (34, 39).
Standard methods were used for DNA purification and cloning (32, 40). Restriction endonucleases (New England Biolabs, Beverly, Mass.) and DNA-modifying enzymes (New England Biolabs and Roche Molecular Biochemicals, Indianapolis, Ind.) were used according to the manufacturers' specifications. Restriction enzyme digestion products and PCR amplification products were transferred from agarose gels to nylon membranes under alkaline conditions (38). Dot blots were prepared by denaturing samples of DNA with 0.5 M NaOH and applying the samples to a nylon membrane filter (Minifold; Schleicher & Schuell, Keene, N.H.). Membranes were hybridized with horseradish peroxidase-labeled probes (described below), washed, and exposed to X-ray film according to the manufacturer's specifications (ECL Direct Labeling and Detection System; Amersham-Pharmacia Biotech, Piscataway, N.J.). Autoradiograms were scanned, and the digital images were annotated with Canvas (Deneba Systems, Inc., Miami, Fla.). The cnfE probe was a 3.0-kb BamHI-EcoRI gel-purified fragment from pHLK102 (39), and the cnfY probe from pHLK602 was a 2.8-kb BsaI-NcoI fragment.
The PCR was done as previously described (39) with commercially synthesized primers (Life Technologies, Inc.) that were designed to amplify cnf sequences from E. coli (19) and Yersinia (37). The following primers were used to amplify E. coli cnfE: forward, 5′-TATTAATCTTCACAGAGGAG-3′; reverse, 5′-CCGGTTATTTATTAAAGGGCTTAG-3′. The following primers were used to amplify Yersinia cnfY: forward, 5′-TGCATCGTCAATAAAAGGAGTGTT-3′; reverse, 5′-CAATTTGGTTTTACTGGTGGTTCA-3′. The sequences of the Yersinia cnfY primers correspond to nucleotides 800 to 823 and 2918 to 2941 of the sequence that was determined in this study.
DNA sequencing was performed with dichlororhodamine terminator cycle-sequencing reagents (Perkin-Elmer, Foster City, Calif.) according to the manufacturer's specifications, and automated sequencing was performed on an ABI Prism 377 instrument. Universal primers were used to sequence the ends of cloned DNAs, and custom primers were designed from the Y. pseudotuberculosis strain YPIII sequences that were obtained. These custom primers were used to complete the sequencing of the cloned DNA, and they were also used in direct sequencing of genomic DNA. The data reported here were obtained by sequencing both strands of the template. BLAST (3) alignments were performed with data at the National Center for Biotechnology Information (NCBI) (at the website http://www.ncbi.nlm.nih.gov/blast/) and from the Sanger Center (at the website http://www.sanger.ac.uk/Projects/Y_pestis/) (37). Additional analyses of DNA sequences were performed with the software packages Lasergene, version 4.0.3 (DNAStar, Inc., Madison, Wis.) and the Wisconsin Package, version 10.1-Unix (Genetics Computer Group, Madison, Wis.).
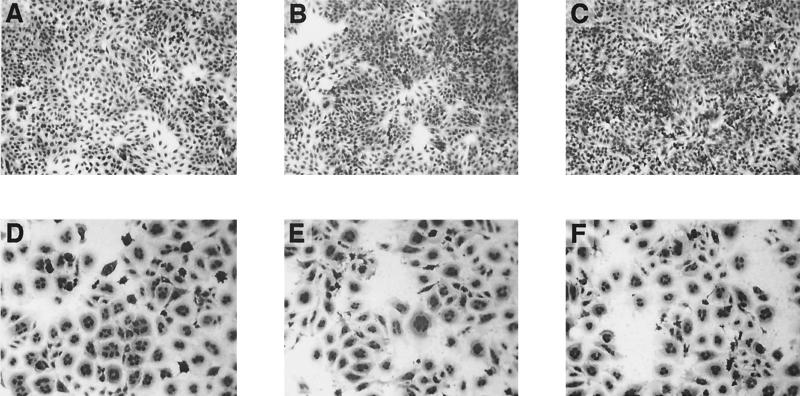
Broth supernatants and cell-free lysates from cultures of different species of Yersinia were tested for cytopathic activity. A total of 58 strains of Yersinia were examined, including 33 strains of Y. pseudotuberculosis. HEp-2 cells treated with extracts from Y. pseudotuberculosis strains YPIII (7, 22) and IP2666c (cured of the 70-kb virulence plasmid [45]) developed into giant, multinucleated cells that remained viable and attached to the culture substrate (Fig. 1). Y. pseudotuberculosis strain YPIIIc, a derivative of strain YPIII that was cured of the virulence plasmid (7, 22), also expressed CNFY activity when grown in vitro at 26 or 37°C. CNFY activity was not detected in other strains of Y. pseudotuberculosis (Table 1 and data not shown) or from additional Yersinia species (Fig. 1B and C). Additional preliminary experiments indicated that CNFY activity was constitutively expressed throughout the growth cycle of Y. pseudotuberculosis and expression was not regulated by temperature or [Ca2+] (not shown). The morphology of cells treated with extracts from CNFY+ Y. pseudotuberculosis was indistinguishable from that caused by E. coli CNFE (compare Fig. 1D with Fig. 1E and F). The specific activity in cell-free lysates of Y. pseudotuberculosis strain YPIII was 38-fold higher than that of the CNFE+ E. coli strain J96, and a substantial amount of CNFY activity (103 CD50s/μg of protein) was found in the supernatants from broth cultures of CNFY+ Y. pseudotuberculosis strains (Fig. 1E). CNFE activity from E. coli strain J96 was cell associated, and no biological activity was detected in filter-sterilized broth-culture supernatants, an observation that was consistent with previous studies of CNFE (11, 23). Y. pseudotuberculosis strains YPIII and IP2666c belong to serogroup III (7, 22, 45), whose strains have been previously reported to produce a dermonecrotic protein exotoxin (9, 24, 26, 29, 41, 47). In the present work, however, additional serogroup III strains did not express CNFY biological activity and these strains contained deletions within the cnfY gene (described below). Thus, no relationship was found between serogroup and toxin production.
FIG. 1.
HEp-2 cell tissue culture assay for CNF biological activity. (A) Untreated control; (B) broth culture supernatant from the CNFY− Y. enterocolitica strain 8081v; (C) cell-free lysate from CNFY− Y. enterocolitica strain 8081v; (D) cell-free lysate from the CNFE+ E. coli strain J96; (E) broth culture supernatant from the CNFY+ strain Y. pseudotuberculosis strain YPIII; (F) cell-free lysate from the CNFY+ strain Y. pseudotuberculosis strain YPIII. All photomicrographs (panels A through F) were taken at the same magnification (10× lens objective) to clearly depict the giant, multinucleated phenotype of cells in panels D to F. Cells were digitally photographed by light microscopy with a Kodak DC120 camera, and the image file was annotated with Canvas.
TABLE 1.
Distribution of cnfY among Y. pseudotuberculosis strains
| Straina | CNFY biological activityb | Approx size (kb) of cnfY allelec | HPId | Serotype | Source (reference) |
|---|---|---|---|---|---|
| YPIII | + | 3.0 | − | III | V. L. Miller (7, 22) |
| YPIIIc | + | 3.0 | − | III | V. L. Miller (7, 22) |
| 713425 | − | 2.1 | − | IA | V. L. Miller |
| 722080 | − | 2.1 | − | IB | V. L. Miller |
| 730317 | − | 2.1 | − | I | V. L. Miller |
| 730440-1 | − | 1.8 | + | IB | V. L. Miller |
| ATCC 29833 | − | 1.8 | NDe | ND | ATCCf |
| PTB1 | − | 1.8 | + | ND | K. A. McDonough (25) |
| PTB4 | − | 1.8 | + | IA | K. A. McDonough (25) |
| PTB6 | − | 1.8 | + | III | K. A. McDonough (25) |
| PTB7 | − | 1.8 | + | ND | K. A. McDonough (25) |
| PTB8 | − | 1.8 | + | III | K. A. McDonough (25) |
| PTB9 | − | 1.8 | + | IA | K. A. McDonough (25) |
| PTB13 | − | 2.1 | − | I | K. A. McDonough (25) |
| PTB16 | − | 1.8 | − | III | K. A. McDonough (25) |
| PTB20 | − | 2.1 | − | IA | K. A. McDonough (25) |
| PTB22 | − | 1.8 | − | IA | K. A. McDonough (25) |
| IP2515c | − | 2.1 | ND | II | J. B. Bliska (45) |
| IP2666c | + | 3.0 | ND | III | J. B. Bliska (45) |
| IP2775c | − | 1.8 | ND | I | J. B. Bliska (45) |
| IP2777c | − | 1.8 | ND | I | J. B. Bliska (45) |
| IP2790c | − | 1.8 | ND | I | J. B. Bliska (45) |
| IP32953 | − | 1.8 | + | I | E. Carniel (10) |
Strains with names ending in ‘c' are cured of the Yersinia virulence plasmid.
Sterile cell extracts induced the formation of giant, multinucleated HEp-2 cells.
Three cnfY alleles were identified in this study: 3.0 is the size of a full-length gene shown in Fig. 2 and 3; 2.1 is the size of a 3′-deleted gene also found in Y. pestis strain CO92 (37) and shown in Fig. 3; 1.8 is the size of a variant with 5′ and 3′ deletions.
HPI, Yersinia high-pathogenicity island.
ND, not determined.
ATCC, American Type Culture Collection, Manassas, Va.
A DNA probe prepared from the cloned E. coli cnfE gene hybridized to a single restriction endonuclease fragment in a Southern blot of total DNA from Y. pseudotuberculosis strain YPIII (not shown). However, PCR primers that were designed to amplify cnfE from E. coli (39) did not amplify sequences from Y. pseudotuberculosis strain YPIII. A large open reading frame (ORF), with a high level of similarity to the amino acid sequence of E. coli CNFE, was identified by a TBLASTN search of the Y. pestis genome sequence (37), and PCR primers designed from the cnfY sequence in Y. pestis were used to amplify a 2.1-kb product from Y. pseudotuberculosis strain YPIII. This PCR product was cloned and used as a probe to identify a 5.4-kb chromosomal fragment from Y. pseudotuberculosis strain YPIII DNA that was digested with BamHI and SalI (not shown). This fragment was cloned into pBRKS (44) to create pHLK602, which expressed CNFY biological activity in E. coli K-12 strain DH5α. Additional evidence that the Y. pseudotuberculosis cnfY sequence was responsible for the observed biological activity was obtained by inserting an Ω-Cmr fragment (20) into the chromosomal cnfY gene of Y. pseudotuberculosis strain YPIII by allelic exchange (17). The derivative with the cnfY:: Ω-Cmr mutation, named strain WEX5000, was confirmed by PCR and Southern blotting and expressed no CNFY activity in the HEp-2 tissue culture assay. The mutation in strain WEX5000 was complemented by pHLK602.
Analysis of the sequence of the Yersinia DNA cloned in pHLK602 identified a large ORF with a predicted translation product that was highly similar to CNFE. There are two alleles of E. coli cnf (cnf1 and cnf2) whose DNA and predicted amino acid sequences are ≥84% identical (19, 36). When the DNA or predicted amino acid sequences of cnfY from Y. pseudotuberculosis were compared to either of the two E. coli sequences, there was ≤1% difference in the results (not shown). Thus, the sequence of cnf1 (19) was used for the comparisons of cnfY and cnfE reported here. The cnfY ORF was 3,045 bp in size and had 65.1% sequence identity with cnfE. The predicted amino acid sequence of CNFY was 60.8% identical and 68.5% similar to that of CNFE (Fig. 2). The homology between CNFY and CNFE was evenly distributed throughout the entire sequence. The two largest areas of dissimilarity were 5 amino acids (aa) in length, there was one region containing four nonconservative amino acid changes, and all of the remaining dissimilarities were 3 aa in length, or smaller. Cysteine and histidine residues that were essential for the biological activity of CNFE (43) were conserved in the predicted sequence of CNFY (Fig. 2). The DNA sequences flanking cnfY had significant similarities to transposases (BLASTX E value, 5e-36) and oxidoreductases (BLASTX E value, 7e-40) (Fig. 3). The overall content of guanosine and cytosine (percent G+C) in the DNA that was sequenced in this study was 39% (Fig. 3), but there was a noticeable demarcation. The percent G+C for cnfY and the DNA 5′ to the gene was 34%, while 3′ to the cnfY gene the sequence contained 51% G+C. Taken together, the sequence data suggested that the cnfY gene was introduced into Y. pseudotuberculosis from another bacterium.
FIG. 2.
Alignment of the predicted amino acid sequences of CNFY (Y) from strain YPIII and CNFE (E) by the method of Lipman and Pearson. Conserved amino acids that were shown to be essential for the activity of CNFE (Cys866 and His881) (43) are bolded and marked by asterisks under those positions, and identical (|) or similar (:, .) residues are indicated.
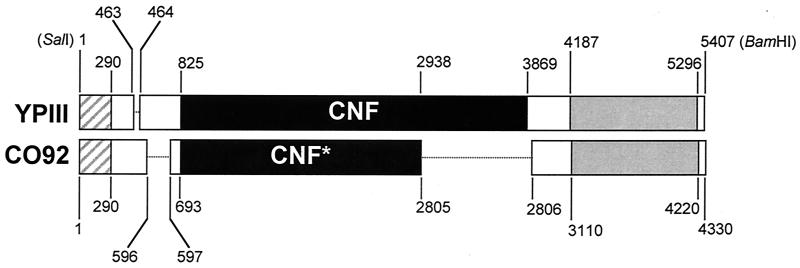
FIG. 3.
Comparison of cnfY DNA sequences from Y. pseudotuberculosis strain YPIII and Y. pestis strain CO92. Boxes indicate sequences that are ≥99% identical in the two species, and dotted lines indicate deletions. Nucleotide positions are numbered according to the sequence present in each species. The BamHI and SalI sites used in the cloning of cnfY from strain YPIII also are shown. ▨, putative transposase; □, intervening regions; ▪, CNFY; ░⃞, putative oxidoreductase.
Despite the considerable identity between the DNA and predicted amino acid sequences of cnfY and cnfE, the CNFY biological activity in extracts from Y. pseudotuberculosis strain YPIII and E. coli K-12 strain DH5α(pHLK602) was not neutralized by goat polyclonal antibodies prepared against purified E. coli CNFE (34). The antibodies completely prevented the multinucleating activity of E. coli CNFE when they were used at a final dilution of up to 1:80, but there was no inhibition of the activity from Y. pseudotuberculosis strain YPIII with final antibody dilutions as low as 1:10 (not shown). Preimmune sera had no effect on the activity of either CNF. Identical results were obtained with a mouse monoclonal antibody (33).
The DNA sequence that was determined from Y. pseudotuberculosis strain YPIII in this study was compared to a similar sequence in the genome database of Y. pestis strain CO92 (37) (Fig. 3). There was ≥99% identity between the DNA sequences that were present in both species. However, there were deletions unique to each species. The sequence from Y. pestis contained the first 2,114 bp of the cnfY ORF, but there was a 931-bp deletion at the 3′ end of the gene. Distal to the deletion, Y. pestis retained the 3′ flanking sequence with similarity to oxidoreductases. The sequences preceding cnfY in both species also contained deletions in the intervening region between the putative transposase and the start of the cnfY gene (Fig. 3).
Chromosomal DNAs from pathogenic and nonpathogenic species of Yersinia were examined for the presence of the cnfY gene. All of the isolates of Y. pseudotuberculosis (n = 33) that were examined contained cnfY DNA that hybridized to the cnfY probe (representative results from seven strains of Y. pseudotuberculosis are shown in Fig. 4) or amplified in a PCR with cnfY-specific primers (not shown). The cnfY gene was detected in Y. pseudotuberculosis strain YPIIIc and seven other Y. pseudotuberculosis strains cured of the 70-kb virulence plasmid. cnfY sequences were not detected in Y. enterocolitica or in nonpathogenic Yersinia species (Fig. 4). A Southern blot of Y. pseudotuberculosis DNAs showed polymorphisms at the cnfY locus (Fig. 5), and further analysis revealed that Y. pseudotuberculosis strains YPIII, YPIIIc, and IP2666c (all CNFYp+) possessed the complete cnfY gene shown in Fig. 3. All of the remaining strains of Y. pseudotuberculosis carried a single deletion like that in Y. pestis strain CO92 (Fig. 3), or they had the Y. pestis deletion and a second deletion between nucleotides 1745 and 2065 with respect to the cnfYp sequence from Y. pseudotuberculosis strain YPIII (not shown). The single cnfY deletion in Y. pestis strain CO92 (Fig. 3) was confirmed in additional independent Y. pestis isolates (H. A. Lockman and P. L. Worsham, unpublished observation). The discovery of identical cnfY deletions in Y. pseudotuberculosis and Y. pestis is consistent with the evidence that the plague bacillus is a recently evolved clone of Y. pseudotuberculosis (1). However, in an examination of a subset of Y. pseudotuberculosis strains, none of the strains that contained a plague-like cnfY allele (n = 6) possessed the Yersinia high-pathogenicity island (HPI) (12) (Table 1), a feature that distinguished them from Y. pestis. In contrast, 8 of 10 Y. pseudotuberculosis strains that were HPI+ also possessed the second cnfY deletion (described above) (Table 1) that was not found in Y. pestis. It was also evident from this analysis that only those strains of Y. pseudotuberculosis with a full-length cnfY gene expressed biological activity, and the strains that did not express CNFY activity had significant deletions of the cnfY ORF.
FIG. 4.
Dot blot of total DNAs from 33 strains of Yersinia. The blot was hybridized at high stringency with a cnfY gene probe. Individual strains of Y. pseudotuberculosis are listed by strain name or number next to the position of the DNA from that strain; +, positive control, the cloned cnfY from Y. pseudotuberculosis strain YPIII; Ye, Y. enterocolitica; Yb, Y. bercovieri; Yf, Y. frederiksenii; Yi, Y. intermedia; Yk, Y. kristensenii; Ym, Y. mollaretii; Yr, Y. rohdei. All samples were applied to the blot in duplicate. The autoradiogram was scanned, and the digital image file was annotated with Canvas.
FIG. 5.
cnfY probe hybridization to a Southern blot of chromosomal DNAs from Y. pseudotuberculosis strains. Lane 1, YPIII; lane 2, YPIIIc; lane 3, 713425; lane 4, 722080; lane 5, 730317; lane 6, 730440-1; lane 7, ATCC 29833. One microgram of each DNA was digested with NsiI. The numbers in the left margin indicate molecular size standards in kilobases. The autoradiogram was scanned, and the digital image file was annotated with Canvas.
The discovery of a chromosomally encoded CNFYp in Y. pseudotuberculosis adds a potential virulence factor to the variety of potent cytotoxins that are produced by these bacteria. Altogether, the results of this study indicated that some Y. pseudotuberculosis strains express a CNF toxin that may, like the E. coli CNF, target eukaryotic small GTPases. Despite the remarkable similarities between the biological activities and the nucleotide and predicted amino acid sequences of the cnf genes from Y. pseudotuberculosis and E. coli, the absence of neutralization of CNFY by anti-CNFE antibodies indicated that there are significant antigenic differences between the proteins. CNFY activity was found in the supernatants of broth cultures of Y. pseudotuberculosis, a result suggesting that CNFY is secreted, unlike the cell-associated E. coli CNFE (11, 23). Pathogenic strains of Yersinia also produce plasmid-encoded cytotoxins (Yops) that are substrates of a type III secretion system (2, 13-15). Like CNFs, some Yop cytotoxins profoundly alter the cytoskeletal architecture of mammalian cells by affecting the activity of GTPases. YopE is a GTPase-activating protein that negatively regulates Rho activity (6, 49), and YopT inactivates Rho via a covalent modification (50). YopH is a potent tyrosine phosphatase that acts on p130Cas and FAK, thereby inhibiting formation of focal adhesion complexes (5). YpkA (YopO) is a serine/threonine kinase that is activated by actin and subsequently inhibits Rho activity (18, 28). YpkA also binds directly to Rho and Rac (4). Understanding the combined roles of all of these toxins in the pathogenic lifestyle of Y. pseudotuberculosis awaits additional studies, but it is relevant to note that YopT is not expressed by the CNFY+ Y. pseudotuberculosis strain YPIII (48). Thus, different strains of this species may express virulence through alternate mechanisms.
Nucleotide sequence accession number.
The cnfY DNA sequence reported here, from Y. pseudotuberculosis strain YPIII, was deposited in GenBank (NCBI) under accession number AF324349.
Acknowledgments
We are indebted to Elisabeth Carniel (Institut Pasteur, Paris, France), Kathleen McDonough (State University of New York, Albany), Virginia Miller (Washington University School of Medicine, St. Louis, Mo.), Karen Birkhead and Nancy Strockbine (Centers for Disease Control and Prevention, Atlanta, Ga.), James Bliska (State University of New York, Stony Brook), and Alexander Sulakvelidze (University of Maryland School of Medicine, Baltimore) for providing the strains of Yersinia that were used in this research. We also thank Virginia Miller and James Bliska for helpful discussions. Anti-E. coli CNF1 antibodies were generously provided by Karen Meysick and Alison O'Brien (Uniformed Services University of the Health Sciences, Bethesda, Md.). We credit an anonymous reviewer for alerting us about the earlier studies on the Y. pseudotuberculosis dermonecrotic exotoxin.
Erica Mersfelder assisted with the DNA sequencing, which was done by the Core Facility at Children's Research Institute and was supported, in part, by National Institutes of Health Grant HD34615.
Editor: J. T. Barbieri
REFERENCES
- 1.Achtman, M., K. Zurth, G. Morelli, G. Torrea, A. Guiyoule, and E. Carniel. 1999. Yersinia pestis, the cause of plague, is a recently emerged clone of Yersinia pseudotuberculosis. Proc. Natl. Acad. Sci. USA 96:14043-14048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aepfelbacher, M., R. Zumbihl, K. Ruckdeschel, C. A. Jacobi, C. Barz, and J. Heesemann. 1999. The tranquilizing injection of Yersinia proteins: a pathogen's strategy to resist host defense. Biol. Chem. 380:795-802. [DOI] [PubMed] [Google Scholar]
- 3.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 4.Barz, C., T. N. Abahji, K. Trulzsch, and J. Heesemann. 2000. The Yersinia Ser/Thr protein kinase YpkA/YopO directly interacts with the small GTPases RhoA and Rac-1. FEBS Lett. 482:139-143. [DOI] [PubMed] [Google Scholar]
- 5.Black, D. S., and J. B. Bliska. 1997. Identification of p130Cas as a substrate of Yersinia YopH (Yop51), a bacterial protein tyrosine phosphatase that translocates into mammalian cells and targets focal adhesions. EMBO J. 16:2730-2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Black, D. S., and J. B. Bliska. 2000. The RhoGAP activity of the Yersinia pseudotuberculosis cytotoxin YopE is required for antiphagocytic function and virulence. Mol. Microbiol. 37:515-527. [DOI] [PubMed] [Google Scholar]
- 7.Bolin, I., L. Norlander, and H. Wolf-Watz. 1982. Temperature-inducible outer membrane protein of Yersinia pseudotuberculosis and Yersinia enterocolitica is associated with the virulence plasmid. Infect. Immun. 37:506-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonacorsi, S. P., O. Clermont, C. Tinsley, I. Le Gall, J. C. Beaudoin, J. Elion, X. Nassif, and E. Bingen. 2000. Identification of regions of the Escherichia coli chromosome specific for neonatal meningitis-associated strains. Infect. Immun. 68:2096-2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown, J. A., W. L. West, W. M. Banks, and J. D. Marshall. 1969. Some characteristics of a heat-labile toxin from Pasteurella pseudotuberculosis. J. Infect. Dis. 119:229-236. [DOI] [PubMed] [Google Scholar]
- 10.Buchrieser, C., R. Brosch, S. Bach, A. Guiyoule, and E. Carniel. 1998. The high-pathogenicity island of Yersinia pseudotuberculosis can be inserted into any of the three chromosomal asn tRNA genes. Mol. Microbiol. 30:965-978. [DOI] [PubMed] [Google Scholar]
- 11.Caprioli, A., V. Falbo, L. G. Roda, F. M. Ruggeri, and C. Zona. 1983. Partial purification and characterization of an Escherichia coli toxic factor that induces morphological cell alterations. Infect. Immun. 39:1300-1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carniel, E. 1999. The Yersinia high-pathogenicity island. Int. Microbiol. 2:161-167. [PubMed] [Google Scholar]
- 13.Cornelis, G. R. 2000. Type III secretion: a bacterial device for close combat with cells of their eukaryotic host. Philos. Trans. R. Soc. Lond. B Biol. Sci. 355:681-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cornelis, G. R. 1998. The Yersinia deadly kiss. J. Bacteriol. 180:5495-5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cornelis, G. R., and H. Wolf-Watz. 1997. The Yersinia Yop virulon: a bacterial system for subverting eukaryotic cells. Mol. Microbiol. 23:861-867. [DOI] [PubMed] [Google Scholar]
- 16.De Rycke, J., A. Milon, and E. Oswald. 1999. Necrotoxic Escherichia coli (NTEC): two emerging categories of human and animal pathogens. Vet. Res. 30:221-233. [PubMed] [Google Scholar]
- 17.Donnenberg, M. S., and J. B. Kaper. 1991. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect. Immun. 59:4310-4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dukuzumuremyi, J. M., R. Rosqvist, B. Hallberg, B. Akerstrom, H. Wolf-Watz, and K. Schesser. 2000. The Yersinia protein kinase A is a host-factor inducible RhoA/Rac-binding virulence factor. J. Biol. Chem. 275:35281-35290. [DOI] [PubMed] [Google Scholar]
- 19.Falbo, V., T. Pace, L. Picci, E. Pizzi, and A. Caprioli. 1993. Isolation and nucleotide sequence of the gene encoding cytotoxic necrotizing factor 1 of Escherichia coli. Infect. Immun. 61:4909-4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fellay, R., J. Frey, and H. Krisch. 1987. Interposon mutagenesis of soil and water bacteria: a family of DNA fragments designed for in vitro insertional mutagenesis of gram-negative bacteria. Gene 52:147-154. [DOI] [PubMed] [Google Scholar]
- 21.Flatau, G., E. Lemichez, M. Gauthier, P. Chardin, S. Paris, C. Fiorentini, and P. Boquet. 1997. Toxin-induced activation of the G protein p21 Rho by deamidation of glutamine. Nature 387:729-733. [DOI] [PubMed] [Google Scholar]
- 22.Gemski, P., J. R. Lazere, T. Casey, and P. Wohlhieter. 1980. Presence of a virulence-associated plasmid in Yersinia pseudotuberculosis. Infect. Immun. 28:1044-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonzalez, E. A., and J. Blanco. 1985. Production of cytotoxin VT in enteropathogenic and non-enteropathogenic Escherichia coli strains of porcine origin. FEMS Microbiol. Lett. 26:127-130. [Google Scholar]
- 24.Haagsma, J. 1970. Enzootic death in mink caused by an exotoxin-producing strain of Yersinia pseudotuberculosis type III. Neth. J. Vet. Sci. 3:77-84. [Google Scholar]
- 25.Hare, J. M., A. K. Wagner, and K. A. McDonough. 1999. Independent acquisition and insertion into different chromosomal locations of the same pathogenicity island in Yersinia pestis and Yersinia pseudotuberculosis. Mol. Microbiol. 31:291-303. [DOI] [PubMed] [Google Scholar]
- 26.Henriksson, K. 1962. An outbreak of pseudotuberculosis in mink. Nord. Vetmed. 14:59. [Google Scholar]
- 27.Johnson, J. R. 1991. Virulence factors in Escherichia coli urinary tract infection. Clin. Microbiol. Rev. 4:80-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Juris, S. J., A. E. Rudolph, D. Huddler, K. Orth, and J. E. Dixon. 2000. A distinctive role for the Yersinia protein kinase: actin binding, kinase activation, and cytoskeleton disruption. Proc. Natl. Acad. Sci. USA 97:9431-9436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lazarus, A. S., and M. M. Nozawa. 1948. The endotoxin of Pasteurella pseudotuberculosis. J. Bacteriol. 56:187-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lennox, E. S. 1955. Transduction of linked genetic characters of the host by bacteriophage P1. Virology 1:190-206. [DOI] [PubMed] [Google Scholar]
- 31.Lerm, M., J. Selzer, A. Hoffmeyer, U. R. Rapp, K. Aktories, and G. Schmidt. 1999. Deamidation of Cdc42 and Rac by Escherichia coli cytotoxic necrotizing factor 1: activation of c-Jun N-terminal kinase in HeLa cells. Infect. Immun. 67:496-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lockman, H. A., and R. Curtiss III. 1992. Isolation and characterization of conditional adherent and non-type 1 fimbriated Salmonella typhimurium mutants. Mol. Microbiol. 6:933-945. [DOI] [PubMed] [Google Scholar]
- 33.Meysick, K. C., M. Mills, and A. D. O'Brien. 2001. Epitope mapping of monoclonal antibodies capable of neutralizing cytotoxic necrotizing factor type 1 of uropathogenic Escherichia coli. Infect. Immun. 69:2066-2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mills, M., K. C. Meysick, and A. D. O'Brien. 2000. Cytotoxic necrotizing factor type 1 of uropathogenic Escherichia coli kills cultured human uroepithelial 5637 cells by an apoptotic mechanism. Infect. Immun. 68:5869-5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oswald, E., J. de Rycke, P. Lintermans, K. van Muylem, J. Mainil, G. Daube, and P. Pohl. 1991. Virulence factors associated with cytotoxic necrotizing factor type two in bovine diarrheic and septicemic strains of Escherichia coli. J. Clin. Microbiol. 29:2522-2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oswald, E., M. Sugai, A. Labigne, H. C. Wu, C. Fiorentini, P. Boquet, and A. D. O'Brien. 1994. Cytotoxic necrotizing factor type 2 produced by virulent Escherichia coli modifies the small GTP-binding proteins Rho involved in assembly of actin stress fibers. Proc. Natl. Acad. Sci. USA 91:3814-3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parkhill, J., B. W. Wren, N. R. Thomson, R. W. Titball, M. T. Holden, M. B. Prentice, M. Sebaihia, K. D. James, C. Churcher, K. L. Mungall, S. Baker, D. Basham, S. D. Bentley, K. Brooks, A. M. Cerdeno-Tarraga, T. Chillingworth, A. Cronin, R. M. Davies, P. Davis, G. Dougan, T. Feltwell, N. Hamlin, S. Holroyd, K. Jagels, A. V. Karlyshev, S. Leather, S. Moule, P. C. Oyston, M. Quail, K. Rutherford, M. Simmonds, J. Skelton, K. Stevens, S. Whitehead, and B. G. Barrell. 2001. Genome sequence of Yersinia pestis, the causative agent of plague. Nature 413:523-527. [DOI] [PubMed] [Google Scholar]
- 38.Reed, K. C., and D. A. Mann. 1985. Rapid transfer of DNA from agarose gels to nylon membranes. Nucleic Acids Res. 13:7207-7221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rippere-Lampe, K. E., A. D. O'Brien, R. Conran, and H. A. Lockman. 2001. Mutation of the gene encoding cytotoxic necrotizing factor type 1 (cnf1) attenuates the virulence of uropathogenic Escherichia coli. Infect. Immun. 69:3954-3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sambrook, J., and D. W. Russell. 2001. Molecular cloning, a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 41.Schar, M., and E. Thal. 1955. Comparative studies on toxins of Pasteurella pestis and Pasteurella pseudotuberculosis. Proc. Soc. Exp. Biol. Med. 88:39-42. [DOI] [PubMed] [Google Scholar]
- 42.Schmidt, G., P. Sehr, M. Wilm, J. Selzer, M. Mann, and K. Aktories. 1997. Gln 63 of Rho is deamidated by Escherichia coli cytotoxic necrotizing factor-1. Nature 387:725-729. [DOI] [PubMed] [Google Scholar]
- 43.Schmidt, G., J. Selzer, M. Lerm, and K. Aktories. 1998. The Rho-deamidating cytotoxic necrotizing factor 1 from Escherichia coli possesses transglutaminase activity. Cysteine 866 and histidine 881 are essential for enzyme activity. J. Biol. Chem. 273:13669-13674. [DOI] [PubMed] [Google Scholar]
- 44.Schmitt, C. K., S. C. Darnell, V. L. Tesh, B. A. Stocker, and A. D. O'Brien. 1994. Mutation of flgM attenuates virulence of Salmonella typhimurium, and mutation of fliA represses the attenuated phenotype. J. Bacteriol. 176:368-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simonet, M., and S. Falkow. 1992. Invasin expression in Yersinia pseudotuberculosis. Infect. Immun. 60:4414-4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sugai, M., K. Hatazaki, A. Mogami, H. Ohta, S. Y. Peres, F. Herault, Y. Horiguchi, M. Masuda, Y. Ueno, H. Komatsuzawa, H. Suginaka, and E. Oswald. 1999. Cytotoxic necrotizing factor type 2 produced by pathogenic Escherichia coli deamidates a Gln residue in the conserved G-3 domain of the Rho family and preferentially inhibits the GTPase activity of RhoA and Rac1. Infect. Immun. 67:6550-6557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thal, E. 1954. Untersuchungen über Pasteurella pseudotuberculosis--unter Berücksichtigung ihres immunologischen Verhalten. Nord. Vetmed. 6:829-832. [Google Scholar]
- 48.Viboud, G. I., and J. B. Bliska. 2001. A bacterial type III secretion system inhibits actin polymerization to prevent pore formation in host cell membranes. EMBO J. 20:5373-5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Von Pawel-Rammingen, U., M. V. Telepnev, G. Schmidt, K. Aktories, H. Wolf-Watz, and R. Rosqvist. 2000. GAP activity of the Yersinia YopE cytotoxin specifically targets the Rho pathway: a mechanism for disruption of actin microfilament structure. Mol. Microbiol. 36:737-748. [DOI] [PubMed] [Google Scholar]
- 50.Zumbihl, R., M. Aepfelbacher, A. Andor, C. A. Jacobi, K. Ruckdeschel, B. Rouot, and J. Heesemann. 1999. The cytotoxin YopT of Yersinia enterocolitica induces modification and cellular redistribution of the small GTP-binding protein RhoA. J. Biol. Chem. 274:29289-29293. [DOI] [PubMed] [Google Scholar]