Abstract
Caspase-11 (Cas11) is a cysteine protease involved in programmed cell death and cytokine maturation. Through activation of Cas1 (interleukin-1β [IL-1β]-converting enzyme), Cas11 is directly involved in the maturation of IL-1β and IL-18. Apoptosis is mediated through Cas3. Given the role of apoptosis and cytokine signaling during the innate immune response in intracellular infection, we examined Cas11-deficient (Cas11−/−) mice during infection with Listeria monocytogenes. Cas11−/− and wild-type C57BL/6 mice were equally susceptible to intravenous infection with L. monocytogenes, resulting in similar bacterial burdens in tissue and similar survival rates. By contrast, enhanced susceptibility was observed in control mice on a mixed genetic 129/C57BL/DBA2 background. Cas11−/− and wild-type mice infected with Listeria had similar hepatic microabscess formation in terms of histologic appearance, size, and number. Apoptosis of L. monocytogenes-infected hepatocytes in vivo and in vitro in primary culture was not altered by the absence of Cas11. Serum IL-18 and IL-1β levels were similar in Cas11−/− mice and controls. Endotoxin (lipopolysaccharide [LPS])-challenged Cas11−/− mice were deficient in the production of gamma interferon. IL-1β responses in Cas11−/− were normal with intravenous administration of LPS but decreased with intraperitoneal administration. Our findings suggest that Cas11 deficiency does not impair the immune response to infection with L. monocytogenes. Apoptosis and maturation of IL-18 and IL-1β were normal despite Cas11 deficiency. LPS-induced proinflammatory pathways are altered by the absence of Cas11. While Cas11-mediated Cas1 and Cas3 activation is crucial for cytokine maturation and apoptosis during inflammation, alternative pathways allow normal inflammatory and apoptotic responses during infection with L. monocytogenes.
Listeria monocytogenes is a facultative intracellular, gram-positive organism which causes severe infections in immunocompromised hosts. In the adult population, Listeria is the third most common pathogen in community-acquired infective meningitis (10, 25). Murine listeriosis has been used to study cell-mediated immunity against intracellular bacterial infections (36). Gamma interferon (IFN-γ)-driven T helper 1 (Th1)-type immune responses are central to the control and clearance of intracellular infections. Interleukin-18 (IL-18) participates with IL-12 in innate and acquired immunity against intracellular organisms (33). IL-18 has been shown to play an important role in responses to intracellular infection with Cryptococcus neoformans and Yersinia enterocolitica (4, 22, 45).
Apoptosis has also been implicated as a mechanism contributing to the host defense against intracellular pathogens (18, 30, 32, 35). Some pathogens, including the mycobacteria, block apoptotic pathways to evade host defenses (31, 44). In murine listeriosis, apoptosis has been demonstrated in hepatocytes involved in microabscesses (11, 35). It has been speculated that programmed cell death of infected hepatocytes serves to limit the spread of infection.
Caspase-11 (Cas11) is a member of a family of cysteine proteases initially identified as the mammalian homologs of the Caenorhabditis elegans death gene family. Caspases act as effector molecules which, when activated, can result in programmed cell death (14, 15). The caspases have been linked to inflammatory response pathways. Cas11 is upregulated upon stimulation with lipopolysaccharide (LPS) and participates in the systemic inflammatory response by cleaving inactive procaspase-1 (interleukin-β-converting enzyme) into the active form (43), acting as an upstream regulator of Cas1 (42). Active Cas1 initiates a complex pathway which can result in cell death and activates the proinflammatory cytokines IL-1β (5, 38) and IL-18 (formerly known as IFN-γ-inducing factor) (13, 16). Cas11-deficient (Cas11−/−) mice are resistant to doses of endotoxin which are lethal in control animals (43). Resistance to LPS is thought to be the result of decreased processing of proinflammatory cytokines. Unlike in wild-type mice, levels of IL-1α and IL-1β in serum do not increase with intraperitoneal LPS challenge in Cas11-deficient mice. In LPS-challenged macrophages, Cas1 is required for IL-18 maturation (16, 39).
Apoptosis is induced in vitro in both Cas1- and Cas11-transfected fibroblasts (27, 42). Both Cas1 and Cas11-deficient mice show only minor defects in apoptosis in vivo and develop normally without early lethality (23, 24, 43). However, in a mouse model of cerebral stroke, Cas11 deficiency has been shown to result in reduced apoptosis (20). In a mouse model of multiple sclerosis, experimental autoimmune encephalomyelitis, oligodendrocytes of Cas11−/− animals were highly resistant to cell death, and susceptibility to experimental autoimmune encephalomyelitis was significantly reduced in Cas11−/− mice (17).
In this study, we assessed the role of Cas11 in Listeria infection in vivo and in vitro. Given the role of Cas11 in cytokine maturation and apoptosis, it was hypothesized that Cas11-deficient mice would not be able to clear infection with L. monocytogenes. Our data demonstrate that the absence of Cas11 did not change the susceptibility in terms of survival and bacterial burden of infection with L. monocytogenes in mice. Accordingly, no difference was observed in the histologic and apoptotic responses of Cas11−/− and wild-type mice. Growth of L. monocytogenes in primary hepatocyte cultures in vitro was not affected by Cas11 deficiency. During L. monocytogenes infection in vivo, IL-1β and IL-18 maturation was not dependent on Cas11. In contrast, IL-1β and IFN-γ production was altered when Cas11-deficient mice were challenged with LPS.
MATERIALS AND METHODS
Reagents.
Escherichia coli LPS serotype 055:B5 was purchased from Sigma (St. Louis, Mo.). Hepatocytes were cultured in Waymouth's medium supplemented with 0.1 μM insulin, 10% heat-inactivated fetal bovine serum (Life Technologies, Rockville, Md.), and 25 μg of gentamicin (Sigma) per ml, when appropriate. Collagenase type 1 was from Worthington Biochemical Corporation (Lakewood, N.J.). Percoll, bovine serum albumin and collagen type 1 were obtained from Sigma.
Mice.
Cas11 knockout (Cas11−/−) mice were from the original breeding population of knockout mice described by Wang et al. (43), maintained at Taconic Farms, Germantown, N.Y. In brief, 129/Sv ES cell clones carrying a null mutant Cas11 allele were injected into C57BL/6 blastocytes. The chimeric males were mated with C57BL/6 × DBA2 females. The homozygous absence of the Cas11 gene in experimental animals was repeatedly checked by PCR using specific primers. Except where mentioned, all studies were conducted with Cas11−/− mice on a mixed background with male inbred C57BL/6 control mice purchased from Charles River Laboratories, Wilmington, Mass. Survival and bacterial burden studies were also performed with Cas11 knockout mice backcrossed to the C57BL/6 background for five generations. Backcrossed mice on a C57BL/6 background (F4 generation) were kindly provided by J. Yuan, Harvard Medical School. After one additional backcrossing, heterozygous offspring of the F5 generation were mated. The resulting offspring were used to create breeding pairs homozygous for the Cas11−/− or wild-type allele. Offspring of these breeding pairs were used at 8 weeks of age, and all animals used in experiments were genotyped. Animals were housed in laminar flow racks in a microisolator (BL2 containment) in the animal care facility of the Massachusetts General Hospital. All animal protocols were approved by the institutional animal use and care committee.
Experimental Listeria infection.
L. monocytogenes was cultivated from a clinical isolate from a patient with Listeria meningitis and bacteremia (Massachusetts General Hospital Clinical Microbiology Laboratory). Bacteria were grown at 37°C overnight in shaking cultures in Luria-Bertani (LB) broth. Aliquots were frozen in 10% glycerol-phosphate-buffered saline (PBS) and stored at −70°C. For use, each inoculum was diluted in 0.9% saline and injected in a lateral tail vein. The inoculum dose was assayed for each inoculation by plating serial dilutions of the injected suspension. For Listeria infection studies in vitro, organisms were added to medium to obtain a multiplicity of infection (MOI) of 1:1 or 10:1 in primary hepatocyte cultures as specified. For apoptosis studies, Listeria-containing medium was replaced by gentamicin-containing medium to kill extracellular bacteria 7 h after infection. At 24 h after the initial infection, cells were washed and fixed in 3.7% paraformaldehyde and stained by terminal deoxynucleotidyltransferase (TdT)-mediated dUTP-biotin nick end labeling (TUNEL) as described below. Using an ocular micrometer, 200 TUNEL-positive and -negative cells per view field were counted to determine the percentage of apoptotic cells. At this time point, no bacterial growth was detectable in the supernatant. For intracellular growth studies, Listeria-inoculated medium was removed and replaced by gentamicin-containing medium after 2 h. At defined time points cells were washed and then lysed once with 0.5% Triton X-100 in 1× PBS, and serial dilutions were cultured on LB plates.
CFU in organ homogenates.
Mice were sacrificed by cervical dislocation while anesthetized. Blood was drawn by heart puncture. Spleens and livers were removed aseptically, and segments were homogenized in PBS after being weighed. Serial dilutions were plated on plates of LB agar. Viable counts were determined after 24 h of incubation at 37°C.
Primary hepatocyte cultures.
Hepatocytes were prepared by a two-step technique. The inferior vena cava of anesthetized animals was cannulated, and the liver was perfused with a modified Krebs-Ringer solution (12.5 mM HEPES, 57.7 mM NaCl, 0.5 mM KH2PO4, 5 mM KCl) containing 0.5 mM EGTA (pH 7.5) (all from Sigma) after clamping of the superior vena cava and incision of the portal vein. The second perfusion consisted of the modified Krebs-Ringer buffer containing 88 U of collagenase type 1 per ml. The livers were transferred to a petri dish and gently teased apart. The resulting cell suspension was centrifuged at 50 × g for 2 min, resuspended in a 45% Percoll-PBS solution, and recentrifuged at 500 × g for 5 min. The final pellet consisted of >98% hepatocytes by morphological criteria and were > 92%viable as determined by the trypan blue exclusion method. Hepatocytes were cultured in Waymouth's medium supplemented with 10% heat-inactivated fetal bovine serum, 0.1 μM bovine insulin, and gentamicin (25 μg/ml), when appropriate.
Cytokine assays.
IL-18 was measured by capture enzyme-linked immunosorbent assay (ELISA) with ELISA development reagents (R&D Systems, Minneapolis, Minn.). Ninety-six-well microtiter plates (Costar, Corning, N.Y.) were coated with monoclonal rat anti-mouse IL-18 antibody (4 μg/ml in PBS) overnight at room temperature (RT). Plates were blocked for 2 h in PBS-1% bovine serum albumin-5% sucrose at RT. Standards consisting of recombinant mouse IL-18 (ranging from 1,000 to 15.6 pg/ml) and samples were loaded in duplicate and incubated at RT for 2 h. The plates were washed, and the second antibody (biotinylated anti-mouse IL-18) was added (0.2 μg/ml). The detection reagent (horseradish peroxidase-conjugated streptavidin [1:200]; 20 min at RT), followed by the substrate solution (tetramethylbenzidine-hydrogen peroxidase, 1:1) (all from R&D Systems), was added. The reaction was stopped after 10 to 15 min with 1 N H2SO4. The optical density was measured at 450 nm. IL-18 concentrations were determined by comparison with a standard curve. IFN-γ, IL-12p70, and IL-1β were measured in the same manner using DuoSet ELISA kits (R&D Systems) according to the manufacturer's recommendations. The lower detection limits of the cytokine assays were as follows: IFN-γ, 30 pg/ml; IL-18, 15 pg/ml; IL-12p70, 25 pg/ml; and IL-1β, 15 pg/ml.
Histopathological analyses and apoptosis detection by in situ TUNEL assay.
The TUNEL assay detects nuclear DNA fragmentation occurring during apoptosis. The assay was performed with the TACS blue label apoptosis detection kit (R&D Systems). Sections of formalin-fixed and paraffin-embedded tissue were rehydrated and deparaffinized. Cells were fixed in 3% formaldehyde. After permeabilization with proteinase K and quenching of endogenous peroxidase with H2O2, DNA fragments were labeled by incorporating biotinylated nucleotides by use of TdT. Using a streptavidin-horseradish peroxidase conjugate followed by TACS blue label substrate, labeled DNA fragments were detected by light microscopy. Eosin was used for counterstaining. Positive controls consisted of samples treated with nuclease, which generates DNA breaks in the majority of cells. Staining with TdT omitted served as a negative control. Microabscess formation was assessed by light microscopy of hematoxylin-eosin-stained sections.
Statistical analysis.
Student's t test was used to determine the significance of differences between controls and experimental groups. The log rank test was used to determine differences in survival curves. A difference was considered statistically significant when the P value was <0.05.
RESULTS
Absence of Cas11 does not alter lethality or bacterial replication of Listeria monocytogenes.
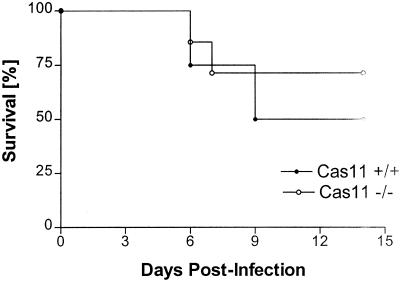
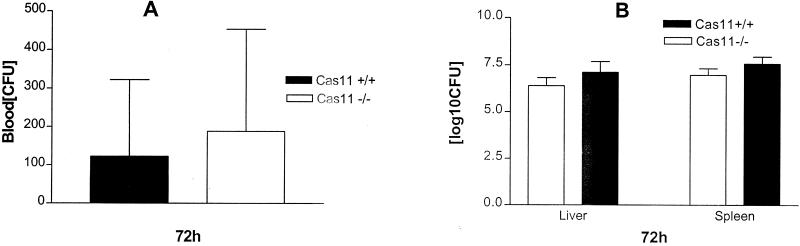
Initial studies compared the survival of Cas11−/− mice on a mixed 129, DBA2, and C57BL/6 background with that of inbred C57BL/6 mice and found that the knockout mice were significantly more susceptible to intravenous and intraperitoneal infection with L. monocytogenes. This difference in survival was reflected in higher bacterial burdens in livers and spleens of Cas11−/− mice and recrudescence of bacteremia at 48 to 72 h after infection. Cas11−/− mice backcrossed to the C57BL/6 background for five generations (n = 7) and wild-type (Cas11+/+) control mice (n = 4) were also infected with 3 × 104 Listeria organisms intravenously. Survival was assessed daily. No statistically significant difference was observed between the two groups (Fig. 1). Blood, liver, and spleen cultures from wild-type and Cas11−/− mice were assessed. Listeria titers in blood, spleen, and liver at 72 h after intravenous infection with 3.4 × 104 (n ≥ 4)) or 4.1 × 105 (n ≥ 4) Listeria organisms resulted in comparable bacterial burdens in liver and spleen and similar blood levels in Cas11−/− and wild-type animals (Fig. 2). In susceptible mouse strains, enhanced bacterial replication was observed in liver, spleen, and blood at 72 h after infection.
FIG. 1.
Cas11−/− (n = 7) and Cas11+/+ (n = 4) mice on a C57BL/6 background (F5) were infected intravenously with 3 × 104 Listeria organisms. Survival was similar in both groups (no significant difference as determined by the log rank test).
FIG. 2.
Blood, livers, and spleens of F5 mice on a C57BL/6 background were infected with 4.1 × 105 Listeria organisms intravenously and harvested at 72 h. Serial dilutions of blood or ground tissues were plated on LB agar plates. The CFU in 30 μl of blood (A) or the log10 CFU (adjusted for total organ weight) in liver and spleen (B) were determined for Cas11−/− mice (n = 11) and wild-type controls (n = 4). Mean values from individual mice (± standard errors of the means) are shown. There was no significant difference for liver, spleen, and blood as determined by the unpaired Student t test.
Cytokine regulation in Listeria-infected Cas11−/− mice.
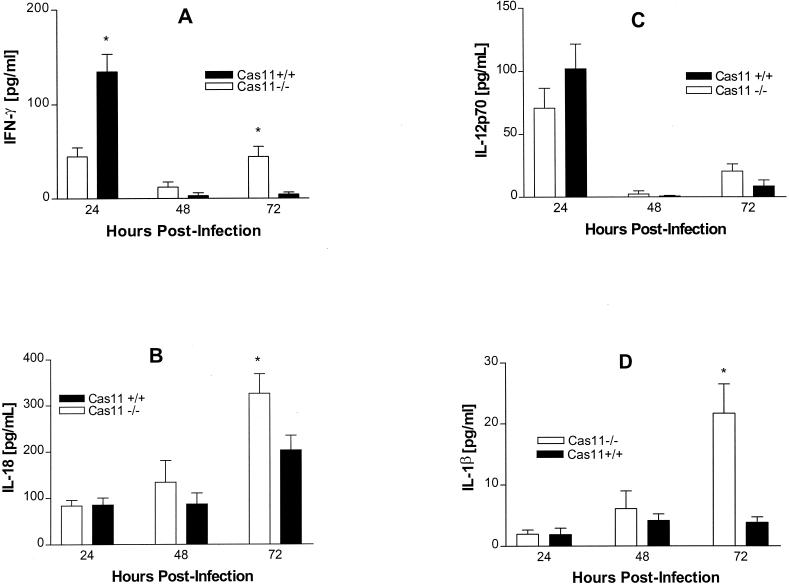
Cas11 is an upstream regulator of Cas1 which has been shown to activate IL-18 and IL-1β (17, 23, 43). IL-18 and IL-12 are key inducers of IFN-γ, a cytokine central to the host response to intracellular infection. In order to assess the basis for observed differences in susceptibility between Cas11−/− (129/DBA2/C57BL/6) and Cas11+/+ (C57BL/6) mice, cytokine profiles were examined in these groups. After infection of Cas11−/− and wild-type mice with 104 Listeria organisms intravenously, serum samples were assessed for cytokine production by ELISA. IFN-γ was first detectable in the serum at 12 h after infection in both Cas11−/− and Cas11+/+ control mice. At 24 h, serum IFN-γ levels in control animals peaked at a level significantly higher than that in Cas11−/− mice. At 48 and 72 h, serum IFN-γ had markedly decreased in the control group. Levels of IFN-γ in Cas11−/− mice remained elevated, however, without attaining the peak level of the control group (Fig. 3A). Serum IL-18 levels at 24 and 48 h were not different in control and Cas11−/− animals (Fig 3B). At 72 h, IL-18 levels were significantly higher in the Cas11−/− group. Levels of IL-12, an inducer of IFN-γ, in serum were not significantly different throughout the infection (Fig. 3C). No difference in serum IL-1β levels was detected at 24 and 48 h. At 72 h, similar to the case for IL-18, IL-1β levels were significantly higher in the knockout mice, possibly due to the higher infectious burden (Fig. 3D).
FIG. 3.
Cas11−/− mice and wild-type controls were infected with 104 Listeria organisms intravenously. At 24, 48, and 72 h, animals were bled by cardiac puncture and levels of IFN-γ (A), IL-18 (B), IL-12p70 (C), and IL-1β (D) in serum were assessed by ELISA. Results are presented as pooled data from at least three independent experiments per point (n = 12 mice total in each group). Mean values from individual mice (± standard errors of the means) are shown. P values were determined by the unpaired Student t test (∗, significantly different from wild-type values [P < 0.05]).
Abscess formation and apoptosis in the liver and spleen during Listeria infection.
Bacteremia due to Listeria is cleared largely by hepatic Kupffer cells. In the setting of excessive infection, Listeria spreads to hepatocytes, which are unable to control intracellular growth (35). Microabscess formation is the histologic hallmark of the Listeria-infected liver (9). Abscesses can be found as early as 16 h after infection. Apoptosis is a mechanism by which the uncontrolled growth of Listeria is interrupted during acute infection (35).
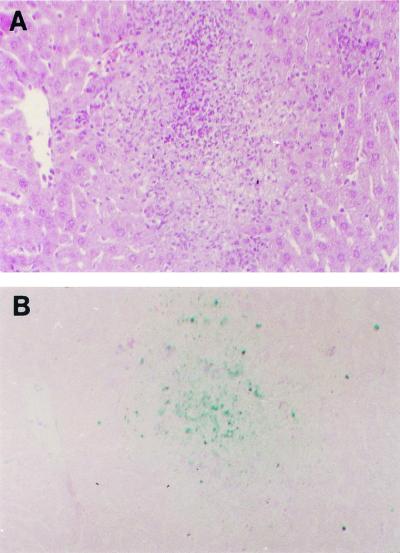
Livers and spleens of Listeria-infected animals were formalin fixed at 72 h after intravenous infection with 104 Listeria organisms, and sections of tissues were stained by the TUNEL method. Infection of livers of both Cas11−/− and wild-type mice resulted in the development of organized microabscesses. The architecture, size, and number of abscesses did not differ between the Cas11−/− mice and controls (Fig. 4). Serial sections were stained with hematoxylin-eosin and TUNEL, allowing a direct comparison between hematoxylin-eosin- and TUNEL-stained sections. Apoptotic cells appeared to be largely hepatocytes. Cas11−/− and wild-type livers had similar distributions and numbers of apoptotic cells. Surrounded by intact liver parenchyma with an occasional apoptotic hepatocyte, the main apoptotic activity that could be detected was within and surrounding the microabscesses. The absence of Cas11 does not appear to impair abscess formation or apoptotic control of infection in the liver (Fig. 4). In the spleen, diffuse apoptosis was observed in Cas11−/− and control mice, with no apparent difference between the two groups.
FIG. 4.
At 72 h postinfection, livers of Cas11−/− and wild-type control mice infected with 104 Listeria organisms intravenously were fixed in 10% formalin and embedded in paraffin. Consecutive sections were stained with hematoxylin-eosin (A) and by the TUNEL method (B), allowing for direct comparison. Both panels show wild-type liver, which was representative for abscesses found in livers of all Listeria-infected Cas11−/− and Cas11+/+ mice. By TUNEL staining, the distributions of apoptotic cells were similar in both groups. Apoptotic cells could be found concentrated at the margin of the abscess. The liver parenchyma between the abscesses appeared normal, and an occasional apoptotic hepatocyte could be found (as in uninfected control livers). The architecture of the abscesses and the sizes and numbers of abscesses per field did not differ between Cas11−/− and Cas11+/+ animals. Magnification, ×100.
Replication and apoptosis in Listeria-infected primary hepatocyte cultures.
To examine possible differences in the ability of primary hepatocytes to contain Listeria or to undergo apoptosis upon infection with Listeria, Cas11−/− and Cas11+/+ primary hepatocyte cultures were infected with Listeria at various MOIs and assessed for intracellular growth and apoptosis.
Two hours after infection of a monolayer of primary hepatocytes with an MOI of 1:1, the inoculum was replaced by gentamicin-containing medium. At various time points after initial infection, the medium was removed and the cells were lysed. Serial dilutions were plated on LB plates. No Listeria was detected in the supernatants of any cultures. Listeria replicated at the same rate in Cas11−/− and wild-type control hepatocytes (Fig. 5).
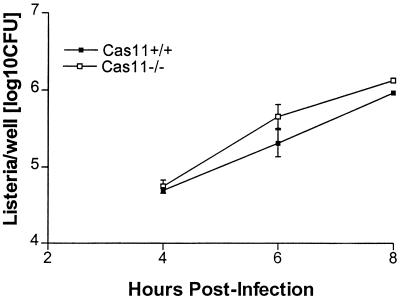
FIG. 5.
Primary Cas11−/− and Cas11+/+ hepatocytes were harvested as described in the text. At 24 h after plating, hepatocytes were infected with Listeria at an MOI of 1:1. After 2 h, the medium was removed and replaced by gentamicin-containing medium to restrict extracellular growth. At 4, 6, and 8 h after infection, cells were lysed and CFU were determined by culturing serial dilutions. Results are the means (± standard errors of the means) from four wells per time point.
Apoptosis of infected hepatocytes was assessed by TUNEL staining. In hepatocytes infected with an MOI of 10:1, all cells in both Cas11−/− and wild-type hepatocytes were apoptotic. Using an MOI of 1:1 in multiple independent experiments, the percentage of apoptotic cells was assessed. In Cas11+/+ hepatocytes, 65% of cells were apoptotic, compared to 67% in Cas11−/− hepatocytes (not a significant difference).
Levels of IFN-γ, IL-1β, IL-12p70, and IL-18 in plasma after LPS challenge.
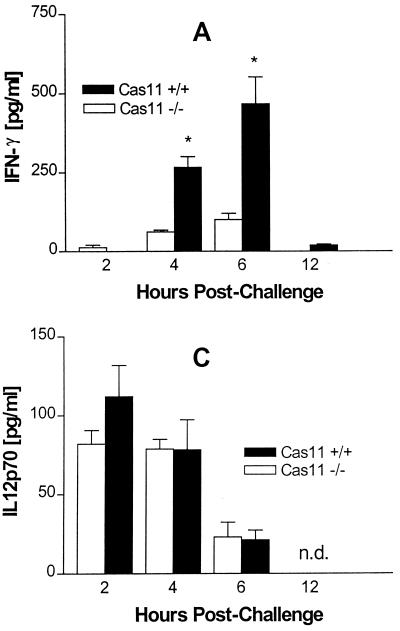
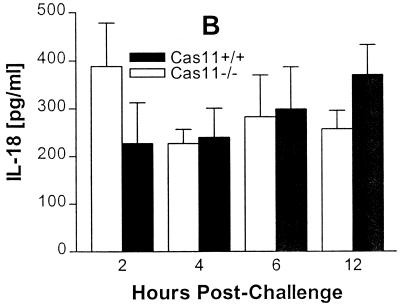
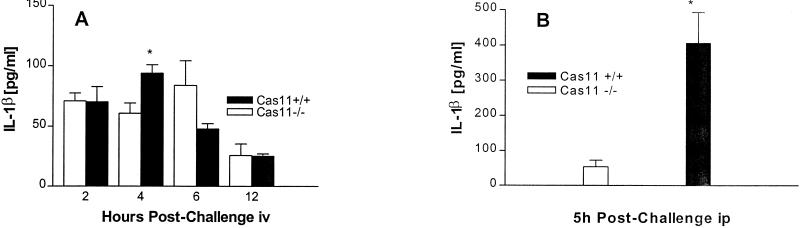
Protection against an otherwise lethal LPS challenge is the phenotypic hallmark of Cas11-deficient mice (43). Cas11 is involved in cytokine processing through the activation of Cas1, which then activates IL-18 and IL-1β. Prior studies by Wang et al. showed that Cas11−/− mice, when challenged with 40 mg/kg of body weight intraperitoneally, produce no detectable IL-1β and very low levels of IL-1α; IL-1β was strongly induced in wild-type mice (43). Cas11−/− and wild-type mice were given a dose of 5 mg of LPS/kg of body weight intravenously or 40 mg/kg of body weight intraperitoneally and sacrificed at various time points. Serum was assessed for IL-1β, IL-12p70, IL-18, and IFN-γ by ELISA. The IFN-γ output was diminished in Cas11−/− mice (Fig. 6A). IL-18 and IL-12 production was not significantly different between Cas11−/− and wild-type mice (Fig. 6B and C). Interestingly, IL-1β was detected in similar amounts in both Cas11−/− and Cas11+/+ mice when LPS was given intravenously (Fig. 7A). However, when LPS was administered intraperitoneally, minimal production of IL-1β was observed in the Cas11−/− mice, consistent with observations by Wang et al. (Fig. 7B) (43). Thus, the Cas11−/− mouse responds differently to peritoneal and intravenous endotoxin, a phenomenon not described previously.
FIG. 6.
Groups of five mice were injected intravenously with 5 mg of E. coli LPS (055:B5)/kg of body weight. At 2, 4, and 6 h after LPS challenge, mice were bled by cardiac puncture and levels of IFN-γ (A), IL-18 (B), and IL-12p70 (C) in serum were measured by ELISA. Results are the means ± standard errors of the means (∗, significantly different from wild-type controls [P < 0.05]).
FIG. 7.
Groups of three to five mice were injected with 5 mg of E. coli LPS (055:B5)/kg of body weight intravenously (iv) (A) or with 40 mg/kg of body weight intraperitoneally (ip) (B). At 2, 4, and 6 h after LPS challenge, mice were bled by cardiac puncture and serum IL-1β was measured by ELISA. Results are means ± standard errors of the means (∗, significantly different from wild-type controls [P < 0.05]).
DISCUSSION
Cas11 has been shown to be involved in both the inflammatory (cytokine maturation) and apoptotic response to pathological stimuli (17, 20, 43). Whereas the importance of cytokines (the most important being IFN-γ) in the host response to Listeria is well established (40), apoptosis has only recently emerged as an important component of host defenses (44). In this study, we investigated the role of Cas11 in the host response to L. monocytogenes. These data indicate that Cas11 is not involved in host defenses against L. monocytogenes. These findings are in marked contrast to the pivotal role of the Cas11 in cytokine maturation and apoptosis during inflammatory processes (17, 20, 43).
Cas11−/− mice on a 129/C57BL/DBA background were more susceptible to listeriosis than were inbred C57BL/6 control mice, in terms of both survival and bacterial burden. This advantage disappeared in resistance studies with F5 backcrossed Cas11−/− mice on a C57BL/6 background. We believe that the observed differences, including the reduced IFN-γ output, are related to the murine genotype. Infectious challenge of mice of different genetic backgrounds has resulted in differences in IFN-γ production and in survival (1, 3, 19). In particular, mice of both backgrounds used for the production of Cas11−/− mice (DBA2 and 129) are more susceptible than C57BL/6 mice when challenged with Listeria (6, 7). The underlying genetic mechanisms of the high susceptibility of inbred 129 and DBA2 mice during Listeria infection have been only partly elucidated. In F5 backcrossed mice, survival was not different between wild-type and knockout animals. In accordance with this, the kinetics of Listeria infection were not altered by Cas11 deletion.
Consistent with these data, Listeria-infected Cas11−/− mice formed microabscesses in liver and spleen, which appeared to be normal in terms of size, number, and architecture. Unanue's group has reported that L. monocytogenes infection results in apoptosis of lymphocytes in spleen and lymph nodes and of 0infected hepatocytes in vivo (26, 35). The absence of Cas11 did not alter the ability of hepatocytes in vivo or in vitro to undergo apoptosis during Listeria infection. The number and distribution of apoptotic cells in infected livers did not differ between Cas11 knockout and control groups and were comparable to findings reported by Rogers et al. (35). Based on histologic appearance, apoptotic cells appeared to be hepatocytes. This is consistent with observations made in other models in which Listeria-associated apoptosis has been observed in hepatocytes but not in macrophages (including Kupffer cells) and in neutrophil-depleted mouse models, in which apoptosis was detectable in hepatocytes (2, 35). In our hands, apoptosis of hepatocytes from lethally infected wild-type animals was more frequent than in studies by Miura et al. (28). These differences may reflect the different mouse strains studied. We conclude that Cas11 is not required to induce apoptosis in hepatocytes in vivo and in vitro during infection with L. monocytogenes. It is possible that direct activation of Cas1 by Listeria or other mediators of the host response is responsible for induction of apoptosis. This has been shown for HeLa cells, in which IFN-γ-mediated cell death is initiated through expression of Cas1 (8). Other apoptosis-inducing pathways (e.g., Fas) may also be involved in the response of Listeria-infected hepatocytes. Of interest is a recent observation that IFN-γ-induced apoptosis in murine hepatocytes was independent of Cas11 (21).
A role for IL-12, tumor necrosis factor alpha, IL-1, IL-6, and IFN-γ has been described previously for early Listeria infection. In addition, macrophages, NK cells, and neutrophils have been implicated in the response to murine Listeria infection (41). During challenge with Listeria, regulation of IL-1β and IL-18 did not differ in Cas11−/− and wild-type mice. In addition, IL-12 levels were similar in both groups. In susceptible strains, the cytokine profile included reduced peak synthesis of IFN-γ despite enhanced production of IL-18. While Cas11 did not seem to be involved in cytokine regulation during Listeria infection, it proved to play an important role in LPS-induced inflammation. Intravenous LPS resulted in diminished IFN-γ production in Cas11−/− mice, while IL-1β, IL-12, and IL-18 production was normal. Previously described diminution of IL-1β production was confirmed in response to intraperitoneal LPS challenge but not with intravenous challenge (43). It is possible that IL-1β and IL-18 are processed via Cas1- and Cas11-independent pathways during Listeria infection. Tsutsui et al. have shown that murine macrophages deficient in Cas1 are capable of mature IL-18 secretion by a Fas-mediated pathway (39). Cas1-independent IL-1β production via Fas ligand activation has also been demonstrated (29). Direct activation of Cas1 has been demonstrated in other systems, including a model of influenza virus infection (34).
The Cas11-Cas1 axis is an important pathway in systemic LPS-induced inflammation. Wang et al. have shown that Cas11−/− mice were resistant to challenge with a lethal dose of LPS intraperitoneally (43). While IL-1β levels were reduced in knockout mice after intraperitoneal challenge with LPS, no differences were found when LPS was administered intravenously. Thus, host response mechanisms in the peritoneum and in the circulation differ. In a related model of local inflammation, turpentine applied locally induced comparable local IL-1β levels in Cas1−/− and wild-type mice (12). When LPS was given systemically in Cas1−/− mice, however, production of mature IL-β was defective, resulting in a resistant phenotype (24). Direct activation of Cas1 by LPS has been demonstrated in cultured monocytes and endothelial cells (37). Thus, the route of application is an important variable in defining the host cytokine response. Cas11 is an important regulator of systemic IFN-γ production. IFN-γ production was diminished in Cas11−/− mice after intravenous LPS exposure. This is a direct reflection of the role of Cas11 in IFN-γ processing, as the levels of IFN-γ inducers (IL-18 and IL-12) were not different in Cas11−/− and wild-type animals. IL-1β and IFN-γ production is also reduced in the brain and spinal cord in Cas11−/− mice during experimental autoimmune encephalomyelitis (17).
In summary, our studies did not establish a role for Cas11 in L. monocytogenes infection. The inflammatory and apoptotic responses to Listeria are normal in Cas11−/− mice. In contrast, the systemic inflammatory and apoptotic responses to endotoxin are at least partially dependent on Cas11.
Acknowledgments
This work was supported by a grant from the Swiss National Science Foundation to N.J.M. The support of the Shriners Hospital for Children is gratefully acknowledged.
The initial Cas11−/− strain and the F4 backcrossed Cas11−/− strain were kindly provided by J. Yuan, Harvard Medical School.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Autenrieth, I. B., M. Beer, E. Bohn, S. H. Kaufmann, and J. Heesemann. 1994. Immune responses to Yersinia enterocolitica in susceptible BALB/c and resistant C57BL/6 mice: an essential role for gamma interferon. Infect. Immun. 62:2590-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barsig, J., and S. H. Kaufmann. 1997. The mechanism of cell death in Listeria monocytogenes-infected murine macrophages is distinct from apoptosis. Infect. Immun. 65:4075-4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bohn, E., J. Heesemann, S. Ehlers, and I. B. Autenrieth. 1994. Early gamma interferon mRNA expression is associated with resistance of mice against Yersinia enterocolitica. Infect. Immun. 62:3027-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bohn, E., A. Sing, R. Zumbihl, C. Bielfeldt, H. Okamura, M. Kurimoto, J. Heesemann, and I. B. Autenrieth. 1998. IL-18 (IFN-gamma-inducing factor) regulates early cytokine production in, and promotes resolution of, bacterial infection in mice. J. Immunol. 160:299-307. [PubMed] [Google Scholar]
- 5.Cerretti, D. P., C. J. Kozlosky, B. Mosley, N. Nelson, N. K. Van, T. A. Greenstreet, C. J. March, S. R. Kronheim, T. Druck, and L. A. Cannizzaro. 1992. Molecular cloning of the interleukin-1 beta converting enzyme. Science 256:97-100. [DOI] [PubMed] [Google Scholar]
- 6.Cheers, C., and I. F. McKenzie. 1978. Resistance and susceptibility of mice to bacterial infection: genetics of listeriosis. Infect. Immun. 19:755-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheers, C., I. F. McKenzie, H. Pavlov, C. Waid, and J. York. 1978. Resistance and susceptibility of mice to bacterial infection: course of listeriosis in resistant or susceptible mice. Infect. Immun. 19:763-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chin, Y. E., M. Kitagawa, K. Kuida, R. A. Flavell, and X. Y. Fu. 1997. Activation of the STAT signaling pathway can cause expression of caspase 1 and apoptosis. Mol. Cell Biol. 17:5328-5337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conlan, J. W., and R. J. North. 1994. Neutrophils are essential for early anti-Listeria defense in the liver, but not in the spleen or peritoneal cavity, as revealed by a granulocyte-depleting monoclonal antibody. J. Exp. Med. 179:259-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Durand, M. L., S. B. Calderwood, D. J. Weber, S. I. Miller, F. S. Southwick, V. S. Caviness, Jr., and M. N. Swartz. 1993. Acute bacterial meningitis in adults. A review of 493 episodes. N. Engl. J. Med. 328:21-28. [DOI] [PubMed] [Google Scholar]
- 11.Ebe, Y., G. Hasegawa, H. Takatsuka, H. Umezu, M. Mitsuyama, M. Arakawa, N. Mukaida, and M. Naito. 1999. The role of Kupffer cells and regulation of neutrophil migration into the liver by macrophage inflammatory protein-2 in primary listeriosis in mice. Pathol. Int. 49:519-532. [DOI] [PubMed] [Google Scholar]
- 12.Fantuzzi, G., G. Ku, M. W. Harding, D. J. Livingston, J. D. Sipe, K. Kuida, R. A. Flavell, and C. A. Dinarello. 1997. Response to local inflammation of IL-1 beta-converting enzyme-deficient mice. J. Immunol. 158:1818-1824. [PubMed] [Google Scholar]
- 13.Ghayur, T., S. Banerjee, M. Hugunin, D. Butler, L. Herzog, A. Carter, L. Quintal, L. Sekut, R. Talanian, M. Paskind, W. Wong, R. Kamen, D. Tracey, and H. Allen. 1997. Caspase-1 processes IFN-gamma-inducing factor and regulates LPS-induced IFN-gamma production. Nature 386:619-623. [DOI] [PubMed] [Google Scholar]
- 14.Green, D. R. 1998. Apoptotic pathways: the roads to ruin. Cell. 94:695-698. [DOI] [PubMed] [Google Scholar]
- 15.Green, D. R., and J. C. Reed. 1998. Mitochondria and apoptosis. Science 281:1309-1312. [DOI] [PubMed] [Google Scholar]
- 16.Gu, Y., K. Kuida, H. Tsutsui, G. Ku, K. Hsiao, M. A. Fleming, N. Hayashi, K. Higashino, H. Okamura, K. Nakanishi, M. Kurimoto, T. Tanimoto, R. A. Flavell, V. Sato, M. W. Harding, D. J. Livingston, and M. S. Su. 1997. Activation of interferon-gamma inducing factor mediated by interleukin-1beta converting enzyme. Science 275:206-209. [DOI] [PubMed] [Google Scholar]
- 17.Hisahara, S., J. Yuan, T. Momoi, H. Okano, and M. Miura. 2001. Caspase-11 mediates oligodendrocyte cell death and pathogenesis of autoimmune-mediated demyelination. J. Exp. Med. 193:111-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang, F. P., D. Xu, E. O. Esfandiari, W. Sands, X. Q. Wei, and F. Y. Liew. 1998. Mice defective in Fas are highly susceptible to Leishmania major infection despite elevated IL-12 synthesis, strong Th1 responses, and enhanced nitric oxide production. J. Immunol. 160:4143-4147. [PubMed] [Google Scholar]
- 19.Iizawa, Y., R. D. Wagner, and C. J. Czuprynski. 1993. Analysis of cytokine mRNA expression in Listeria-resistant C57BL/6 and Listeria-susceptible A/J mice during Listeria monocytogenes infection. Infect. Immun. 61:3739-3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang, S. J., S. Wang, H. Hara, E. P. Peterson, S. Namura, S. Amin-Hanjani, Z. Huang, A. Srinivasan, K. J. Tomaselli, N. A. Thornberry, M. A. Moskowitz, and J. Yuan. 2000. Dual role of caspase-11 in mediating activation of caspase-1 and caspase-3 under pathological conditions. J. Cell Biol. 149:613-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kano, A., T. Haruyama, T. Akaike, and Y. Watanabe. 1999. IRF-1 is an essential mediator in IFN-gamma-induced cell cycle arrest and apoptosis of primary cultured hepatocytes. Biochem. Biophys. Res. Commun. 257:672-677. [DOI] [PubMed] [Google Scholar]
- 22.Kawakami, K., M. H. Qureshi, T. Zhang, H. Okamura, M. Kurimoto, and A. Saito. 1997. IL-18 protects mice against pulmonary and disseminated infection with Cryptococcus neoformans by inducing IFN-gamma production. J. Immunol. 159:5528-5534. [PubMed] [Google Scholar]
- 23.Kuida, K., J. A. Lippke, G. Ku, M. W. Harding, D. J. Livingston, M. S. Su, and R. A. Flavell. 1995. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science 267:2000-2003. [DOI] [PubMed] [Google Scholar]
- 24.Li, P., H. Allen, S. Banerjee, S. Franklin, L. Herzog, C. Johnston, J. McDowell, M. Paskind, L. Rodman, and J. Salfeld. 1995. Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell 80:401-411. [DOI] [PubMed] [Google Scholar]
- 25.Lorber, B. 1997. Listeriosis. Clin. Infect. Dis. 24:1-9. [DOI] [PubMed] [Google Scholar]
- 26.Merrick, J. C., B. T. Edelson, V. Bhardwaj, P. E. Swanson, and E. R. Unanue. 1997. Lymphocyte apoptosis during early phase of Listeria infection in mice. Am. J. Pathol. 151:785-792. [PMC free article] [PubMed] [Google Scholar]
- 27.Miura, M., H. Zhu, R. Rotello, E. A. Hartwieg, and J. Yuan. 1993. Induction of apoptosis in fibroblasts by IL-1 beta-converting enzyme, a mammalian homolog of the C. elegans cell death gene ced-3. Cell 75:653-660. [DOI] [PubMed] [Google Scholar]
- 28.Miura, T., D. Mizuki, S. Sasaki, S. Hasegawa, H. Sashinami, and A. Nakane. 2000. Host resistance to Listeria monocytogenes infection is enhanced but resistance to Staphylococcus aureus infection is reduced in acute graft-versus-host disease in mice. Infect. Immun. 68:4340-4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miwa, K., M. Asano, R. Horai, Y. Iwakura, S. Nagata, and T. Suda. 1998. Caspase 1-independent IL-1beta release and inflammation induced by the apoptosis inducer Fas ligand. Nat. Med. 4:1287-1292. [DOI] [PubMed] [Google Scholar]
- 30.Monack, D. M., B. Raupach, A. E. Hromockyj, and S. Falkow. 1996. Salmonella typhimurium invasion induces apoptosis in infected macrophages. Proc. Natl. Acad. Sci. USA 93:9833-9838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moore, K. J., and G. Matlashewski. 1994. Intracellular infection by Leishmania donovani inhibits macrophage apoptosis. J. Immunol. 152:2930-2937. [PubMed] [Google Scholar]
- 32.Ojcius, D. M., P. Souque, J. L. Perfettini, and A. Dautry-Varsat. 1998. Apoptosis of epithelial cells and macrophages due to infection with the obligate intracellular pathogen Chlamydia psittaci. J. Immunol. 161:4220-4226. [PubMed] [Google Scholar]
- 33.Okamura, H., H. Tsutsui, S. Kashiwamura, T. Yoshimoto, and K. Nakanishi. 1998. Interleukin-18: a novel cytokine that augments both innate and acquired immunity. Adv. Immunol. 70:281-312. [DOI] [PubMed] [Google Scholar]
- 34.Pirhonen, J., T. Sareneva, I. Julkunen, and S. Matikainen. 2001. Virus infection induces proteolytic processing of IL-18 in human macrophages via caspase-1 and caspase-3 activation. Eur. J. Immunol. 31:726-733. [DOI] [PubMed] [Google Scholar]
- 35.Rogers, H. W., M. P. Callery, B. Deck, and E. R. Unanue. 1996. Listeria monocytogenes induces apoptosis of infected hepatocytes. J. Immunol. 156:679-684. [PubMed] [Google Scholar]
- 36.Schaible, U. E., H. L. Collins, and S. H. Kaufmann. 1999. Confrontation between intracellular bacteria and the immune system. Adv. Immunol. 71:267-377. [DOI] [PubMed] [Google Scholar]
- 37.Schumann, R. R., C. Belka, D. Reuter, N. Lamping, C. J. Kirschning, J. R. Weber, and D. Pfeil. 1998. Lipopolysaccharide activates caspase-1 (interleukin-1-converting enzyme) in cultured monocytic and endothelial cells. Blood 91:577-584. [PubMed] [Google Scholar]
- 38.Thornberry, N. A., H. G. Bull, J. R. Calaycay, K. T. Chapman, A. D. Howard, M. J. Kostura, D. K. Miller, S. M. Molineaux, J. R. Weidner, and J. Aunins. 1992. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature 356:768-774. [DOI] [PubMed] [Google Scholar]
- 39.Tsutsui, H., N. Kayagaki, K. Kuida, H. Nakano, N. Hayashi, K. Takeda, K. Matsui, S. Kashiwamura, T. Hada, S. Akira, H. Yagita, H. Okamura, and K. Nakanishi. 1999. Caspase-1-independent, Fas/Fas ligand-mediated IL-18 secretion from macrophages causes acute liver injury in mice. Immunity 11:359-367. [DOI] [PubMed] [Google Scholar]
- 40.Unanue, E. R. 1997. Inter-relationship among macrophages, natural killer cells and neutrophils in early stages of Listeria resistance. Curr. Opin. Immunol. 9:35-43. [DOI] [PubMed] [Google Scholar]
- 41.Unanue, E. R. 1996. Macrophages, NK cells and neutrophils in the cytokine loop of Listeria resistance. Res. Immunol. 147:499-505. [DOI] [PubMed] [Google Scholar]
- 42.Wang, S., M. Miura, Y. Jung, H. Zhu, V. Gagliardini, L. Shi, A. H. Greenberg, and J. Yuan. 1996. Identification and characterization of Ich-3, a member of the interleukin-1beta converting enzyme (ICE)/Ced-3 family and an upstream regulator of ICE. J. Biol. Chem. 271:20580-20587. [DOI] [PubMed] [Google Scholar]
- 43.Wang, S., M. Miura, Y. K. Jung, H. Zhu, E. Li, and J. Yuan. 1998. Murine caspase-11, an ICE-interacting protease, is essential for the activation of ICE. Cell. 92:501-509. [DOI] [PubMed] [Google Scholar]
- 44.Weinrauch, Y., and A. Zychlinsky. 1999. The induction of apoptosis by bacterial pathogens. Annu. Rev. Microbiol. 53:155-187. [DOI] [PubMed] [Google Scholar]
- 45.Zhang, T., K. Kawakami, M. H. Qureshi, H. Okamura, M. Kurimoto, and A. Saito. 1997. Interleukin-12 (IL-12) and IL-18 synergistically induce the fungicidal activity of murine peritoneal exudate cells against Cryptococcus neoformans through production of gamma interferon by natural killer cells. Infect. Immun. 65:3594-3599. [DOI] [PMC free article] [PubMed] [Google Scholar]