Abstract
Innate immunity serves as a first line defense in vertebrate organisms by providing an initial barrier to microorganisms and triggering antigen-specific responses. Antimicrobial peptides are thought to be effectors of innate immunity through their antibiotic activity and direct killing of microorganisms. Evidence to support this hypothesis in vertebrates is indirect, based on expression profiles and in vitro assays using purified peptides. Here we investigated the function of antimicrobial peptides in vivo using mice deficient in an antimicrobial peptide, mouse β-defensin-1 (mBD-1). We find that loss of mBD-1 results in delayed clearance of Haemophilus influenzae from lung. These data demonstrate directly that antimicrobial peptides of vertebrates provide an initial block to bacteria at epithelial surfaces.
The airways and lungs are protected from infection by an elaborate host defense system (23). This includes mechanical and physical clearance accomplished by coughing and the mucociliary ladder. Pathogens not immediately cleared by these mechanisms encounter the innate and adaptive immune system. A genetic breach in innate immunity of the respiratory system was recently described by Smith et al., who showed decreased bacterial killing of airway surface fluid (ASF) from epithelia derived from patients with cystic fibrosis (21). This observation led to a more detailed characterization of the molecules in ASF with antibiotic activity.
The innate host defense system includes numerous secreted peptides and proteins with antimicrobial activity (6) including lysozyme, lactoferrin, secretory leukocyte protease inhibitor, secretory phospholipase A2, defensins (2, 5), cathelicidins (3), proteases, and pre-existing antibodies and complement (23). Many of these substances are secreted by serous cells of airway submucosal glands. Others, such as defensins and cathelicidins, are also secreted by surface epithelial cells and appear to be up-regulated in response to infection or exposure to lipopolysaccharide. These substances have been shown to kill a spectrum of bacteria in vitro (2, 3, 5). It is believed that this mixture facilitates the maintenance of a sterile environment in the lungs. However, elucidating the importance of individual components and defining specific functions is a difficult task. Individual components may have synergistic or antagonistic activities; studies to date have been limited to functional characterization of purified components in vitro.
We chose to develop an animal model of defensin deficiency to better define the role of this family of peptides. Murine β-defensin 1 (mBD-1), a murine homologue of human β-defensin 1 (hBD-1), is expressed at epithelial surfaces, with highest levels found in kidneys (1, 14, 19). Recombinant mBD-1 exhibits antimicrobial activity in vitro, although its function in vivo is unknown (1). When expression of hBD-1 was blocked in a human bronchial xenograft model, the ASF lost some of its antimicrobial activity (10). We used established murine models of bacterial lung clearance to study the role of mBD-1 in pulmonary host defense.
MATERIALS AND METHODS
Generation of mBD-1-deficient mice.
A genomic clone of β-defensin-1 was isolated from a phage library, and a restriction map was produced. The targeting vector was designed to delete exon 1 by replacing it with the positive selection cassette containing neo. A negative selection cassette containing herpes simplex virus thymidine kinase was placed outside the homologous sequence. Lex1 embryonic stem (ES) cells (generated from 129SvEv mice) were transfected with the targeting vector. Positive-negative selection was performed, and colonies were analyzed by Southern analysis. Mice were generated by injecting mutated ES cells into C57BL/6 blastocysts. Three chimeric mice were born and all three were transmitted to the germ line. Lexicon Genetics Inc. (The Woodlands, Tex.) performed the process of generating and selecting heterozygote males.
The founder animals were backcrossed with C57BL/6 mice by using a marker-assisted selection protocol (Charles River Laboratories) which yielded congenic heterozygous C57BL/6 mice after five generations. Matings of heterozygous animals were established to generate homozygous mBD-1-deficient (mBD-1−/−) and wild-type (mBD-1+/+) animals. All animals were genotyped by PCR using genomic DNA extracted from tail snips as a template. For the experiments, 8- to 11-week-old animals were used, either homozygote littermates from heterozygote parents or sex- and age-matched animals from matings between homozygote parents of the mBD-1+/+ or the mBD-1−/− genotype. Animals were housed in the Animal Facility of the Wistar Institute under standard care conditions in microisolators. All procedures were in accordance with and approved by the Institutional Animal Care and Use Committee.
Detection of mBD-1 mRNA.
Total RNA was extracted from kidney, trachea, and differentiated primary airway cultures (7) by using Trizol (Life Technologies). An RNase protection assay was performed with an RNase protection assay (RPA) kit (RPA III; Ambion) and digoxigenin-labeled RNA probes, using the DIG OMNI system (Roche) for probe synthesis and detection. The sense and antisense primers for reverse transcription-PCR (RT-PCR) featured SP6 and T7 promoter overhangs, respectively, which served for in vitro transcription of probes for RPA. RT-PCR was performed in two steps; cDNA was synthesized with OmniScript RT (Qiagen) and an oligo(dT15) primer. The cDNA was purified through MicroSpin S-200HR columns (Amersham-Pharmacia) before PCR was performed using specific primers for glyceraldehyde-3-phosphate dehydrogenase mRNA (GAPDH-SP6-L, 5′ ATTTAGGTGACACTATAGAATATCATCTTGGGCTACACTGAGG; GAPDH-T7-R, 5′ TAATACGACTCACTATAGGGCGATGGAGGCCATGTAGGCCATG) and for mBD-1 (mBD-1-SP6-L, 5′ATTTAGGTGACACTATAGAATATTCTCCTGGTGATGATATGTTTTCT; mBD-1-T7-R, 5′ TAATACGACTCACTATAGGGCGATTACAATCCATCGCTCGTCC). In order to enhance the sensitivity for detection of mBD-1 in trachea, the PCR products were labeled with digoxigenin and visualized after electrophoresis and blotting, with a PCR DIG probe synthesis kit and DIG OMNI detection system (Roche).
Bacterial challenge and analysis.
Haemophilus influenzae strain H338 is a mutant of a type b clinical isolate, Eagan, with a kanamycin resistance gene inserted in oapB, which has been shown to have no contribution to in vivo survival (8). Streptococcus pneumoniae strain D39 is a virulent capsular type 2 strain used in studies on the pathogenesis of pneumococcal infections (15). Frozen bacteria were thawed and cultured overnight on agar plates. The bacteria in a single arbitrarily selected colony were grown in liquid brain heart infusion medium supplemented with 1% Fildes enrichment (Becton-Dickinson) and 2 μg of NAD (Sigma) per ml. Bacteria were grown to mid-log phase, pelleted, and resuspended in PBS, and the optical density at 600 nm was determined. Based on a previously established standard curve, the bacteria were further diluted to obtain the desired concentration, which was confirmed by quantitative culture for each experiment. Anesthetized mice were dosed intranasally with 50 μl of bacterial suspension.
For clearance experiments, animals were euthanized 24 h after bacterial challenge unless otherwise indicated. Bronchoalveolar lavage was performed in situ with a 20-gauge catheter inserted into the proximal trachea, flushing the lower airways twice with 0.5 ml of phosphate-buffered saline (PBS) containing 5 mM EDTA and 1% (vol/vol) protease inhibitor cocktail (P8340; Sigma). Serial dilutions of bronchoalveolar lavage (BAL) fluid were plated for bacterial counts. The BAL cells were separated from the BAL fluid by centrifugation, resuspended in PBS containing 4% bovine serum albumin, and counted, and a fraction was cytospun on microscopic slides for staining with Kwik-Diff (Shandon) and subsequent differential counts. Cleared BAL fluid samples were assayed for tumor necrosis factor alpha (TNF-α), interleukin 6 (IL-6), and IL-1β by using commercial enzyme-linked immunosorbent assay kits (R&D Systems). The left lung together with the trachea were homogenized and used for serial plating to quantify viable bacteria. The right lung was inflated and embedded by using optimal cutting temperature embedding medium (Sakura Finetek) for preparation of hematoxylin- and eosin-stained tissue sections. Sections were analyzed in a blinded fashion by a single observer and graded (0 to 10) for severity of inflammation in the peribronchiolar, perivascular, and alveolar compartments.
For survival experiments, three groups of five or more animals of each genotype, C57BL/6, mBD-1+/+, and mBD-1−/−, were infected intranasally with increasing doses of S. pneumoniae. Infected mice were monitored twice a day for 14 days. Animals that appeared to be terminally ill were euthanized. The spleens of dead and euthanized animals were removed aseptically and homogenized in PBS. A macroscopic pathological examination was performed, focusing on lungs, spleens, and general signs of sepsis, such as petechial bleeding in internal organs. The bacterial load was determined by plating serial dilutions of the spleen homogenate on selective agar medium in order to confirm S. pneumoniae sepsis as cause of death. The 50% lethal doses were extrapolated for each genotype from the respective mortality rates of three dosage groups.
Statistical analysis.
The breeding data were analyzed by chi-square goodness-of-fit test for a 1:2:1 distribution. Differences in bacterial load, cytokine concentrations, and BAL cell counts were analyzed by rank sum test. Results were considered significant when P values were <0.05.
RESULTS
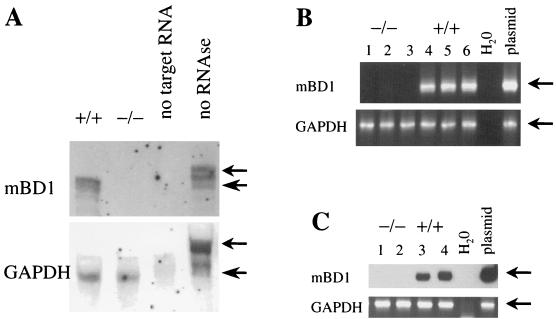
mBD-1 knockout mice were generated by homologous recombination in ES cells, which were injected into blastocysts, yielding male animals transmitting the disrupted gene to the germ line. These founder animals were backcrossed in order to obtain a congenic C57BL/6 colony. Expression of mBD-1 was analyzed by Northern blotting, RPA, and RT-PCR, showing the absence of mBD-1-specific mRNA obtained from the kidneys and tracheas of homozygous mBD-1-deficient mice (mBD-1−/−) as well as in differentiated primary airway cultures derived from these mice (Fig. 1).
FIG. 1.
Absence of mRNA specific for mBD-1 in mBD-1−/− mice. (A) RNase protection assay for mRNA specific for mBD-1 and the housekeeping GAPDH gene with 10 μg of total kidney RNA from mBD-1+/+ and mBD-1−/− mice. Small and large arrows indicate the full-length probe and the specifically protected fragment, respectively. (B) RT-PCR for mBD-1 and GAPDH from fully differentiated primary airway cultures derived from mBD-1−/− (lanes 1 to 3) and mBD-1+/+ mice (lanes 4 to 6). Lanes 1 and 4, PBS; lanes 2 and 5, LPS; lanes 3 and 6, H. influenzae. (C) RT-PCR for mBD-1 and GAPDH with total RNA from mouse tracheas collected from mBD-1−/− (lanes 1 and 2) and mBD-1+/+ (lanes 3 and 4) animals, either uninfected (lanes 1 and 3) or 24 h after infection with H. influenzae (lanes 2 and 4). The mBD-1 PCR products were visualized by chemiluminescence.
Homozygous mBD-1-deficient mice appeared to be normal and healthy. Histologic analysis of tissues from mBD-1−/− mice revealed no gross differences from those of mBD-1+/+ mice (data not shown). The fertility of mBD-1−/− animals was unchanged compared to that of mBD-1+/+ mice (Table 1). However, matings between heterozygous mice yielded significantly fewer mBD-1−/− offspring than expected according to Mendelian inheritance (Table 1).
TABLE 1.
Summary of the breeding data from the congenic mBD-1−/− colonya
| Mating type | Total no. of offspring | Litter size | No. (%) of:
|
No. (%) with genotype:
|
|||
|---|---|---|---|---|---|---|---|
| Females | Males | wt | het | KO | |||
| het × het | 275 | 6.7 ± 1.7 | 135 (49.1) | 140 (50.9) | 70 (25.4) | 155 (56.5) | 50 (18.2) |
| wt × wt | 124 | 5.6 ± 2.4 | 65 (52.4) | 59 (47.6) | 124 (100) | ||
| KO × KO | 133 | 7.0 ± 1.8 | 70 (52.6) | 63 (47.4) | 133 (100) | ||
het, heterozygous; wt, wild type; KO, knockout. Significantly fewer mBD-1−/− mice were obtained from heterozygote parents than expected according to Mendelian inheritance (Pearson chi-square goodness-of-fit test, 1:2:1 distribution; P = 0.025). However, the fertility of homozygous parents of the mBD-1+/+ and mBD-1−/− genotypes was found to be indistinguishable.
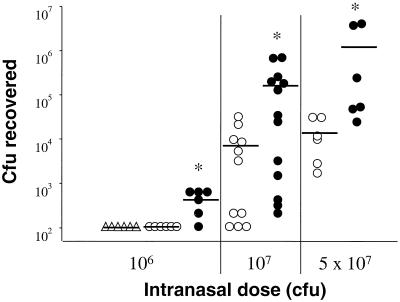
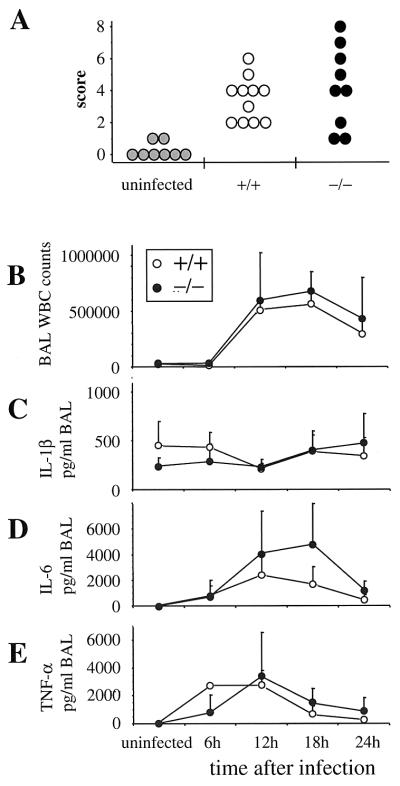
Mice were infected intranasally with H. influenzae type b strain Eagan (12) and S. pneumoniae D39 type 2 (15). Animals were euthanized at different times after administration of the bacteria to assay for clearance of the pathogens and for indices of inflammation. BAL was performed in situ, followed by removal of lungs and trachea en bloc. Samples were collected aseptically and used to determine the number of viable bacteria and cytokine levels in BAL fluid and lung homogenate and histopathology in the lower respiratory tract. Both mBD-1−/− and mBD-1+/+ mice are able to eventually clear high doses of bacteria (data not shown). However, clearance of bacteria was significantly less efficient in mBD-1−/− mice 24 h after bacterial challenge at three different doses (Fig. 2). Animals heterozygous for mBD-1 deficiency demonstrated no abnormality in clearance of H. influenzae at lower doses of bacteria and a slight delay in clearance at high doses of bacteria that was less substantial than that observed in homozygotes (data not shown). Microscopic evaluation of lung tissue sections, using a blinded scoring system to assess the amount of pathological changes, revealed no differences between the mBD-1−/− and mBD-1+/+ mice (Fig. 3A). In both groups, there was a predominantly neutrophilic infiltration in the peribronchiolar and alveolar compartments. The total numbers and the differentials of cells obtained from BAL did not differ between mBD-1−/− and mBD-1+/+ mice (Fig. 3B). Measurement of cytokines in BAL fluid revealed no significant differences in the levels of IL-6, TNF-α, and IL-1β between mBD-1−/− and mBD-1+/+ animals, either in uninfected mice or at 6, 12, 18, and 24 h after infection (Fig. 3C, D, and E).
FIG. 2.
Clearance of H. influenzae 24 h after infection. mBD-1−/− mice have significantly higher loads at all three doses than mBD-1+/+ mice, as indicated by asterisks (Mann-Whitney rank sum test, with P values for increasing doses of 0.015, 0.022, and 0.009, respectively). Symbols represent individual animals (▵, C57B1/6; ○, mBD-1+/+; •, mBD-1−/−); bars show group averages. The limit of detection for bacteria in this assay was 100 CFU per lung; lungs with fewer bacteria are therefore depicted as having 100 CFU.
FIG. 3.
Histopathology and inflammatory response are unchanged in mBD-1−/− mice. (A) Histopathological assessment of lung sections obtained from uninfected mice and from mBD-1+/+ and mBD-1−/− animals 24 h after infection with 107 CFU of H. influenzae. Individual sections were scored in a blinded fashion for inflammatory responses, which predominantly consisted of neutrophilic infiltration around airways and into the alveolar space. (B to E) Kinetics of the inflammatory response to infection with 5 × 107 CFU H. influenzae. BAL fluid was collected at the indicated time points, and total BAL white cell (WBC) counts were determined (B), as well as levels of IL-1β (C), IL-6 (D), and TNF-α (E). Three animals per group were analyzed per time point, except for 12 h (11 mBD-1+/+ and 9 mBD-1−/− animals in each group) and 24 h (6 animals in each group).
S. pneumoniae infects mice efficiently, and the animals develop severe pneumonia followed by a lethal sepsis. Groups of animals were infected intranasally with increasing doses of S. pneumoniae and were monitored for survival over 14 days in order to determine the dose-dependent mortality. No significant differences were observed between C57BL/6, congenic mBD-1+/+, and mBD-1−/− mice, with extrapolated 50% lethal doses of 107.0, 106.6, and 106.8 CFU per animal, respectively (five to seven animals/group and three to five groups/strain). Necropsy results showed evidence of sepsis in all groups.
DISCUSSION
The response of an organism to infection at a mucosal surface, such as the airway, is a complex process with several phases of attack and potential redundancy at each phase. The role of one β-defensin in mediating the clearance of H. influenzae and S. pneumoniae in the lung was the focus of this study. The function of the innate immune system is better understood in insects, where few signaling pathways regulate the expression of several classes of antimicrobial peptides (17). For example, mutations in the Toll-signaling pathway in Drosophila reduced the expression of a number of antimicrobial peptides and resulted in significant mortality after fungal infection (18). At least two distinct functions of β-defensins in mammals have been proposed based on in vitro assays, including direct antibiotic activity and chemoattractant activity that could contribute to the initial wave of inflammation and trigger the acquired immune system (25).
Evaluating the specific contribution of individual molecules such as mBD-1 to the host response following pathogenic challenge is potentially complicated by the existence of redundant pathways. Six different β-defensins have been identified in mice (1, 4, 16, 19, 20, 24), and four similar proteins have been identified in humans (2, 5, 9, 13). A subset of the β-defensins are found in lungs, in addition to a number of other proteins (e.g., lysozyme and lactoferrin) and processes (e.g., mucociliary clearance) that contribute to pulmonary host defense (22). Our data demonstrated that elimination of mBD-1 results in a defect in the ability of the host to clear H. influenzae from the lung, which is most consistent with this peptide functioning as an antibiotic at the airway surface. H. influenzae is eventually cleared, however, and the initial inflammatory response, as measured by cytokines and neutrophils in BAL, is not compromised. This illustrates the importance of redundancy in host defense.
The nature of the host response to bacterial pathogens in the mBD-1-deficient mouse also was evaluated in lieu of its proposed role in chemotaxis (25). No significant deviations in the cellular inflammatory response to H. influenzae were detected in the absence of mBD-1, failing to confirm its role as a chemoattractant. However, biological redundancy could complicate the interpretation of a negative result.
Considering the panel of antibacterial peptides and proteins present on the airway surface, we did not expect to see a broad compromise in host defense as a result of the deficiency of a single β-defensin. In fact, mortality due to intrapulmonary S. pneumoniae was independent of mBD-1 expression, confirming the selectivity of the phenotype in these animals. This apparent selectivity does not rule out in vivo activity of mBD-1 to S. pneumoniae if redundant effector pathways participate in host defense to this pathogen.
It is our desire to further utilize these animal models to better understand the host defense of the airways in vivo. This knowledge of normal host defense, we hope, will allow better insight into abnormalities of host defense such as those seen in cystic fibrosis.
Acknowledgments
We thank Tim Miller, Lance Dunlop, Ruth Qian, and the Animal Models Group and the Cell and Morphology Core of the Institute for Human Gene Therapy for excellent technical assistance. Many thanks also go to Donald Davidson and Rainer Wiewrodt for technical support and helpful discussions and to Kathleen Propert for advice in biostatistics.
This work was supported by the Cystic Fibrosis Foundation and the NIH (R01 HL49040 and P30 DK47757).
Editor: J. D. Clements
REFERENCES
- 1.Bals, R., M. J. Goldman, and J. M. Wilson. 1998. Mouse beta-defensin 1 is a salt-sensitive antimicrobial peptide present in epithelia of the lung and urogenital tract. Infect. Immun. 66:1225-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bals, R., X. Wang, Z. Wu, T. Freeman, V. Bafna, M. Zasloff, and J. M. Wilson. 1998. Human beta-defensin 2 is a salt-sensitive peptide antibiotic expressed in human lung. J. Clin. Investig. 102:874-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bals, R., X. Wang, M. Zasloff, and J. M. Wilson. 1998. The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc. Natl. Acad. Sci. USA 95:9541-9546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bals, R., X. Wang, R. L. Meegalla, S. Wattler, D. J. Weiner, M. C. Nehls, and J. M. Wilson. 1999. Mouse beta-defensin 3 is an inducible antimicrobial peptide expressed in the epithelia of multiple organs. Infect. Immun. 67:3542-3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bensch, K. W., M. Raida, H. J. Magert, P. Schulz-Knappe, and W. G. Forssmann. 1995. hBD-1: a novel beta-defensin from human plasma. FEBS Lett. 368:331-335. [DOI] [PubMed] [Google Scholar]
- 6.Bevins, C. L. 1999. Scratching the surface: inroads to a better understanding of airway host defense. Am. J. Respir. Cell Mol. Biol. 20:861-863. [DOI] [PubMed] [Google Scholar]
- 7.Davidson, D. J., F. M. Kilanowski, S. H. Randell, D. N. Sheppard, and J. R. Dorin. 2000. A primary culture model of differentiated murine tracheal epithelium. Am. J. Physiol. Lung Cell Mol. Physiol. 279:L766-L778. [DOI] [PubMed]
- 8.Fan, X., H. Goldfine, E. Lysenko, and J. N. Weiser. 2001. The transfer of choline from the host to bacterial cell surface requires glpQ in Haemophilus influenzae. Mol. Microbiol. 41:1029-1036. [DOI] [PubMed] [Google Scholar]
- 9.Garcia, J. R., A. Krause, S. Schulz, F. J. Rodriguez-Jimenez, E. Kluver, K. Adermann, U. Forssmann, A. Frimpong-Boateng, R. Bals, and W. G. Forssmann. 2001. Human beta-defensin 4: a novel inducible peptide with a specific salt-sensitive spectrum of antimicrobial activity. FASEB J. 15:1819-1821. [PubMed] [Google Scholar]
- 10.Goldman, M. J., G. M. Anderson, E. D. Stolzenberg, U. P. Kari, M. Zasloff, and J. M. Wilson. 1997. Human beta-defensin-1 is a salt-sensitive antibiotic in lung that is inactivated in cystic fibrosis. Cell 88:553-560. [DOI] [PubMed] [Google Scholar]
- 11.Hagberg, L., I. Engberg, R. Freter, J. Lam, S. Olling, and C. Svanborg Eden. 1983. Ascending, unobstructed urinary tract infection in mice caused by pyelonephritogenic Escherichia coli of human origin. Infect. Immun. 40:273-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansen, E. J., and G. B. Toews. 1992. Animal models for the study of noninvasive Haemophilus influenzae disease: pulmonary clearance systems. J. Infect. Dis. 165(Suppl. 1):S185-S187. [DOI] [PubMed]
- 13.Harder, J., J. Bartels, E. Christophers, and J. M. Schroder. 2001. Isolation and characterization of human beta-defensin-3, a novel human inducible peptide antibiotic. J. Biol. Chem. 276:5707-5713. [DOI] [PubMed] [Google Scholar]
- 14.Huttner, K. M., C. A. Kozak, and C. L. Bevins. 1997. The mouse genome encodes a single homolog of the antimicrobial peptide human beta-defensin 1. FEBS Lett. 413:45-49. [DOI] [PubMed] [Google Scholar]
- 15.Iannelli, F., B. J. Pearce, and G. Pozzi. 1999. The type 2 capsule locus of streptococcus pneumoniae. J. Bacteriol. 181:2652-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jia, H. P., S. A. Wowk, B. C. Schutte, S. K. Lee, A. Vivado, B. F. Tack, C. L. Bevins, and P. B. McCray, Jr. 2000. A novel murine beta-defensin expressed in tongue, esophagus, and trachea. J. Biol. Chem. 275:33314-33320. [DOI] [PubMed] [Google Scholar]
- 17.Lehrer, R. I., and T. Ganz. 1999. Antimicrobial peptides in mammalian and insect host defence. Curr. Opin. Immunol. 11:23-27. [DOI] [PubMed] [Google Scholar]
- 18.Lemaitre, B., E. Nicolas, L. Michaut, J. M. Reichhart, and J. A. Hoffmann. 1996. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 86:973-983. [DOI] [PubMed] [Google Scholar]
- 19.Morrison, G. M., D. J. Davidson, F. M. Kilanowski, D. W. Borthwick, K. Crook, A. I. Maxwell, J. R. Govan, and J. R. Dorin. 1998. Mouse beta defensin-1 is a functional homolog of human beta defensin-1. Mamm. Genome 9:453-457. [DOI] [PubMed] [Google Scholar]
- 20.Morrison, G. M., D. J. Davidson, and J. R. Dorin. 1999. A novel beta defensin, Defb2, which is upregulated in the airways by lipopolysaccharide. FEBS Lett. 442:112-116. [DOI] [PubMed] [Google Scholar]
- 21.Smith, J. J., S. M. Travis, E. P. Greenberg, and M. J. Welsh. 1996. Cystic fibrosis airway epithelia fail to kill bacteria because of abnormal airway surface fluid. Cell 85:229-236. (Erratum, 87:following 355.) [DOI] [PubMed]
- 22.Travis, S. M., P. K. Singh, and M. J. Welsh. 2001. Antimicrobial peptides and proteins in the innate defense of the airway surface. Curr. Opin. Immunol. 13:89-95. [DOI] [PubMed] [Google Scholar]
- 23.Wilmott, R., M. Fiedler, and J. M. Stark. 1998. Disorders of the respiratory tract in children. W. B. Saunders, Philadelphia, Pa.
- 24.Yamaguchi, Y., S. Fukuhara, T. Nagase, T. Tomita, S. Hitomi, S. Kimura, H. Kurihara, and Y. Ouchi. 2001. A novel mouse beta-defensin, mBD-6, predominantly expressed in skeletal muscle. J. Biol. Chem. 276:31510-31514. [DOI] [PubMed] [Google Scholar]
- 25.Yang, D., O. Chertov, S. N. Bykovskaia, Q. Chen, M. J. Buffo, J. Shogan, M. Anderson, J. M. Schroder, J. M. Wang, O. M. Howard, and J. J. Oppenheim. 1999. Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science 286:525-528. [DOI] [PubMed] [Google Scholar]