Abstract
Cell surface protein antigen (PAc) and glucosyltransferases (GTF) of Streptococcus mutans are major colonization factors of the organism. We prepared bovine milk containing antibodies against a fusion of the saliva-binding alanine-rich region of PAc with the glucan-binding domain of GTF-I. This study examined the effect of the immune milk on the cariogenicity of S. mutans in a rat model. Concentrated immune milk was fed to rats once a day for 55 days. The group that received immune milk had significantly less caries development than controls.
Streptococcus mutans has been strongly implicated as a cause of dental caries, a common human disease (22, 23). Colonization of tooth surfaces by S. mutans is mediated by both sucrose-independent and sucrose-dependent mechanisms. The former involves interaction between a 190-kDa surface protein antigen (PAc) of S. mutans, which is variously designated as antigen I/II, B, IF, P1, SR, and MSL-1 (22), and acquired pellicles formed on tooth surfaces (1, 10, 13). The latter involves the synthesis of water-insoluble glucan from sucrose catalyzed by glucosyltransferases (GTF) (11). Simultaneous inhibition of these colonization factors may result in the protection of tooth surfaces against dental caries. In previous studies, PAcA-GB, which is a fusion of the saliva-binding alanine-rich region (PAcA) of PAc and the glucan-binding (GB) domain of GTF-I, was constructed (29) and used to immunize Holstein cows (19).
In this study, we examined the effects of immune milk containing antibodies to PAcA-GB on dental caries induction in specific-pathogen-free (SPF) rats infected with S. mutans.
Preparation of concentrated immune milk.
Immune milk containing antibodies to PAcA-GB was prepared according to previously described methods (19). In this study, we modified the immunization method and obtained immune milk which possesses approximately antibody titers 10 times higher than those obtained in a previous study (19). Nonimmune milk was collected from a Holstein cow that had not received any injection of immunogen. After pasteurization at 65°C for 30 min, milk from each cow was defatted with a cream separator, concentrated by freeze-drying, and stored at 4°C until used. Approximately 17.7 kg of milk powder was obtained from 230 liters of pooled immune milk.
The concentrations of specific antibodies to recombinant PAc (rPAc) and GTF-I in milk were determined by enzyme-linked immunosorbent assay as described by Oho et al. (19) using purified specific immunoglobulin G (IgG) antibodies to PAcA-GB as standards. rPAc was purified from culture supernatants of transformant S. mutans TK18 (10). GTF-I, which is encoded by gtfB, was purified from whole cells of transformant S. mutans UA130B+ (29). Specific IgG antibodies were purified from pooled immune milk with a 5-ml HiTrap protein G column (Pharmacia, Uppsala, Sweden) as described previously (19). Milk samples were prepared by dissolving powdered milk in distilled water (0.5 g/ml). The mean concentrations of specific antibodies to rPAc and GTF-I in the reconstituted immune milk were 613.5 and 194.0 μg/ml, respectively. The mean concentrations of specific antibodies to rPAc and GTF-I in the reconstituted nonimmune milk were 6.5 and 8.0 μg/ml, respectively.
Dental caries experiment in rats.
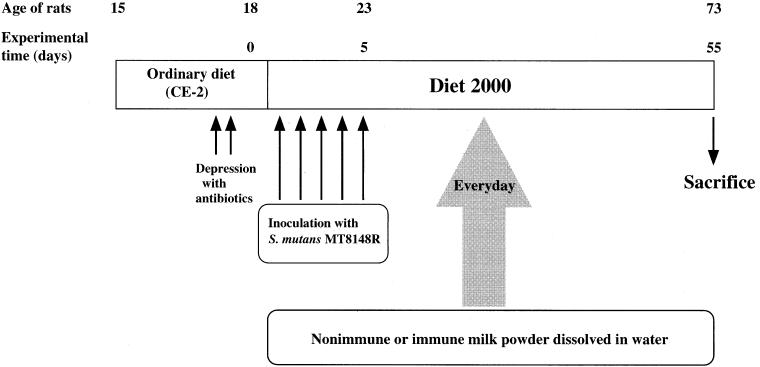
S. mutans MT8148R was used as a representative strain of S. mutans serotype c, which was made resistant to streptomycin (4). Thirty SPF Sprague-Dawley rats (male, 15 days of age; Seac Yoshitomi, Fukuoka, Japan) were divided randomly into three groups of 10 rats each. These rats were treated with tetracycline (4 mg/g of powdered diet CE-2; CLEA, Osaka, Japan) and penicillin G (4,000 U/ml of water) to enable the inoculated organisms to establish themselves in the oral cavity. All the rats (19 days old) were infected daily for 5 consecutive days with 0.1 ml of a cell suspension containing 6 × 109 CFU of S. mutans MT8148R and provided with powdered caries-inducing diet (diet 2000; CLEA), which contained 56% sucrose (9). During the experimental period, rats in the control group received only distilled water, and each rat in the experimental groups received 200 μl (from day 1 to 21) or 400 μl (from day 22 to 55) of reconstituted nonimmune or immune milk (0.5 g of milk powder dissolved in 1 ml of distilled water) from a micropipette once a day until the end of the experiment (Fig. 1). In a preliminary experiment, we determined the milk volume required to fill the oral cavity of rats, enabling teeth to make contact with the milk as thoroughly as possible, and these doses were chosen. The dose was doubled on day 22 following the growth of rats. An oral swab was taken from individual rats at weekly intervals, which permitted semiquantitative microbiological examination (20). At the end of the experiment (73 days of age), the rats were sacrificed and the mandibles were removed aseptically. The jaws were immersed in 5 ml of phosphate-buffered saline and ultrasonicated for 20 s to disperse the dental plaque on the tooth surface. The suspensions were decimally diluted with sterile phosphate-buffered saline and streaked on mitis salivarius agar plates containing 500 μg of streptomycin per ml. The caries induced in the 12 molar teeth on both maxilla and mandibles were scored by the method of Keyes (8). These experiments were performed according to the guidelines for animal experiments of the Faculty of Dental Science, Kyushu University, and the Law (no. 105) and Notification (no. 6) of the Japanese Government.
FIG. 1.
Experimental design for examining inhibitory effects of immune milk on dental caries induction in SPF rats infected with S. mutans MT8148R.
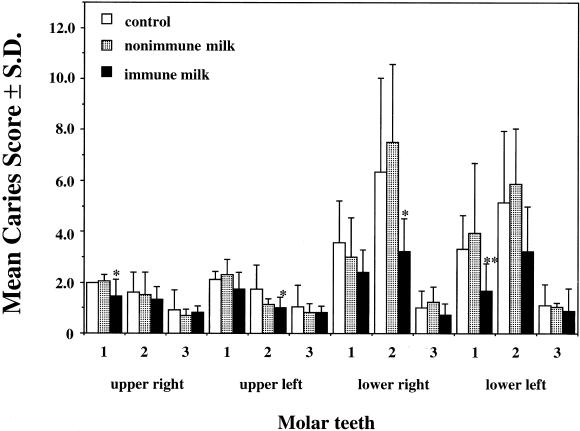
The caries scores of each group are shown in Table 1. The group that received immune milk had a significant reduction in caries development compared to the control group. However, there were no significant differences between the two groups in the recovery of S. mutans MT8148R from the mandibles at the end of the experiment (Table 1) or from the oral swabs during the experimental period (data not shown). The mean caries scores of individual teeth in the group that received immune milk were lower than those in the control group (Fig. 2). The differences in caries scores were significant for 4 of 12 molar teeth in this experiment. However, there were no significant differences between the control and nonimmune-milk groups.
TABLE 1.
Caries-inhibitory effects of immune and nonimmune milk in SPF rats infected with S. mutans MT8148R
| Group | Caries score (mean ± SD)
|
Recovery of strain MT8148R (105 CFU, mean ± SD) | ||
|---|---|---|---|---|
| Occlusal | Buccal + lingual | Total | ||
| Control | 25.8 ± 4.6 | 4.1 ± 4.2 | 29.9 ± 8.3 | 7.3 ± 12.0 |
| Nonimmune milk | 26.0 ± 6.3 | 5.1 ± 3.6 | 31.1 ± 9.3 | 23.5 ± 18.5a |
| Immune milk | 18.6 ± 3.5b | 0.7 ± 1.5a | 19.3 ± 4.2b | 5.6 ± 4.2 |
P < 0.05 compared with the control.
P < 0.01 compared with the control.
FIG. 2.
Mean caries scores on molar teeth of SPF rats after 55 days of infection with S. mutans MT8148R. Data are means and standard deviations. ∗ and ∗∗, P < 0.05 and P < 0.01 (compared with the control group), respectively.
Active immunization of experimental animals with cell surface polymers of S. mutans, such as PAc (I/II, B, or SR), GTF, and serotype-specific carbohydrate antigen, provides protection against dental caries (14, 15, 26). However, it may be impractical to introduce active immunization in humans to prevent dental caries because a vaccine targeting a nonlethal disease, such as dental caries, must be safer than vaccines developed for life-threatening infections (3). Therefore, passive immunization for the prevention of dental caries has received much attention (3, 5, 25), and monoclonal antibodies (17), chicken egg yolk antibodies (6, 24), bovine milk antibodies (2, 16), and synthetic peptide (7) have been used. In those experiments, IgG, chicken egg yolk IgY, and synthetic peptides were purified and applied directly to the oral cavity or applied as a mixture with diet and drinking water. Bovine milk has several advantages for use in humans as a means of passive immunization, because it is a common food that is easily delivered to the oral cavity and it is produced on a large scale at low cost. For the delivery of milk to the oral cavity, one can rinse with milk or apply powdered milk with a toothbrush. In this study, we prepared concentrated milk powder after freeze-drying immune milk, and rat dental caries was significantly inhibited by the application of reconstituted immune milk once a day. Powdered milk can be stored for a long period without any loss of antibody. After freeze-drying, almost no reductions in the antibody titers were observed compared with those in fresh milk, and high antibody titers were present even after 1 year.
In this study, immune milk clearly suppressed caries development in a rat model. This is the first study to demonstrate the efficacy of passive immunization with bovine milk containing antibodies to PAcA-GB for protection against dental caries in vivo. This effect might be ascribed to the simultaneous inhibition of the virulence factors PAc and GTF-I of S. mutans. In the assessment of caries scores of individual teeth, we found that lower first and second molars were heavily decayed although other teeth were moderately affected. This result correlates well with the observations of Smith et al. (24), although their procedures for passive immunization (e.g., the antibodies and immunization period used) differed from ours. These results might be due to the innate factors affecting caries susceptibility of individual teeth, such as the anatomical location or eruption period in the oral cavity. In the group that received nonimmune milk, the recovery of S. mutans at the end of the experiment was about three times higher than that in the control group, although the caries score of the nonimmune-milk group was approximately the same as that of the control group. Bovine milk has several components which possess cariostatic properties, such as caseins, immunoglobulins, lactoferrin, lactoperoxidase, and lysozyme (12, 18, 21, 27, 28). The finding that caries development in the nonimmune-milk group was suppressed might be ascribed to the effects of such milk components.
In conclusion, we demonstrated a reduction in caries development in a rat model using bovine milk containing antibodies against the fusion protein PAcA-GB. This immune milk might be an effective tool for controlling dental caries in humans.
Acknowledgments
This work was supported in part by Grants-in-Aid for Developmental Scientific Research (A)12357013 (T.K.) and (C)13672158 (T.O.) from the Ministry of Education, Science, Sports and Culture of Japan.
Editor: J. D. Clements
Footnotes
This paper is dedicated to the memory of Toshihiko Koga.
REFERENCES
- 1.Bowen, W. H., K. Schilling, E. Giertsen, S. Pearson, S. F. Lee, A. Bleiweis, and D. Beeman. 1991. Role of a cell surface-associated protein in adherence and dental caries. Infect. Immun. 59:4606-4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Filler, S. J., R. L. Gregory, S. M. Michalek, J. Katz, and J. R. McGhee. 1991. Effect of immune bovine milk on Streptococcus mutans in human dental plaque. Arch. Oral Biol. 36:41-47. [DOI] [PubMed] [Google Scholar]
- 3.Hajishengallis, G., and S. M. Michalek. 1999. Current status of a mucosal vaccine against dental caries. Oral Microbiol. Immunol. 14:1-20. [DOI] [PubMed] [Google Scholar]
- 4.Hamada, S., T. Horikoshi, T. Minami, S. Kawabata, J. Hiraoka, T. Fujiwara, and T. Ooshima. 1991. Oral passive immunization against dental caries in rats by use of hen egg yolk antibodies specific for cell-associated glucosyltransferase of Streptococcus mutans. Infect. Immun. 59:4161-4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamada, S., and Y. Kodama. 1996. Passive immunity for protection against mucosal infections and vaccination for dental caries, p. 187-197. In H. Kiyono, P. L. Ogra, and J. R. McGhee (ed.), Mucosal vaccines. Academic Press, San Diego, Calif.
- 6.Hatta, H., K. Tsuda, M. Ozeki, M. Kim, T. Yamamoto, S. Otake, M. Hirasawa, J. Katz, N. K. Childers, and S. M. Michalek. 1997. Passive immunization against dental plaque formation in humans: effect of a mouth rinse containing egg yolk antibodies (IgY) specific to Streptococcus mutans. Caries Res. 31:268-274. [DOI] [PubMed] [Google Scholar]
- 7.Kelly, C. G., J. S. Younson, B. Y. Hikmat, S. M. Todryk, M. Czisch, P. I. Haris, I. R. Flindall, C. Newby, A. I. Mallet, J. K. Ma, and T. Lehner. 1999. A synthetic peptide adhesion epitope as a novel antimicrobial agent. Nat. Biotechnol. 17:42-47. [DOI] [PubMed] [Google Scholar]
- 8.Keyes, P. 1944. A method of recording and scoring caries lesions in the molar teeth of Syrian hamsters. J. Dent. Res. 23:439-444. [Google Scholar]
- 9.Keyes, P., and H. Jordan. 1964. Periodontal lesions in the Syrian hamster. III. Findings related to an infectious and transmissible component. Arch. Oral Biol. 9:377-400. [DOI] [PubMed] [Google Scholar]
- 10.Koga, T., N. Okahashi, I. Takahashi, T. Kanamoto, H. Asakawa, and M. Iwaki. 1990. Surface hydrophobicity, adherence, and aggregation of cell surface protein antigen mutants of Streptococcus mutans serotype c. Infect. Immun. 58:289-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuramitsu, H. K., M. Smorawinska, Y. J. Nakano, A. Shimamura, and M. Lis. 1995. Analysis of glucan synthesis by Streptococcus mutans. Dev. Biol. Stand. 85:303-307. [PubMed] [Google Scholar]
- 12.Lassiter, M. O., A. L. Newsome, L. D. Sams, and R. R. Arnold. 1987. Characterization of lactoferrin interaction with Streptococcus mutans. J. Dent. Res. 66:480-485. [DOI] [PubMed] [Google Scholar]
- 13.Lee, S. F., A. Progulske-Fox, G. W. Erdos, D. A. Piacentini, G. Y. Ayakawa, P. J. Crowley, and A. S. Bleiweis. 1989. Construction and characterization of isogenic mutants of Streptococcus mutans deficient in major surface protein antigen P1 (I/II). Infect. Immun. 57:3306-3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lehner, T., M. W. Russell, J. Caldwell, and R. Smith. 1981. Immunization with purified protein antigens from Streptococcus mutans against dental caries in rhesus monkeys. Infect. Immun. 34:407-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lett, E., S. Gangloff, M. Zimmermann, D. Wachsmann, and J. P. Klein. 1994. Immunogenicity of polysaccharides conjugated to peptides containing T- and B-cell epitopes. Infect. Immun. 62:785-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loimaranta, V., J. Tenovuo, S. Virtanen, P. Marnila, E. L. Syväoja, T. Tupasela, and H. Korhonen. 1997. Generation of bovine immune colostrum against Streptococcus mutans and Streptococcus sobrinus and its effect on glucose uptake and extracellular polysaccharide formation by mutans streptococci. Vaccine 15:1261-1268. [DOI] [PubMed] [Google Scholar]
- 17.Ma, J. K., B. Y. Hikmat, K. Wycoff, N. D. Vine, D. Chargelegue, L. Yu, M. B. Hein, and T. Lehner. 1998. Characterization of a recombinant plant monoclonal secretory antibody and preventive immunotherapy in humans. Nat. Med. 4:601-606. [DOI] [PubMed] [Google Scholar]
- 18.Mitoma, M., T. Oho, Y. Shimazaki, and T. Koga. 2001. Inhibitory effect of bovine milk lactoferrin on the interaction between a streptococcal surface protein antigen and human salivary agglutinin. J. Biol. Chem. 276:18060-18065. [DOI] [PubMed] [Google Scholar]
- 19.Oho, T., Y. Shimazaki, M. Mitoma, M. Yoshimura, Y. Yamashita, K. Okano, Y. Nakano, H. Kawagoe, M. Fukuyama, N. Fujihara, and T. Koga. 1999. Bovine milk antibodies against cell surface protein antigen PAc-glucosyltransferase fusion protein suppress cell adhesion and alter glucan synthesis of Streptococcus mutans. J. Nutr. 129:1836-1841. [DOI] [PubMed] [Google Scholar]
- 20.Ooshima, T., T. Fujiwara, T. Takei, A. Izumitani, S. Sobue, and S. Hamada. 1988. The caries inhibitory effects of GOS-sugar in vitro and in rat experiments. Microbiol. Immunol. 32:1093-1105. [DOI] [PubMed] [Google Scholar]
- 21.Roger, V., J. Tenovuo, M. Lenander-Lumikari, E. Söderling, and P. Vilja. 1994. Lysozyme and lactoperoxidase inhibit the adherence of Streptococcus mutans NCTC 10449 (serotype c) to saliva-treated hydroxyapatite in vitro. Caries Res. 28:421-428. [DOI] [PubMed] [Google Scholar]
- 22.Russell, M. W. 1992. Immunization against dental caries. Curr. Opin. Dent. 2:72-80. [PubMed] [Google Scholar]
- 23.Russell, R. R. 1994. The application of molecular genetics to the microbiology of dental caries. Caries Res. 28:69-82. [DOI] [PubMed] [Google Scholar]
- 24.Smith, D. J., W. F. King, and R. Godiska. 2001. Passive transfer of immunoglobulin Y antibody to Streptococcus mutans glucan binding protein B can confer protection against experimental dental caries. Infect. Immun. 69:3135-3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith, D. J., and M. A. Taubman. 1997. Vaccines against dental caries infection, p. 913-930. In M. M. Levine, G. C. Woodrow, J. B. Kaper, and G. S. Cobon (ed.), New generation vaccines. Marcel Dekker, New York, N.Y.
- 26.Taubman, M. A., C. J. Holmberg, and D. J. Smith. 1995. Immunization of rats with synthetic peptide constructs from the glucan-binding or catalytic region of mutans streptococcal glucosyltransferase protects against dental caries. Infect. Immun. 63:3088-3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vacca-Smith, A. M., and W. H. Bowen. 1995. The effect of milk and kappa casein on streptococcal glucosyltransferase. Caries Res. 29:498-506. [DOI] [PubMed] [Google Scholar]
- 28.Vacca-Smith, A. M., B. C. Van Wuyckhuyse, L. A. Tabak, and W. H. Bowen. 1994. The effect of milk and casein proteins on the adherence of Streptococcus mutans to saliva-coated hydroxyapatite. Arch. Oral Biol. 39:1063-1069. [DOI] [PubMed] [Google Scholar]
- 29.Yu, H., Y. Nakano, Y. Yamashita, T. Oho, and T. Koga. 1997. Effects of antibodies against cell surface protein antigen PAc-glucosyltransferase fusion proteins on glucan synthesis and cell adhesion of Streptococcus mutans. Infect. Immun. 65:2292-2298. [DOI] [PMC free article] [PubMed] [Google Scholar]