Abstract
Immunity against Mycobacterium tuberculosis depends largely on activation of cell-mediated responses, and gamma interferon has been shown to play a crucial role in this process in both humans and animal models. Since the lung is normally the organ in which infection is initiated and is the major site of pathology, immune responses in the lung play a significant role in restricting initial infection with M. tuberculosis. The aim of the present study was to stimulate efficient immunity in the lung by targeting the gut mucosa. Detoxified monophosphoryl lipid A (MPL) has been shown to be a relatively nontoxic adjuvant which efficiently promotes the induction of type 1 responses when it is given by the traditional subcutaneous route. We have therefore compared subcutaneous immunization of mice to oral immunization by using a model subunit vaccine carrying two immunodominant proteins from M. tuberculosis, in combination with MPL-based adjuvants. While less effective when used to prime a response, a heterologous priming and boosting vaccination strategy employing oral boosting induced significant systemic type 1 responses which equaled and surpassed those attained by subcutaneous immunization protocols. Moreover, the increased immune responses observed correlated with the induction of substantial protection against subsequent aerosol infection with virulent M. tuberculosis at levels comparable to, or better than, those obtained by multiple subcutaneous vaccinations. These results demonstrate that booster vaccinations via mucosal surfaces, by combining efficient subunit vaccines with the potent adjuvant MPL, may be an effective method of addressing some of the shortcomings of current vaccination strategies.
Despite decades of effort and enormous expenditure, tuberculosis (TB) remains one of the world's most devastating diseases. A recent World Health Organization report estimated that there may be more than 100 million new cases of TB in the first decade of the 21st century (4). While Mycobacterium bovis BCG vaccination has remained a mainstay of control programs for more than half a century, neither vaccination campaigns nor improved drug treatment regimens have prevented the recent resurgence of TB (3). Together with the variable efficacy of the BCG vaccine (16) against adult pulmonary TB, this resurgence has driven the search for a vaccine with high efficacy to combat the TB epidemic. However, the apparent efficacy of neonatal vaccination against pediatric manifestations of TB (10, 32) and other associated health benefits of BCG vaccination (21) mean that BCG is unlikely to be retired in the immediate future. The benefit in children appears to be most evident for systemic disease (24) (suggesting the induction of effective systemic immunity) but is clearly unable to prevent pulmonary disease in adults, suggesting that the key to decreasing TB mortality may be to enhance immunity in the lung, the target organ for both pathogen entry and disease-associated pathology. BCG itself seems to be a poor candidate as a booster vaccine, since multiple BCG vaccinations do not appear to offer significant benefits (16). This may be because residual immunity after the first BCG vaccination prevents subsequent inoculations of BCG from replicating, which appears to be necessary to induce a strong immune response (9).
Previously published work has shown that recombinant antigens can induce good systemic immunity against tuberculosis in animal models (26). However, nothing is known about the ability of these vaccines to stimulate immunity at mucosal surfaces, at which there may be a more efficient route of boosting immune responses in the lung (25). There are obvious advantages to choosing a simple oral administration over injection as the route of vaccination for a vaccine that may be administered to a very large number of recipients, especially in areas where human immunodeficiency virus is endemic and the use of needles entails increased risk. However, few vaccines which have been administered by the mucosal route are able to stimulate effective cell-mediated immune responses, the exceptions being invasive bacterial vaccines (23, 35). Indeed, most studies have assessed efficacy solely by the ability to induce a humoral response (12). We have therefore studied the oral delivery of a model subunit vaccine based on immunogenic antigens derived from Mycobacterium tuberculosis as a strategy for inducing immunity to TB in animal models.
The efficacy of vaccination with recombinant proteins is known to be critically dependent upon the choice of adjuvant (8, 22). Unfortunately, none of the adjuvants generally approved for human vaccination promote the development of the type 1 immune responses that are crucial for the establishment of protective immunity to M. tuberculosis (22, 31). Even among adjuvants used in animal studies, few that induce type 1 immune responses are suitable for, or have even been studied by, anything other than the subcutaneous route. However, some microbial components are able to stimulate strong cell-mediated responses by stimulating antigen-presenting cells (APC) to produce proinflammatory cytokines (13) and this makes these molecules potential adjuvant candidates for TB vaccination. The toxicity of one such molecule, detoxified monophosphoryl lipid A (MPL), is sufficiently low that it has been tested as a potential adjuvant in humans (31). We have previously shown that MPL can function as an effective adjuvant if it is delivered subcutaneously in the depot-forming agent dioctadecylammonium bromide (DDA) (8). In this study, we have combined MPL-containing adjuvants with a prototypic subunit recombinant vaccine and shown that the use of this combination via the oral route gives levels of protection against aerosol infection in mice and guinea pigs which are equivalent to, or better than, those reported for subcutaneous vaccination.
MATERIALS AND METHODS
Animals.
Female pathogen-free C57BL/6 mice, 6 to 8 weeks of age, were obtained from Bomholtegaard, Ry, Denmark, while female outbred Dunkin Hartley guinea pigs were obtained from Møllegaard Breeding and Research Center A/S, Lille Skensved, Denmark. Animals were housed in isolator cages in the Statens Serum Institute's animal facilities. M. tuberculosis-infected animals were housed in a separate BSL3 facility. All animals were allowed a 1-week rest period after delivery before the initiation of experiments.
Immunization.
As a control, a single dose of BCG Danish 1331 (5 × 104 CFU) was injected subcutaneously at the base of the tail. Recombinant vaccine (a fusion protein composed of Ag85B and the early secretory antigenic target ESAT-6 (designated hybrid) was delivered in adjuvant, except where indicated below, as two doses, with a 2-week interval between vaccinations. For subcutaneous vaccination, the mice were injected at the base of the tail with antigen in 25 μg of MPL (Corixa, Seattle, Wash.) emulsified in DDA (250 μg/dose; Eastman Kodak Co., Rochester, N.Y.) in a total volume of 200 μl, as recently described (26). For oral vaccination, mice were anesthetized by intraperitoneal injection of 100 μl of Metofane (Pitman-Moore, Mundelein, Ill.), a soft plastic tube was inserted into the lower throat (past the tracheal opening), and 50 μl of a solution containing adjuvant with or without antigen was administered. Adjuvants used for oral vaccination were a stable emulsified MPL (MPLSE) (Corixa) diluted 1:4 in phosphate-buffered saline (PBS), an aqueous-formulation MPL (MPLAF) (Corixa), cholera toxin (CT) (Sigma-Aldrich, St. Louis, Mo.), or modified, heat-labile toxin (LT) (kindly provided by J. Clements, Tulane University Health Sciences Center).
M. tuberculosis infection.
Animals were infected with M. tuberculosis (Erdman) by the aerosol route in a Glas-Col inhalation exposure system. Exposure times were calibrated to deliver approximately 20 to 25 bacilli into the lungs of each guinea pig and 20 CFU into the lungs of each infected mouse. These numbers were tested by the sacrifice of test animals shortly after exposure (data not shown). For experiments, animals were sacrificed 6 weeks after infection and bacterial numbers in the lung and spleen were determined by triplicate serial titrations on 7H11 agar plates. All results were based on four to eight animals per time point.
Antigen preparation.
The recombinant fusion protein ESAT-6-Ag85B (hybrid) was produced in Escherichia coli cells and purified using a His tag purification system as described previously (26). The lipopolysaccharide (LPS) content of the purified protein was below 0.3 ng/μg of protein, and the preparation had no significant nonspecific activity on naïve cells. The protein was diluted into a PBS-0.1% Tween 20 buffer and kept at −80°C until use.
Lymphocyte cultures.
Organs were homogenized by maceration through a fine-mesh stainless steel sieve into complete RPMI medium (2 mM glutamine, a 100-U/ml concentration [each] of penicillin 6-potassium and streptomycin sulfate, 10% fetal calf serum, and 50 mM 2-mercaptoethanol; GIBCO, Grand Island, N.Y.). The cells were counted, an aliquot was diluted to 8 × 106 cells/ml, and the cells were dispensed in 100-μl volumes into 96-well plates containing twofold serial dilutions of either hybrid (from 10 μg/ml) or concanavalin A (from 5 μg/ml) in 100 μl of complete RPMI medium. Cultures were incubated at 37°C in 10% CO2 for 3 days before the removal of 100 μl of supernatant for cytokine determination by enzyme-linked immunosorbent assay (ELISA).
ELISA for IFN-γ.
A double-sandwich ELISA method was used to quantify the levels of gamma interferon (IFN-γ) in duplicate titrations of culture supernatants by using a commercial kit for IFN-γ assay in accordance with the instructions of the manufacturer (Mabtech, AB, Stockholm, Sweden). Concentrations of IFN-γ in the samples were calculated by a standard curve generated from recombinant IFN-γ (Life Technologies), and results were expressed in picograms per milliliter. The differences in results between the duplicate wells were consistently less than 10% of the mean.
FACS analysis of lymphocytes.
Cells were isolated as described above from the lungs and spleens of mice perfused with 15 ml of cold saline containing 50 U of heparin sulfate/ml. Red blood cells were lysed by a 2-min incubation in lysis buffer (0.15 mM NH4Cl, 1.0 mM KHCO3), and the lymphocytes were washed and resuspended in complete RPMI medium at 107 cells/ml. Cells were transferred in volumes of 100 μl to 96-well U-bottom plates with 100 μl of medium containing 10 μg of hybrid/ml. Control wells without antigen were also prepared. Cells were incubated for 2 h, and then brefeldin A (Løwens Kemisk Fabrik, Copenhagen, Denmark) was added to a final concentration of 5 μg/ml and cells were incubated a further 2 h. Cells were washed twice with fluorescence-activated cell sorter (FACS) buffer and then stained for surface markers with CD4 APC (Pharmingen, San Diego, Calif.). Cells were washed and then fixed by incubation in PBS containing 4% paraformaldehyde for 30 min at room temperature, washed twice, and stored overnight in PBS at 4°C. The next day, cells were washed in PBS and then permeabilized and stained with a Cytofix/Cytoperm kit (Pharmingen) according to the manufacturer's instructions. Cells were resuspended in a final volume of 300 ml, and 50,000 events were counted with a FACScan (Becton Dickinson Immunocytometry Systems, Mountain View, Calif.).
Statistics.
The data obtained were tested for analysis of variance. Differences between means were assessed by Student's t test for IFN-γ assays, whereas log-transformed CFU data were assessed by Tukey's test. In both instances, a P value of <0.05 was considered significant.
RESULTS
Oral immunization does not efficiently prime immune responses.
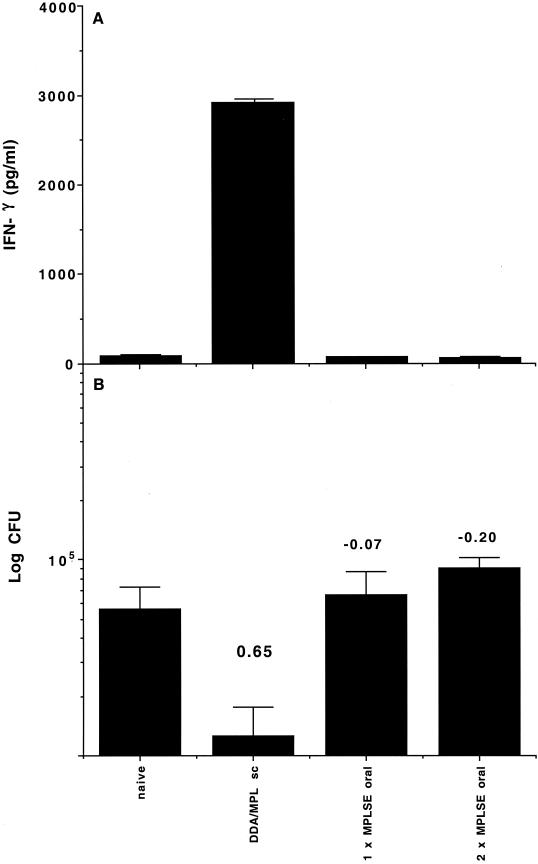
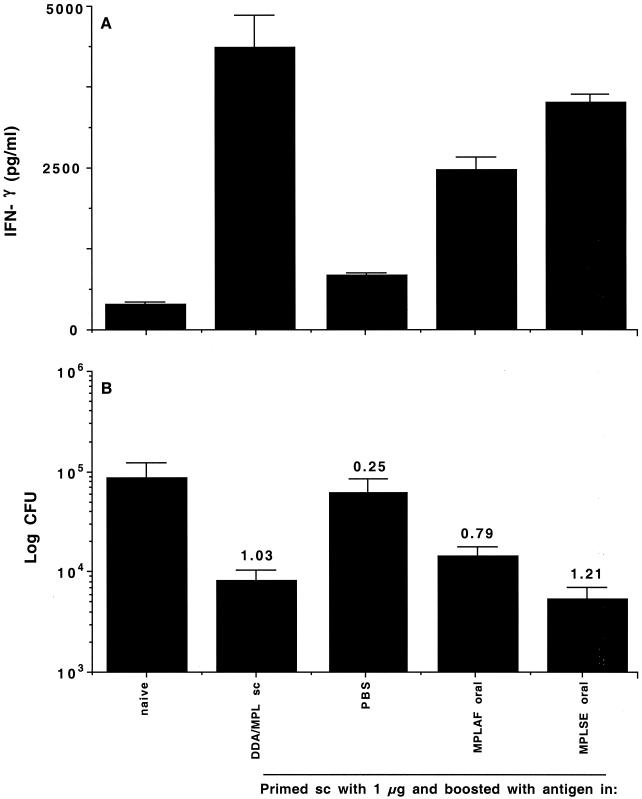
To determine the ability of oral vaccination to generate immune responses, groups of C57BL/6 mice were lightly anesthetized and given one or two doses of 10 μg of antigen in 50 μl of MPLSE adjuvant (containing 12.5 μg of MPL) delivered directly into the stomach with a soft plastic tube. MPLSE is a stable, oil-based emulsion suitable for administration by the oral route. Mice receiving one vaccination were inoculated at the same time that mice receiving two vaccinations were given the second dose. As controls, mice either were left untreated, received adjuvant without antigen, or were vaccinated subcutaneously with two doses of 1 μg of antigen in 200 μl of DDA-MPL adjuvant (250 μg of DDA, 25 μg of MPL), a protocol which has been shown to generate strong systemic immune responses (8). The ability of the vaccination protocols to generate systemic immune responses was assessed by harvesting spleen cells 2 weeks after the last vaccination and restimulating them in vitro with the antigen. As can be seen in Fig. 1A, two subcutaneous vaccinations stimulated strong immune responses, with high levels of antigen-specific IFN-γ being produced. However, the response after one or two oral vaccinations was no different from that observed in naïve mice (Fig. 1A). To ensure that the lack of a response was not due to adjuvant dose, the adjuvant and antigen were titrated over a 100-fold range. No responses were seen at any dose tested by this regimen, and adjuvant controls without antigen likewise did not stimulate significant responses (data not shown).
FIG. 1.
Oral vaccination with MPL-based adjuvants does not prime effective immune responses. (A) Systemic immune responses as assessed from IFN-γ levels after in vitro restimulation of spleen cells from mice harvested 2 weeks after the booster vaccination. Results are IFN-γ levels (in picograms per milliliter) from four mice per experimental group ± standard deviations. (B) Protective efficacy as assessed by determining reductions in the numbers of CFU in the lungs of mice 6 weeks after aerosol infection. Results are mean log10 numbers of CFU ± standard deviations from four mice per experimental group. Protection expressed as the log reduction in numbers of CFU in vaccinated versus naïve animals is shown above each group. sc, subcutaneous.
It was considered possible that immunization by the oral route might not stimulate detectable systemic immune responses but still generate some protective responses in the lung. To assess this possibility, mice were infected with M. tuberculosis cells via the aerosol route 6 weeks after the final vaccination. The infection was allowed to develop for a further 6 weeks, and then the lungs were harvested and the protective efficacy of the vaccination was measured by determining the reduction in bacterial load. As shown in Fig. 1B, neither one nor two doses of the hybrid vaccine given by the oral route were able to stimulate significant protection against subsequent infection. However, two doses of antigen given subcutaneously induced a strong immune response, leading to significant protection in the lung.
Comparison of orally administered MPL adjuvant with classic oral adjuvants.
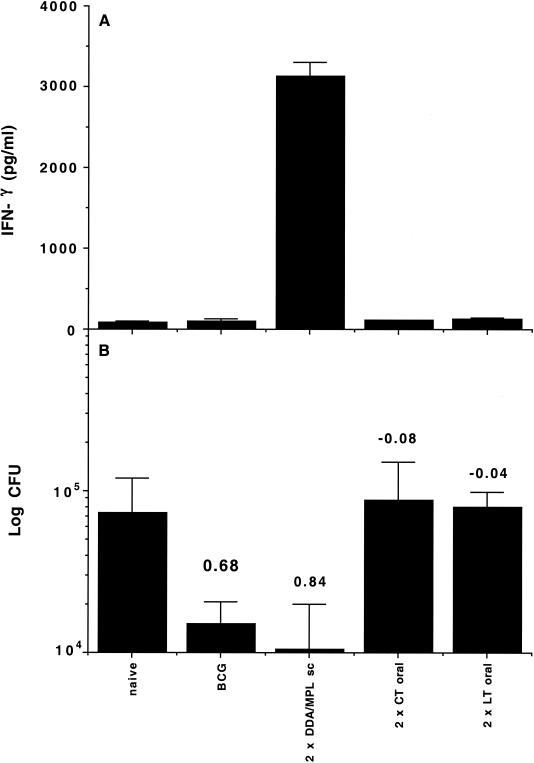
To determine whether the failure of oral vaccination with MPL-based adjuvants was specific to the adjuvant or concerned a more general fault with the protocol tested, we repeated the experiment with two adjuvants known to be active by the mucosal route, CT and E. coli LT (6, 12). We used the same protocol as before, with mice receiving either two doses of 10 μg of antigen and 1 μg of either adjuvant by the oral route or 1 μg of antigen with the same adjuvant formation as in the experiment described above by the subcutaneous route. As an additional positive control group, mice were vaccinated subcutaneously with 5 × 104 CFU of the vaccine, BCG Danish 1331, which is known to induce strong protection in the murine model (17).
Two weeks after the final vaccination, the induction of systemic immune responses by the different vaccines was assessed by measurement of IFN-γ responses after in vitro restimulation of spleen cells (Fig. 2A). As previously observed, only subcutaneous vaccination with antigen in DDA-MPL generated significant type 1 immune responses. While LT is associated with the generation of Th1 responses, CT is associated with the generation of Th2 responses and might have been expected to not be detected in this assay. However, neither LT nor CT generated significant hybrid-specific antibody, and they did not cause detectable antigen-specific T-cell proliferation, suggesting that deviation of the response to Th2 was not the reason for the vaccine failure (data not shown). This hypothesis was confirmed by later work (see Table 1).
FIG. 2.
The failure of MPL to prime effective immune responses by the oral route is shared by classic oral adjuvants. (A) Systemic immune responses as assessed from IFN-γ levels after in vitro restimulation of spleen cells from mice harvested 2 weeks after the booster vaccination. Results are IFN-γ levels (in picograms per milliliter) from four mice per experimental group ± standard deviations. (B) Protective efficacy as assessed by the log reduction in numbers of CFU from the lungs of mice 6 weeks after aerosol infection. Results are mean log10 numbers of CFU ± standard deviations from four mice per experimental group. Protection expressed as the log reduction in numbers of CFU in vaccinated compared to naïve animals is shown above each group. sc, subcutaneous.
TABLE 1.
Relative cytokine levels in murine spleens 2 weeks after vaccinationa
| Vaccine group (route) | IFN-γ | IL-4 | IL-10 | |
|---|---|---|---|---|
| Priming agent | Boosting agent | |||
| PBS | PBS | 169 ± 13 | 75 ± 11 | 211 ± 45 |
| MPL-DDA (s.c.) | MPL-DDA (s.c.) | 2,499 ± 133*** | 94 ± 5 | 583 ± 51* |
| MPLAF (oral) | MPLAF (oral) | 131 ± 6 | 58 ± 18 | 188 ± 57 |
| MPLSE (oral) | MPLSE (oral) | 280 ± 12 | 94 ± 4 | 163 ± 54 |
| MPL-DDA (s.c.) | MPLAF (oral) | 420 ± 154 | 78 ± 9 | 211 ± 47 |
| MPL-DDA (s.c.) | MPLSE (oral) | 630 ± 10* | 85 ± 45 | 275 ± 83 |
Values are expressed as picograms of cytokine per milliliter calculated from a standard curve according to the manufacturer's instructions and are peak values calculated from triplicate spleen cultures stimulated with antigen over a range of 10 to 1.25 μg/ml in vitro. The values are derived from mice administered 1 μg of the hybrid antigen by subcutaneous (s.c.) vaccination and 10 μg by oral vaccination, doses which were found to be optimal to stimulate a response by these routes. Variation between the results of replicate experiments was less than 15%. *, P < 0.05; ***, P < 0.001.
Furthermore, when the efficacies of the different vaccination strategies were determined 6 weeks after aerosol infection, both subcutaneous vaccination with antigen in DDA-MPL and subcutaneous vaccination with BCG were shown to induce strong protective immunity in the lung (Fig. 2B). As previously shown, subcutaneous vaccination with the hybrid vaccine was as good as, or even better than, that with BCG (26), although the difference between the two was not significant. The very low IFN-γ responses in the BCG-vaccinated group demonstrate that early induction of a strong systemic type 1 immune response is not a prerequisite for the induction of immunity. Nonetheless, it is clear that oral vaccination failed to induce significant immunity against M. tuberculosis either systemically or at mucosal surfaces.
Oral delivery of MPL-based adjuvants effectively boosts immune responses.
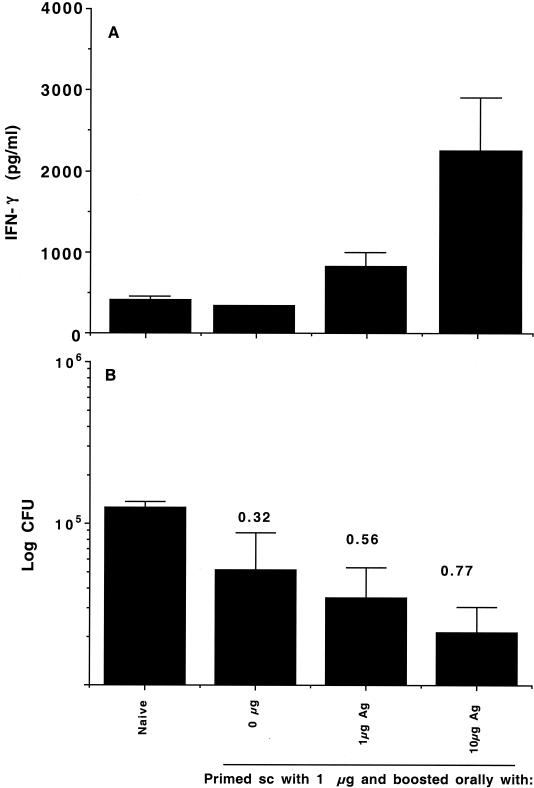
It was hypothesized that the failure of the oral vaccination protocols tested could have been due to a failure to prime a response (either because of the lack of antigen depot, inefficient acquisition of soluble antigen from the gut, or the induction of an inappropriate response) or perhaps due to the different microenvironments in which antigen was presented. To test this hypothesis, groups of mice were given a single subcutaneous vaccination with 1 μg of antigen in DDA-MPL and 2 weeks later given an oral booster vaccination in MPLSE containing either 0, 1, or 10 μg of antigen. It has been previously shown that the hybrid antigen can generate responses at doses as low as 0.01 μg (26) when given subcutaneously, so it was considered unlikely that these doses would prove to be insufficient by the oral route.
The effect of priming the immune response with antigen in DDA-MPL is shown in Fig. 3A. A single subcutaneous vaccination failed to stimulate a significant immune response in the spleen, as assessed by IFN-γ production by spleen cells in response to in vitro restimulation with antigen. However, boosting orally with 1 μg of antigen in MPLSE induced a small but significant (P < 0.05) antigen-specific response in the spleen whereas boosting orally with 10 μg of antigen induced a highly significant increase in IFN-γ production (P < 0.001) comparable to that seen with two subcutaneous vaccinations. In other experiments, doses tested above 10 μg were superoptimal, inducing lower levels of IFN-γ and poorer protection (unpublished data). This effect has also been observed with other strong immunogens (2).
FIG. 3.
Oral delivery of MPL-based adjuvants effectively boosts immune responses in a dose-dependent manner. (A) Systemic immune responses as assessed from IFN-γ levels after in vitro restimulation of spleen cells from mice harvested 2 weeks after the booster vaccination. Results are IFN-γ levels (in picograms per milliliter) from four mice per experimental group ± standard deviations. (B) Protective efficacy as assessed by the log reduction in numbers of CFU from the lungs of mice 6 weeks after aerosol infection. Results are mean log10 numbers of CFU ± standard deviations from four mice per experimental group. Protection expressed as the log reduction in numbers of CFU in vaccinated versus naïve animals is shown above each group. sc, subcutaneous; Ag, antigen.
The immunogenicities of the different vaccination strategies were paralleled by the efficacy data from mice infected 6 weeks after the final vaccination (Fig. 3B). A single subcutaneous vaccination induced a small but not significant reduction in bacterial load, whereas oral boosting with either 1 or 10 μg induced significant protection against infection.
Mucosal vaccination with CT is typically used to induce humoral rather than cell-mediated immunity responses, and recent results suggest that it may bias responses toward type 2 responses (19). It has also been suggested (1, 34) that induction of interleukin 10 (IL-10) by mucosal immunization can lead to a systemic immunosuppressive effect. To test whether these factors played a role in the failure of oral priming with the hybrid-MPLSE vaccine combination, systemic immune responses from mice primed either orally or subcutaneously were analyzed for the levels of the type 1-associated cytokine IFN-γ, the type 2-associated cytokine IL-4, or the immunosuppressive cytokine IL-10. As shown in Table 1, oral priming does not appear to induce systemic type 2 responses or significant levels of IL-10. None of the protocols used induced significant increases in IL-4 (Table 1), and only subcutaneous priming was able to induce significant increases in antigen-specific IFN-γ.
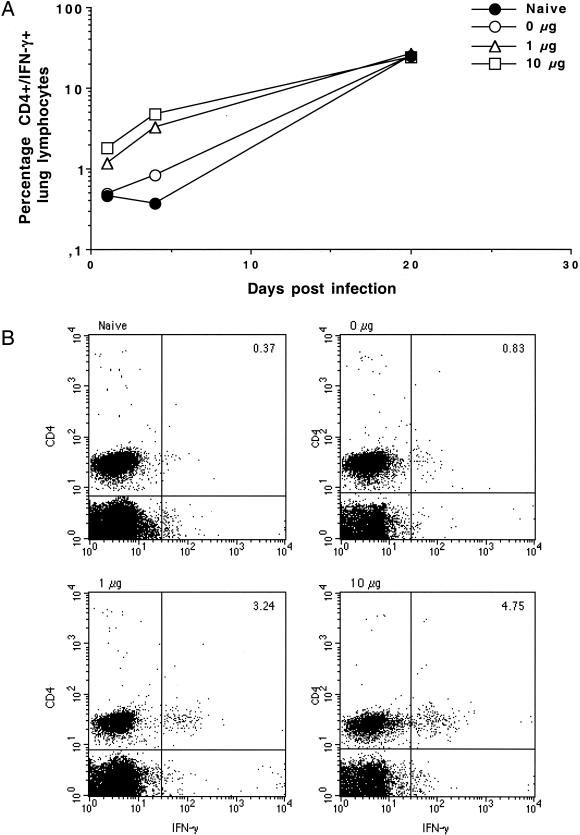
The induction of protective immune responses in the lung by a heterologous priming and boosting vaccination was further quantified by FACS analysis of the T-cell response in the lung. Lymphocytes circulating in the blood have been shown to have a significant degree of reactivity to M. tuberculosis-derived antigens after infection or vaccination (26). Mice were therefore perfused with cold, heparin-containing PBS to remove blood before the lungs were taken at 1, 4, and 20 days postinfection for analysis of infiltrating lymphocytes. These cells were stimulated with antigen and stained for intracellular IFN-γ. As early as day 1 after infection, increased numbers of IFN-γ and CD4+ cells were detectable in the lungs of mice given oral booster doses but not in the lungs of control mice (Fig. 4A). By day 4 postinfection, this difference was quite marked (Fig. 4). At 20 days postinfection, the numbers of IFN-γ and CD4 cells had increased in all mice and were not different between the groups tested (Fig. 4A), suggesting that mobilization of an early response against bacteria in the lung was key to the reduction of bacterial load subsequently observed. These data clearly show that even relatively small amounts of antigen can be acquired via the gut mucosa and stimulate strong systemic immune responses, with activated lymphocytes being primed for efficient migration into the lung.
FIG. 4.
(A) Percentages of total lung lymphocytes positive for both CD4 and IFN-γ isolated from the lungs of perfused mice after aerosol infection. Results are from six mice per experimental group. (B) Results of staining cells from mice which had received 0, 1, or 10 μg of the hybrid antigen as a booster vaccine 4 days postinfection for CD4 and IFN-γ.
A heterologous priming/boosting vaccination is as effective as subcutaneous vaccination.
Given the demonstrated ability of oral vaccination to boost a single subcutaneous immunization, a direct comparison of the efficacy of this approach to that of conventional subcutaneous priming and boosting was carried out. Groups of mice were vaccinated subcutaneously with 1 μg of antigen as before and 2 weeks later given a booster dose of 1 μg of antigen either subcutaneously in DDA-MPL or orally in one of two MPL-containing adjuvants: an aqueous formulation (MPLAF) or the oil emulsion previously used (MPLSE). Immune responses were assessed 2 weeks after vaccination, and the animals were infected by the aerosol route 6 weeks after the final vaccination. As shown in Fig. 5, responses to oral booster doses in either adjuvant both stimulated an early systemic type 1 immune response and induced protection in the lung. However, antigen delivered orally in the absence of adjuvant did not boost the response significantly above that of a single subcutaneous vaccination (Fig. 4 and 5). The magnitudes of the responses from MPL-containing booster vaccines were not significantly different (P < 0.7 for MPLSE, P < 0.6 for MPLAF) whether the vaccine was given subcutaneously or orally.
FIG. 5.
(A) Systemic immune responses as assessed from IFN-γ levels after in vitro restimulation of spleen cells from mice harvested 2 weeks after the booster vaccination. Results are IFN-γ levels (in picograms per milliliter) from four mice per experimental group ± standard deviations. (B) Protective efficacy as assessed by measuring the numbers of CFU from the lungs of mice 6 weeks after aerosol infection. Results are mean log10 numbers of CFU ± standard deviations from four mice per experimental group. Protection expressed as the log reduction in the numbers of CFU in vaccinated versus naïve animals is shown above each group. sc, subcutaneous.
To determine the general applicability of these findings, the heterologous priming/boosting vaccination strategy was also tested in the highly sensitive guinea pig model. Guinea pigs were either immunized with 5 × 104 CFU of BCG or given a single subcutaneous immunization with 20 μg of the hybrid antigen mixed with MPL-DDA (500 μg of DDA, 50 μg of MPL) followed by two booster vaccinations with either 2 or 20 μg of the hybrid antigen by either the oral (in MPLSE, containing 50 μg of MPL) or the subcutaneous route (with antigen and DDA-MPL as described above). As shown in Table 2, animals that received oral boosting had a greatly reduced number of CFU in the lung, and as seen in the previous experiments with mice, oral boosting appeared to generate responses equivalent to, or marginally better than, those obtained by an optimal subcutaneous protocol. While BCG vaccination still induced stronger responses than those of subunit vaccination over the relatively short time interval shown here (Table 2), it has been shown that over longer periods, subunit vaccination induces protection and survival that is equivalent to that induced by BCG vaccination (26; unpublished data). Reagents are not available to monitor IFN-γ values in guinea pigs, but the systemic nature of the response is indicated by the induction of very strong delayed-type hypersensitivity (DTH) responses, especially with the higher dose of antigen given orally. Thus, it appears that oral boosting may be a generally applicable, simple method of enhancing the immunogenicity of prior vaccination.
TABLE 2.
Oral-vaccine-induced protection in guinea pigs
| Treatment | DTHa | Log10 no. of CFU in spleen and lungb | Log10 resistancec |
|---|---|---|---|
| None | 2.0 (2, 2.5) | 6.70 ± 0.32 | NA |
| BCG | 19.4 (19.2, 19.6) | 4.87 ± 0.15 | 1.84 ± 0.36 |
| One s.c.d dose | 12.6 (11.8, 14.5) | 6.20 ± 0.10 | 0.50 ± 0.34 |
| Three s.c. doses | 19.4 (19.2, 19.6) | 6.14 ± 0.11 | 0.56 ± 0.34 |
| One s.c. + two oral (2 μg) doses | 5.5 (4.3, 7.7) | 5.72 ± 0.48 | 0.98 ± 0.58 |
| One s.c. + two oral (20 μg) doses | 19.6 (15.3, 22.3) | 5.83 ± 0.14 | 0.87 ± 0.35 |
DTH is expressed as the median width (in millimeters) of the areas of erythema induced by intradermal injection 6 weeks after vaccination with 100 μg of short-term culture filtrate derived from culture of M. tuberculosis. Short-term culture filtrate contains multiple proteins, including antigen 85B and ESAT-6. Results are median values from groups of four guinea pigs each, and 25th and 75th quartiles are shown in that order in parentheses.
Values are mean log10 numbers of CFU ± standard deviations of M. tuberculosis isolated from the spleens and lungs of five animals per group 13 weeks postchallenge and are representative of two experiments performed.
Data are expressed as levels of protection (log10 resistance), calculated by subtracting the log10 mean number of bacilli in the organs of vaccinated animals from the log10 mean number of bacilli in the organs of naïve guinea pigs. NA, not applicable.
s.c., subcutaneous.
DISCUSSION
The dramatic rise in the number of new TB cases over recent decades has stimulated research in all areas of TB immunology, with particular emphasis on vaccine development. As a result, a number of promising vaccine candidates have been developed and are in the process of being approved for human clinical trials (13, 27). Nonetheless, there remain a number of practical difficulties. While some recombinant protein vaccines have been shown to stimulate good immunity in animal models, this has generally required either multiple vaccinations, adjuvants that are not acceptable for human use because of potential side effects, or both. However, adjuvant protein vaccines offer significant advantages, such as safety in immunocompromised recipients and effectiveness in recipients who are already BCG vaccinated (unpublished data) or sensitized to environmental mycobacteria (9). A major focus of vaccine research has therefore been the development of delivery systems that reduce the resources required for vaccination while at the same time stimulating an optimal immune response.
Adjuvant protein subunit vaccines against M. tuberculosis have conventionally been delivered by subcutaneous or intradermal injection. It has already been shown that subunit vaccines delivered subcutaneously with an adjuvant combining DDA and MPL can effectively initiate a protective response in small-animal models of M. tuberculosis infection, such as mice and guinea pigs (8, 26), and this has recently been duplicated in cattle and primates (unpublished data). Here, we show that MPL-containing adjuvants can also be used for oral vaccination of mice and guinea pigs and that they are effective even with relatively small amounts of antigen (Fig. 5; Table 2).
Mucosal vaccination has obvious advantages, both physical and immunological. Needleless vaccination systems are an attractive alternative to conventional subcutaneous vaccination due to the decreased risk of exposure to potentially infectious blood, the level of technical skill required for reproducible subcutaneous vaccination, and the negative attitudes of many recipients to injection. Equally important, the mucosa is the normal portal of entry for M. tuberculosis and the lung mucosa is monitored by specialized lymphoid tissues (the bronchus-associated lymphoid tissue) dedicated to antigen uptake and immune surveillance (7, 11). Bronchus-associated lymphoid tissue is only one of a number of mucosa-associated lymphoid tissues, and it has been shown that differential expression of integrins that bind to ligands such as endothelial vascular cell adhesion molecule 1 or mucosal addressin cell adhesion molecule-1 by lymphocytes leads to a lymphocyte population that efficiently circulates to sites of mucosal inflammation (29). The rapid induction of these cells appears to play a crucial role in acquired immunity at mucosal surfaces (15), which is consistent with the correlation between protection and an early, rapid increase in the number of IFN-γ-positive CD4+ cells in the vaccinated animals in our experiments (Fig. 4). It is not yet clear if this increase is due to migration from other tissues or to expansion of T cells already primed in the lung. It has long been hoped that immunization via one mucosal surface will lead to enhanced immunity at other mucosal surfaces, and the high levels of protection obtained in the lung in the work presented here suggest that this is, in fact, true. However, much work remains to be done before the utility of mucosal vaccination against human TB is clear. In particular, while it is assumed that MPL's effect is due to its ability to stimulate the production of proinflammatory cytokines by APC, the mechanism of uptake and the target cells involved in oral delivery of the adjuvant remain unclear.
Adjuvants which are both approved for clinical use and able to induce cell-mediated responses via the oral route are simply not available, although work in this area is rapidly increasing. Recent work has clearly demonstrated that distinct subsets of APC differentially express receptors for molecules from microbial pathogens (20) and that stimulation even with similar LPS molecules can lead to divergent outcomes (28, 30). Moreover, the activating effect of MPL on the innate response, even in mice unresponsive to LPS, has also been shown (18). Nonetheless, it remains unclear precisely how the vaccine antigen is acquired via the gut and why MPL-based adjuvants function in an environment that is already filled with gram-negative bacteria and presumably rich in bacterial lipids.
It is also unclear why oral delivery of the vaccine fails to effectively prime primary immune responses, although it boosts systemic immune responses at least as effectively as multiple subcutaneous immunizations. Since our experiments have focused largely on the assessment of the type 1 responses required for protection against mycobacterial infection, it was considered that priming via the oral route might lead to either tolerance or the induction of type 2 responses. However, assays for induction of antigen-specific IL-4 or IL-10 did not show any significant increase in the levels of these cytokines after priming responses via the oral route (Table 1). Nonetheless, the data presented suggest that antigen is efficiently acquired through the gut mucosa and can be effectively presented to an already-existing population of primed T cells. Activation of APC by appropriate adjuvants is still required for this process, however, as administration of antigen orally in the absence of MPL-containing adjuvants did not increase either systemic IFN-γ production or immunity in the lung, even when the animals had been primed by subcutaneous vaccination (Fig. 5).
MPL-containing adjuvants have already been tested in human volunteers and found to be well tolerated (14, 33). We have also tested them extensively in animal models. While there was no evidence of discomfort or pathology in the animals used for the experiments described here, we have found that MPL-based adjuvants can induce severe pathology if they are delivered onto sensitive surfaces such as the nasal mucosa (data not shown). It is for this reason that we have concentrated the work presented here on oral delivery. Based on these results, the use of these adjuvants in an oral delivery system, where the risk of adverse side effects is reduced further since administration is noninvasive, has definite potential for human vaccination. The possibility of boosting immunity via the oral route is particularly attractive in the case of TB vaccination, as it has been suggested that BCG (the current vaccine and the most widely administered vaccine in the world) offers some protection in the decade following vaccination (10, 32), although this protection subsequently wanes. BCG itself is a poor booster vaccine, and multiple BCG vaccinations are not recommended, for both this reason and the significant local side effects that often result (5). However, we have already shown that the hybrid vaccine is unaffected by the existence of prior immune responses (9) and that it can boost immunity induced by prior BCG vaccination (Olsen et al., unpublished data). Thus, the approach outlined in this report offers the possibility of a simply administered oral booster vaccine specifically targeted to the prevention of adult pulmonary TB. We are therefore continuing to investigate the efficacies of different delivery systems and adjuvants for oral booster vaccines.
Acknowledgments
This study was supported by the Danish Research Council and the European Commission, contract number QLRT-PL1999-01093.
We thank Lene Rasmussen and Tina Lerche for excellent technical assistance.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Akbari, O., R. H. DeKruyff, and D. T. Umetsu. 2001. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat. Immunol. 2:725-731. [DOI] [PubMed] [Google Scholar]
- 2.Andersen, P. 1994. Effective vaccination of mice against Mycobacterium tuberculosis infection with a soluble mixture of secreted mycobacterial proteins. Infect. Immun. 62:2536-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersen, P. 2001. TB vaccines: progress and problems. Trends Immunol. 22:160-168. [DOI] [PubMed] [Google Scholar]
- 4.Anonymous. 2001. Global tuberculosis control. World Health Organization report W. H. O./CDS/TB/2001.287.
- 5.Anonymous. 1995. Global tuberculosis programme and global programme on vaccines: statement on BCG revaccination for the prevention of tuberculosis. Wkly. Epidemiol. Rec. 70: 229-236. [PubMed] [Google Scholar]
- 6.Bonenfant, C., I. Dimier-Poisson, F. Velge-Roussel, D. Buzoni-Gatel, G. Del Giudice, R. Rappuoli, and D. Bout. 2001. Intranasal immunization with SAG1 and nontoxic mutant heat-labile enterotoxins protects mice against Toxoplasma gondii. Infect. Immun. 69:1605-1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyaka, P. N., M. Marinaro, J. L. Vancott, I. Takahashi, K. Fujihashi, M. Yamamoto, F. W. van Ginkel, R. J. Jackson, H. Kiyono, and J. R. McGhee. 1999. Strategies for mucosal vaccine development. Am. J. Trop. Med. Hyg. 60:35-45. [DOI] [PubMed] [Google Scholar]
- 8.Brandt, L., M. Elhay, I. Rosenkrands, E. B. Lindblad, and P. Andersen. 2000. ESAT-6 subunit vaccination against Mycobacterium tuberculosis. Infect. Immun. 68:791-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brandt, L., J. Feino Cunha, A. Weinreich Olsen, B. Chilima, P. Hirsch, R. Appelberg, and P. Andersen. 2002. Failure of the Mycobacterium bovis BCG vaccine: some species of environmental mycobacteria block multiplication of BCG and induction of protective immunity to tuberculosis. Infect. Immun. 70:672-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colditz, G. A., C. S. Berkey, F. Mosteller, T. F. Brewer, M. E. Wilson, E. Burdick, and H. V. Fineberg. 1995. The efficacy of bacillus Calmette-Guerin vaccination of newborns and infants in the prevention of tuberculosis: meta-analyses of the published literature. Pediatrics 96:29-35. [PubMed] [Google Scholar]
- 11.Czerkinsky, C., F. Anjuere, J. R. McGhee, A. George-Chandy, J. Holmgren, M. P. Kieny, K. Fujiyashi, J. F. Mestecky, V. Pierrefite-Carle, C. Rask, and J. B. Sun. 1999. Mucosal immunity and tolerance: relevance to vaccine development. Immunol. Rev. 170:197-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Del Giudice, G., A. Podda, and R. Rappuoli. 2001. What are the limits of adjuvanticity? Vaccine 20:S38-S41. [DOI] [PubMed]
- 13.Diamond, G., D. Legarda, and L. K. Ryan. 2000. The innate immune response of the respiratory epithelium. Immunol. Rev. 173:27-38. [DOI] [PubMed] [Google Scholar]
- 13a.Doherty, T. M., and P. Andersen, 2002. Tuberculosis vaccine development. Curr. Opin. Pulm. Res., in press. [DOI] [PubMed]
- 14.Drachenberg, K. J., A. W. Wheeler, P. Stuebner, and F. Horak. 2001. A well-tolerated grass pollen-specific allergy vaccine containing a novel adjuvant, monophosphoryl lipid A, reduces allergic symptoms after only four preseasonal injections. Allergy 56:498-505. [DOI] [PubMed] [Google Scholar]
- 15.Feng, C. G., W. J. Britton, U. Palendira, N. L. Groat, H. Briscoe, and A. G. Bean. 2000. Up-regulation of VCAM-1 and differential expansion of beta integrin-expressing T lymphocytes are associated with immunity to pulmonary Mycobacterium tuberculosis infection. J. Immunol. 164:4853-4860. [DOI] [PubMed] [Google Scholar]
- 16.Fine, P. E. 1995. Variation in protection by BCG: implications of and for heterologous immunity. Lancet 346:1339-1345. [DOI] [PubMed] [Google Scholar]
- 17.Gheorghiu, M., and P. H. Lagrange. 1983. Viability, heat stability and immunogenicity of four BCG vaccines prepared from four different BCG strains. Ann. Immunol. (Paris) 134C:125-147. [DOI] [PubMed]
- 18.Haziot, A., N. Hijiya, S. C. Gangloff, J. Silver, and S. M. Goyert. 2001. Induction of a novel mechanism of accelerated bacterial clearance by lipopolysaccharide in CD14-deficient and Toll-like receptor 4-deficient mice. J. Immunol. 166:1075-1078. [DOI] [PubMed] [Google Scholar]
- 19.Jones, H. P., L. M. Hodge, K. Fujihashi, H. Kiyono, J. R. McGhee, and J. W. Simecka. 2001. The pulmonary environment promotes Th2 cell responses after nasal-pulmonary immunization with antigen alone, but Th1 responses are induced during instances of intense immune stimulation. J. Immunol. 167:4518-4526. [DOI] [PubMed] [Google Scholar]
- 20.Kadowaki, N., S. Ho, S. Antonenko, R. W. Malefyt, R. A. Kastelein, F. Bazan, and Y. J. Liu. 2001. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J. Exp. Med. 194:863-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kristensen, I., P. Aaby, and H. Jensen. 2000. Routine vaccinations and child survival: follow up study in Guinea-Bissau, West Africa. BMJ 321:1435-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindblad, E. B., M. J. Elhay, R. Silva, R. Appelberg, and P. Andersen. 1997. Adjuvant modulation of immune responses to tuberculosis subunit vaccines. Infect. Immun. 65:623-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Medina, E., and C. A. Guzman. 2000. Modulation of immune responses following antigen administration by mucosal route. FEMS Immunol. Med. Microbiol. 27:305-311. [DOI] [PubMed] [Google Scholar]
- 24.Mittal, S. K., V. Aggarwal, A. Rastogi, and N. Saini. 1996. Does B.C.G. vaccination prevent or postpone the occurrence of tuberculous meningitis? Indian J. Pediatr. 63:659-664. [DOI] [PubMed] [Google Scholar]
- 25.Ogra, P. L., H. Faden, and R. C. Welliver. 2001. Vaccination strategies for mucosal immune responses. Clin. Microbiol. Rev. 14:430-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olsen, A. W., L. A. van Pinxteren, L. Meng Okkels, P. Birk Rasmussen, and P. Andersen. 2001. Protection of mice with a tuberculosis subunit vaccine based on a fusion protein of antigen 85B and ESAT-6. Infect. Immun. 69:2773-2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orme, I. M., D. N. McMurray, and J. T. Belisle. 2001. Tuberculosis vaccine development: recent progress. Trends Microbiol. 9:115-118. [DOI] [PubMed] [Google Scholar]
- 28.Pulendran, B., P. Kumar, C. W. Cutler, M. Mohamadzadeh, T. Van Dyke, and J. Banchereau. 2001. Lipopolysaccharides from distinct pathogens induce different classes of immune responses in vivo. J. Immunol. 167:5067-5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rott, L. S., M. J. Briskin, D. P. Andrew, E. L. Berg, and E. C. Butcher. 1996. A fundamental subdivision of circulating lymphocytes defined by adhesion to mucosal addressin cell adhesion molecule-1. Comparison with vascular cell adhesion molecule-1 and correlation with beta 7 integrins and memory differentiation. J. Immunol. 156:3727-3736. [PubMed] [Google Scholar]
- 30.Schnare, M., G. M. Barton, A. C. Holt, K. Takeda, S. Akira, and R. Medzhitov. 2001. Toll-like receptors control activation of adaptive immune responses. Nat. Immunol. 2:947-950. [DOI] [PubMed] [Google Scholar]
- 31.Singh, M., and D. O'Hagan. 1999. Advances in vaccine adjuvants. Nat. Biotechnol. 17:1075-1081. [DOI] [PubMed] [Google Scholar]
- 32.Thilothammal, N., P. V. Krishnamurthy, D. K. Runyan, and K. Banu. 1996. Does BCG vaccine prevent tuberculous meningitis? Arch. Dis. Child. 74:144-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thoelen, S., P. Van Damme, C. Mathei, G. Leroux-Roels, I. Desombere, A. Safary, P. Vandepapeliere, M. Slaoui, and A. Meheus. 1998. Safety and immunogenicity of a hepatitis B vaccine formulated with a novel adjuvant system. Vaccine 16:708-714. [DOI] [PubMed] [Google Scholar]
- 34.Tsuji, N. M., K. Mizumachi, and J. Kurisaki. 2001. Interleukin-10-secreting Peyer's patch cells are responsible for active suppression in low-dose oral tolerance. Immunology 103:458-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Viret, J.-F., D. Favre, B. Wegmüller, C. Herzog, J. U. Que, S. J. Cryz, Jr., and A. B. Lang. 1999. Mucosal and systemic immune responses in humans after primary and booster immunizations with orally administered invasive and noninvasive live attenuated bacteria. Infect. Immun. 67:3680-3685. [DOI] [PMC free article] [PubMed] [Google Scholar]