Abstract
Host defense functions of nitric oxide (NO) are known for many bacterial infections. In this study, we investigated the antimicrobial effect of NO in murine salmonellosis by using inducible NO synthase (iNOS)-deficient mice infected with an avirulent or virulent Salmonella enterica serovar Typhimurium strain. All iNOS-deficient mice died of severe septicemia within 6 days after intraperitoneal injection with an avirulent strain (LT2) to which wild-type mice were highly resistant; 50% lethal doses (LD50s) of the LT2 strain for iNOS-deficient and wild-type mice were 30 CFU and 7 × 104 CFU, respectively. Lack of NO production in iNOS-deficient mice was verified directly by electron spin resonance spectroscopy. Bacterial yields in liver and blood were much higher in iNOS-deficient mice than in wild-type mice throughout the course of infection. Very small amounts of a virulent strain of serovar Typhimurium (a clinical isolate, strain Gifu 12142; LD50, 50 CFU) given orally caused severe septicemia in iNOS-deficient animals; wild-type mice tolerated higher doses (LD50, 6 × 102 CFU). Histopathology of livers from infected iNOS-deficient mice revealed extensive damage, such as diffuse hepatocellular apoptosis and increased neutrophil infiltration, but livers from infected wild-type mice showed a limited number of microabscesses, consisting of polymorphonuclear cells and macrophages and low levels of apoptotic change. The LT2 strain was much more susceptible to the bactericidal effect of peroxynitrite than the Gifu strain, suggesting that peroxynitrite resistance may contribute to Salmonella pathogenicity. These results indicate that NO has significant host defense functions in Salmonella infections not only because of its direct antimicrobial effect but also via cytoprotective actions for infected host cells, possibly through its antiapoptotic effect.
The enterobacterial Salmonella species are a significant cause of morbidity and mortality among human populations, especially those in regions of the world lacking adequate sanitation and health care delivery systems (8, 26). Salmonella species are gram-negative, motile, facultative, intracellular bacilli, and their invasion of and multiplication within mononuclear phagocytic cells in the liver, spleen, lymph nodes, and Peyer's patches are the hallmark events of typhoid fever (7, 20, 50). Salmonella enterica serovar Typhimurium is a leading cause of bacterial gastroenteritis, and it can also produce salmonellosis, which is characterized by progressive multiple microabscess formations and septicemia (57, 58).
Nitric oxide (NO) is a gaseous, inorganic, free radical, molecular species; produced in biological systems, it regulates a diverse array of physiological functions and acts as an inter- and extracellular messenger in most mammalian organs (21, 40). Many types of cells, such as leukocytes, hepatocytes, vascular smooth muscle cells, and endothelial cells, can produce NO during enzymatic conversion of l-arginine to l-citrulline by NO synthase (NOS). A large amount of NO generated by the inducible isoform of NOS (iNOS) has been demonstrated to have a beneficial effect in host defense mechanisms against various pathogenic bacteria and protozoa (18, 22, 41, 42). It has been presumed that iNOS expression can provide antimicrobial activity through formation of reactive nitrogen oxides derived from NO (6, 13, 30, 37, 61). For example, peroxynitrite (ONOO−), a potent oxidant formed from NO and superoxide radical (O2−), is microbicidal for various bacteria, including Salmonella enterica serovar Typhimurium (6, 12, 13, 30), and nitrosothiols, one-electron oxidized derivatives of NO, have potent bacteriostatic activity against serovar Typhimurium (1, 12, 13, 37).
To obtain a better understanding of the pathogenesis of Salmonella infections, including typhoid fever, it is essential to elucidate the NO-dependent antimicrobial mechanism of the host. It was previously shown that pharmacological inhibition of either NO or superoxide production resulted in a remarkable enhancement of Salmonella growth and increased mortality in murine salmonellosis, suggesting that both NO and superoxide contribute critically to host defense against serovar Typhimurium (57). A similar exacerbation of Salmonella pathogenesis by in vivo blockage of NO biosynthesis was recently reported (32). It was also demonstrated that mice deficient in both NADPH phagocyte oxidase (phox) and iNOS were more susceptible to various bacterial infections than were mice deficient in either single enzyme (54). Other earlier studies using iNOS knockout mice clearly illustrated the contribution of NO to antimicrobial defense of macrophages against Salmonella (35, 59). Nevertheless, the in vivo antimicrobial mechanisms involving NO are not fully understood.
It was recently revealed that Salmonella-infected host cells such as macrophages, hepatocytes, and intestinal epithelial cells undergo apoptosis during the infection, which in turn may accelerate bacterial invasion and dissemination leading to septicemia (17, 23, 27, 38, 39). Strong antiapoptotic activity has been documented for NO and especially for its derivative nitrosothiols, possibly through inhibition of a cascade composed of intracellular cysteine proteases known as caspases, via S nitrosylation of the active site cysteine of the enzymes (28, 33, 44). It is thus of considerable interest to see whether NO modulates the host response during murine salmonellosis by protecting host cells from the toxic effects of the bacterial infection rather than functioning as a simple antimicrobial agent.
This study was undertaken to clarify the host defense function of NO in vivo, in view of its antimicrobial effect against serovar Typhimurium and its cytoprotective effect on host cells during Salmonella infection, by using iNOS-deficient and wild-type mice infected with virulent or avirulent serovar Typhimurium.
MATERIALS AND METHODS
Animals.
Male wild-type C57BL/6 (B6) and C57BL/6-NOS2tm1Lau (iNOS−/−) (31) mice, 5 or 7 weeks old, were produced in Jackson Laboratory (West Grove, Pa.). Wild-type (iNOS+/+), heterozygous (iNOS+/−), and homozygous (iNOS−/−) iNOS-deficient littermate mice were bred at the Center for Animal Resources and Development, Kumamoto University. All experiments were carried out according to the Guidelines in the Laboratory Protocol of Animal Handling, Kumamoto University School of Medicine.
Bacteria and media.
Two serovar Typhimurium strains were used: an avirulent LT2 strain and a virulent Gifu 12142 strain (provided by Takayuki Ezaki, Gifu University), which had been isolated from a patient with septicemia caused by serovar Typhimurium. Bacteria were grown overnight in brain heart infusion (BHI) broth (Difco Laboratories, Detroit, Mich.) at 37°C for routine culture. M9 minimal medium (7 mg of Na2HPO4, 3 mg of KH2PO4, 0.5 mg of NaCl, 1 mg of NH4Cl, 5 μg of thiamine, 0.12 mg of MgSO4, 0.015 mg of CaCl2, and 2 mg of glucose per ml) was used for the peroxynitrite susceptibility assay to avoid any potential antagonism against peroxynitrite by thiol-containing substances in the nutrient medium. The number of bacteria was determined by means of a colony-forming assay with the use of deoxycholate-hydrogen sulfate-lactose agar (Nissui, Tokyo, Japan) plates as described previously (57).
Serovar Typhimurium infection in mice.
Serovar Typhimurium strain LT2 or Gifu 12142 in 0.2 ml of 0.01 M phosphate-buffered 0.15 M saline (PBS, pH 7.4) was given intraperitoneally (i.p.) or orally (p.o.) to the mice. At various times after inoculation with serovar Typhimurium, the body weights and survival rates of the mice were monitored, and mice were killed to obtain liver and blood samples for determining bacterial growth. For the oral challenge with both LT2 and Gifu 12142 strains, mice fasted for 24 h before inoculation. The number of bacteria was quantified by use of the colony-forming assay as just described. Briefly, the liver was weighed and homogenized in ice-cold PBS (PBS/liver ratio of 9:1, vol/wt) by using a Polytron homogenizer (Kinematica GmbH, Lucerne, Switzerland). The resultant liver homogenate was then serially diluted and was subjected to the colony-forming assay after culture for 18 h at 37°C.
Measurement of NO generation in vivo.
NO generated in mouse liver was determined by electron spin resonance (ESR) spectroscopy with the use of N-dithiocarboxy(sarcosine) (DTCS)-Fe complex as a spin trap for NO (3). Specifically, a mouse was injected subcutaneously with the DTCS-Fe complex (180 mg of DTCS and 40 mg of FeSO4·7H2O per kg of body weight). Thirty minutes after DTCS-Fe administration, the mouse liver was perfused via the portal vein with 20 ml of saline containing 10 U of heparin. The perfused liver was resected and cut into small pieces, which were then transferred to the ESR sample tube and rapidly frozen in liquid nitrogen. The NO-DTCS-Fe adduct formed after administration of the complex was then quantified by ESR spectroscopy (Bruker Instruments, Inc., Rheinstetten, Germany) at 110 K.
Measurement of NOx levels in plasma.
Plasma samples were obtained by centrifugation of blood collected on different days after infection and were kept at −80°C until use. After appropriate dilution of the plasma, 10 μl of each aliquot was analyzed for NOx (NO2− + NO3−) by using a high-performance liquid chromatography-based flow reactor with Griess reagent (NOx analyzer and ENO-10; Eicom, Kyoto, Japan) (1).
Histological examination.
The livers of the mice were fixed with 10% buffered neutral formalin solution, embedded in paraffin, and cut into 3-μm-thick sections. Sections were stained with hematoxylin and eosin. For morphometric analysis of the pathological lesions, the total area of microabscesses and granulomatous lesions was measured in more than three different visual fields for each liver section, after photographs of the fields were taken at low magnification (×20).
Immunohistochemistry.
For immunohistochemical analysis, tissues were fixed in 2% periodate-lysine-paraformaldehyde fixative at 4°C for 4 h. After 12 h of successive washing with PBS containing 10, 15, and 20% sucrose, tissues were embedded in Tissue-Tek OCT compound (Miles, Elkhart, Ind.), frozen in dry ice-acetone, and kept at −80°C until use. The 6-μm-thick sections were prepared with a cryostat, and cryosections were air dried overnight. Sections were stained by the indirect immunoperoxidase method (3), with specific antibody for iNOS (1:100; Santa Cruz Biotechnology, Santa Cruz, Calif.), antineutrophil monoclonal antibody (1:100; Serotec Inc., Raleigh, N.C.), or a polyclonal antinitrotyrosine antibody (1:500; Upstate Biotechnology, Lake Placid, N.Y.) as a primary antibody. Tissue-bound peroxidase activity was visualized by reaction with the substrate 3,3′-diaminobenzidine; hematoxylin was used for the nuclear staining. The morphometric analysis was performed by light microscopy examination of neutrophils infiltrated in the liver tissue. The number of neutrophils was counted by using 100 photographs for each group taken at a magnification of ×20 and was expressed per square millimeter of liver section.
Identification of apoptotic change in the liver occurring during infection.
The apoptotic change in the liver occurring during Salmonella infection was analyzed by use of the terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assay (43), with an in situ apoptosis detection kit (TACS; Trevigen, Inc., Gaithersburg, Md.) according to the manufacturer's instructions. After the sections were prepared as just described, the TUNEL procedure was performed with 6-μm-thick sections, followed by a streptavidin-labeled peroxidase reaction with TACS Blue Label for blue coloration. The sections were examined by light microscopy, and the TUNEL-positive cells were counted and expressed per square millimeter of liver section.
Bactericidal assays.
We used stationary-phase bacteria grown in BHI broth (17- to 18-h culture) or M9 medium for analysis of the bactericidal action of peroxynitrite. Peroxynitrite was synthesized from nitrite and hydrogen peroxide (H2O2) in a quenched-flow reactor, as previously described (4). The constant-flux infusion method was used to treat the bacteria with steady concentrations of peroxynitrite (46). During the constant-flux infusion process, the effective and constant concentration of peroxynitrite is determined on the basis of a balance between the rates of supply and decomposition of peroxynitrite in the system. The effective concentration of peroxynitrite maintained in the reaction mixture was estimated by the dihydrorhodamine 123 oxidation assay, as described earlier (30, 53). By infusion of 10, 25, and 50 mM peroxynitrite in 10 mM NaOH into M9 medium, pH 7.2 (1.9 ml), at a flow rate of 3.3 μl/min, the concentrations of peroxynitrite remained constant at 9.7, 15.34, and 21.7 μM, respectively. Accordingly, the suspension of serovar Typhimurium (108 CFU/ml) was treated with constant concentrations of peroxynitrite of 9.7, 15.34, and 21.7 μM, and aliquots (20 μl) were removed from the reaction mixture at different intervals and were immediately diluted with PBS. Viable bacteria were then quantified by use of the colony-forming assay, as just described. Similarly, decomposed peroxynitrite was used for treatment of bacteria in M9 medium. The susceptibility of serovar Typhimurium strains to H2O2 or tert-butyl hydroperoxide (t-BuOOH; Sigma Chemical Co., St. Louis, Mo.) was also determined by adding various concentrations of H2O2 or t-BuOOH at final concentrations of 1, 5, and 10 mM or 25 and 50 mM to stationary-phase bacteria in BHI broth and then obtaining the viable cell count, after various incubation periods, by the colony-forming assay.
Statistical analysis.
All data are expressed as means ± standard errors of the means (SEM). The statistical difference was determined by the two-tailed unpaired t test. A P of <0.05 was considered statistically significant.
RESULTS
Effect of iNOS depletion on mortality of mice infected with Salmonella.
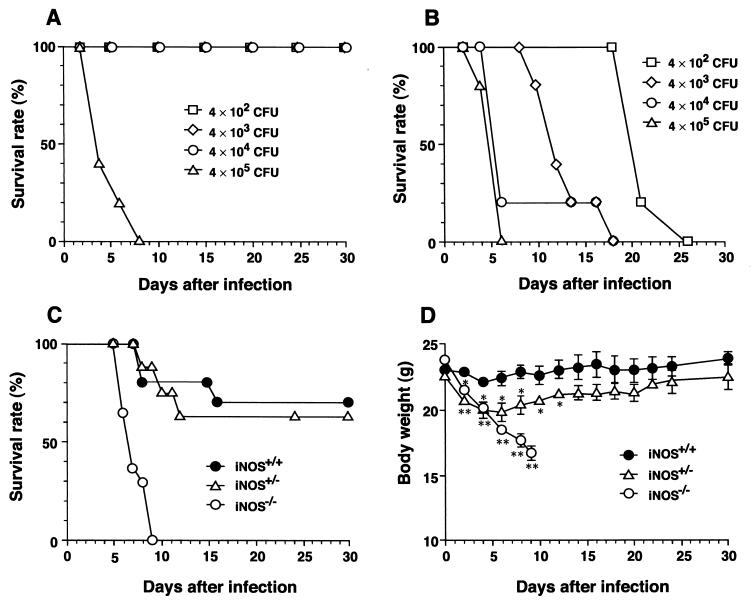
To explore the role of iNOS in the host response to Salmonella infection, we infected wild-type and iNOS-deficient mice with an i.p. dose of serovar Typhimurium LT2 ranging from 4 × 102 to 4 × 105 CFU/mouse. Mice lacking iNOS showed much lower survival rates and died more quickly after infection with the LT2 strain than did iNOS-competent mice (Fig. 1A and B). Even with the lowest dose (4 × 102 CFU/mouse), all iNOS-deficient mice succumbed within 30 days after infection (Fig. 1B). When littermate mice having different iNOS genotypes were infected with the LT2 strain at 5 × 104 CFU/mouse, the mortality rate of the heterozygote was slightly higher than that of the wild-type mouse (Fig. 1C). Heterozygotes also lost more body weight than did wild-type mice during the early phase (days 3 to 6) of infection (Fig. 1D). Body weight loss by iNOS-deficient homozygotes was most remarkable, and they were greatly emaciated within 10 days after infection. Because just less than 10 CFU/mouse of the Gifu 12142 strain injected i.p. produced rapidly progressive and fatal infections in both wild-type and iNOS-deficient mice (all mice died within 10 days after infection), we found no difference in susceptibility of mice with different iNOS genotypes to this highly virulent strain (Table 1).
FIG. 1.
Percent survival of wild-type and iNOS-deficient mice infected with serovar Typhimurium. Both wild-type (A) and iNOS−/− (B) mice (7-week-old males) were infected with i.p. doses of serovar Typhimurium LT2 ranging from 4 × 102 to 4 × 105 CFU/mouse. n = 6 for each dose. Survival rate was monitored until 30 days after infection. Survival curve (C) and changes in body weight (D) of wild-type (iNOS+/+; n = 10), heterozygous (iNOS+/−; n = 8), and iNOS-deficient homozygous (iNOS−/−; n = 14) littermate mice infected with 5 × 104 CFU/mouse i.p. at different time points after infection. Body weight data are expressed as means ± SEM (∗, P < 0.05, and ∗∗, P < 0.01, versus iNOS+/+ mice).
TABLE 1.
LD50s for two serovar Typhimurium strains in wild-type and iNOS-deficient mice
| Serovar Typhimurium strain | LD50 (no. of CFU/mouse) for each inoculation routea
|
|||
|---|---|---|---|---|
| i.p.b
|
p.o.b
|
|||
| iNOS+/+ | iNOS−/− | iNOS+/+ | iNOS−/− | |
| LT2 | 7 × 104 | 30 | >1 × 1010 | 7 × 107 |
| Gifu 12142 | <10 | <10 | 6 × 102 | 50 |
LD50s were determined by the method of Reed and Muench (48).
Male 7- and 5-week-old mice were used for i.p. and p.o. inoculations, respectively.
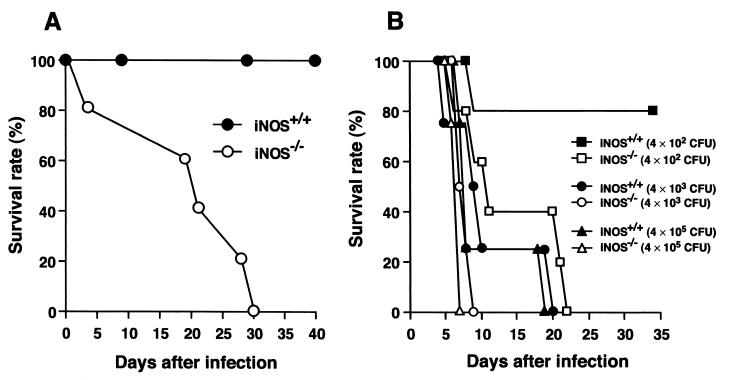
It is believed that the natural route of infection of enteric Salmonella is oral and that Salmonella species traverse intestinal mucosal M cells to reach the lymphoid follicle of Peyer's patches, where they proliferate and then spread to deeper tissues via lymphatics and the bloodstream (11, 24). To examine how iNOS is involved in Salmonella infection in this natural route, we challenged wild-type and iNOS-deficient mice with an oral dose of the serovar Typhimurium LT2 or Gifu 12142 strain. As shown in Fig. 2A, the LT2 strain did not cause lethal infections in wild-type mice even when it was given p.o. at a very high dose (109 CFU/mouse); the same strain showed apparently increased pathogenicity in iNOS-deficient mice, although relatively high doses were needed for p.o. compared with i.p. inoculation. In contrast, not only iNOS-deficient mice but also wild-type mice were susceptible to p.o. infection with the Gifu 12142 strain. However, iNOS-deficient mice infected p.o. with the Gifu 12142 strain died more quickly and in greater numbers than did iNOS-competent mice (Fig. 2B), as was the case with the LT2 p.o. infections.
FIG. 2.
Percent survival of wild-type and iNOS-deficient mice after oral challenge with serovar Typhimurium LT2 or Gifu 12142 strain. (A) Five-week-old male iNOS+/+ (n = 5) and iNOS−/− (n = 5) mice were orally infected with the LT2 strain at 109 CFU/mouse. (B) iNOS+/+ and iNOS−/− mice (5 weeks old, males; n = 4) were orally infected with various doses of the Gifu strain.
The LD50s for the two different serovar Typhimurium strains in wild-type and iNOS-deficient mice are summarized in Table 1. These results indicate that, regardless of inoculation route, iNOS-deficient mice are more susceptible to Salmonella infection than are wild-type animals, suggesting a protective role of NO during Salmonella infection.
Bacterial growth in wild-type and iNOS−/− mice infected with Salmonella.
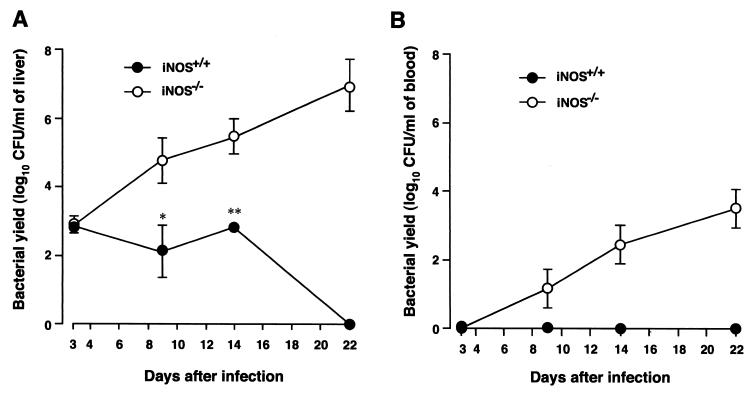
To clarify the mechanism of enhanced lethality of Salmonella infection in iNOS−/− mice, we next evaluated the yield of bacteria in liver and blood in both wild-type and iNOS−/− animals. Mice were infected i.p. with the LT2 strain at 4 × 102 CFU/mouse. They were killed at various time points after infection, and liver homogenate and blood samples obtained were subjected to the colony-forming assay. Higher bacterial yields were observed for iNOS−/− mice than for wild-type mice, with no viable bacteria being found in the blood of wild-type mice throughout the course of infection (Fig. 3). There was a 100-fold-higher bacterial count in the livers of iNOS−/− mice than in those of wild-type mice on days 9 and 14 after infection. On day 22, the bacterial counts in the liver and blood of iNOS-deficient mice reached 107 and 103 CFU, respectively; in wild-type mice, however, no bacteria could be detected in both liver and blood (Fig. 3A and B). The same trend of higher bacterial yield in iNOS−/− than in wild-type mice was observed in infection produced with the serovar Typhimurium Gifu 12142 strain given p.o. (data not shown). These data illustrate that septicemia caused by serovar Typhimurium occurred much more extensively in iNOS-deficient mice than in wild-type mice, indicating again an important defense function for NO formed from iNOS in Salmonella infection.
FIG. 3.
Bacterial growth in liver (A) and blood (B) of wild-type and iNOS-deficient mice infected with serovar Typhimurium LT2. Both iNOS−/− and iNOS+/+ mice were infected i.p. with 4 × 102 CFU of the LT2 strain per mouse. Bacterial counts in liver and blood samples were determined at different time points after infection. The number of bacteria was determined by the colony-forming assay. Data are means ± SEM (n = 3 or 4); ∗, P < 0.05, and ∗∗, P < 0.01, versus iNOS+/+ mice.
In vivo NO production and levels in plasma of NOx in Salmonella-infected mice.
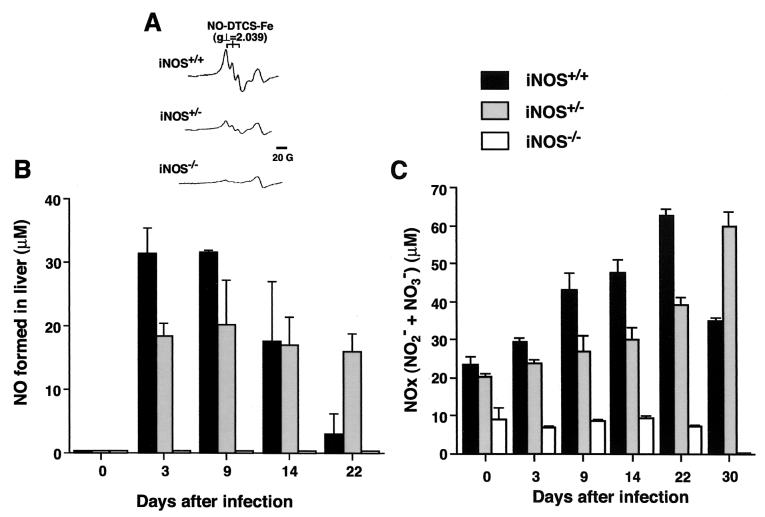
We further analyzed the time profile of NO production in Salmonella-infected livers of wild-type mice and their heterozygous and homozygous iNOS-deficient littermates by ESR spectroscopy, with the DTCS-Fe complex as a spin trap for NO (Fig. 4A and B). As shown in Fig. 4A, typical ESR spectra of the NO-DTCS-Fe adduct consisting of a triplet hyperfine structure were observed with infected livers of wild-type mice but not with those of iNOS-deficient mice after i.p. infection with the LT2 strain (4 × 102 CFU/mouse). The amount of NO generated in the livers of wild-type mice was elevated at days 3 and 9 after infection and decreased thereafter (Fig. 4B). The time profile of NO adduct production in wild-type mouse livers was consistent with that of bacterial growth (Fig. 3A). iNOS−/− mice did not show an appreciable ESR signal for NO production at any time points examined, and the amount of NO adduct formed in the heterozygous (iNOS+/−) mice was almost half of that in the wild-type (iNOS+/+) mice throughout the course of infection, except for day 22 after infection (Fig. 4B).
FIG. 4.
In vivo NO generation detected by ESR spectroscopy and production of NOx (NO2− + NO3−) in the plasma during the course of infection. iNOS+/+, iNOS+/−, and iNOS−/− littermate mice were infected i.p. with 4 × 102 CFU of serovar Typhimurium LT2. (A) For each group of littermate mice, typical ESR spectra of the NO-DTCS-Fe complex produced in the liver on day 3 after infection are shown. For each group of mice, the amount of NO generated in the liver was determined directly by ESR spectroscopy (B), and the level in plasma of NOx (C) was measured by use of a high-performance liquid chromatography-based flow reactor with Griess reagent at various time points after infection. Data are expressed as means ± SEM (n = 3 to 6 per group at each time point).
The level in plasma of NOx (NO2− + NO3−) after Salmonella infection was quantified, as was the ESR analysis (Fig. 4C). In wild-type mouse plasma, the NOx level peaked on day 22 after infection and declined 30 days after infection, which may reflect the total clearance of bacteria from blood and liver and recovery of the mice from infection, as just described. The time course of NOx formation in the plasma, however, was not consistent with that of NO production as assessed by ESR analysis. This result may be due to in vivo accumulation of NOx (the final oxidized products of NO) converted from NO generated primarily in the major organs, including the liver, infected with Salmonella during the course of the infection. In heterozygous mice, the NOx level was high even at 30 days after infection (Fig. 4C), possibly because of a somewhat delayed recovery from the infection compared with that of wild-type mice, as shown in Fig. 1C and D. The same trend was observed for the level of NO production in the liver as determined by ESR spin trapping (Fig. 4B). Although a very low level in plasma for NOx was observed for iNOS−/− mice, there was no time-dependent increase in the level to correlate with the progression of septicemia after Salmonella infection.
Extensive liver damage and apoptotic changes in iNOS-deficient mice infected with Salmonella.
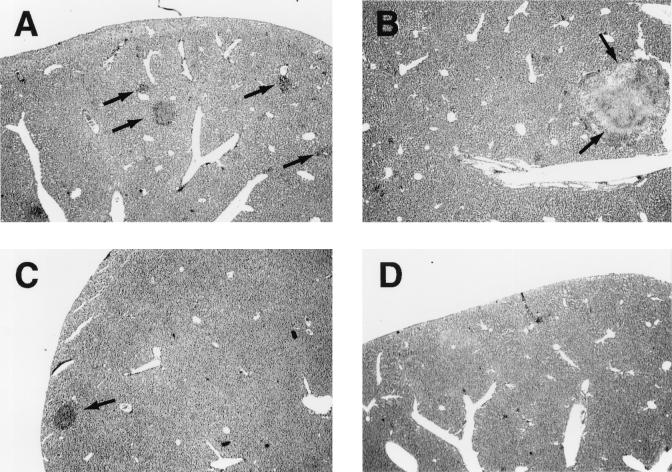
The pathological changes in the Salmonella-infected liver were evaluated by histological and immunohistochemical analyses. Apoptotic change in liver lesions induced by the Salmonella infection was also identified by the TUNEL method. On days 3 and 14 after infection (LT2 strain given i.p. at 4 × 102 CFU/mouse), the livers of iNOS−/− mice showed progressive formation of microabscesses (granulomatous lesions) together with extensive infiltration of inflammatory cells, such as neutrophils and macrophages (Fig. 5A and B). In contrast, in wild-type mice the pathological lesions were less evident and microabscesses were much reduced in both number and size compared with those in iNOS−/− mice (Fig. 5C and D).
FIG. 5.
Histopathology of livers of wild-type and iNOS-deficient mice infected with serovar Typhimurium strain LT2. Liver sections obtained at days 3 and 14 after infection (i.p.; 4 × 102 CFU/mouse) were stained with hematoxylin and eosin. Liver sections from iNOS−/− mice (A and B) and iNOS+/+ mice (C and D) were obtained on day 3 (A and C) and day 14 (B and D). The most typical result from at least six mice of each group is shown. Arrows indicate microabscess formations. Magnification, ×16.
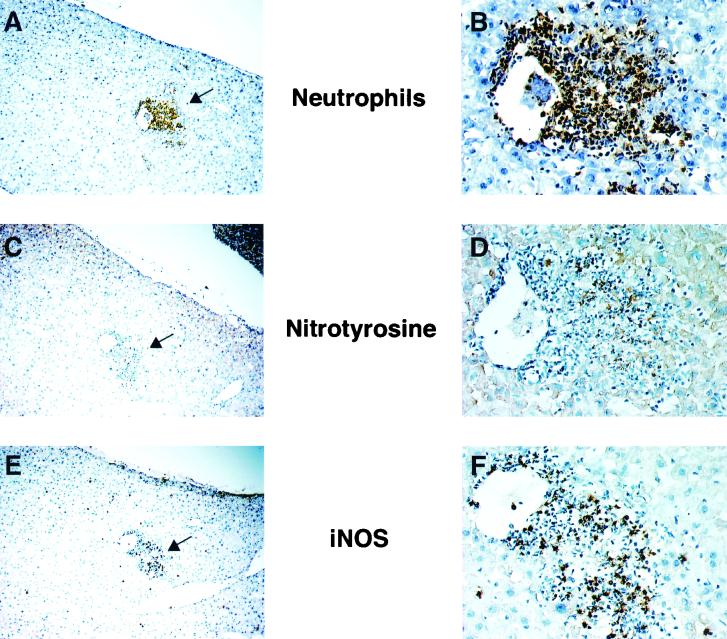
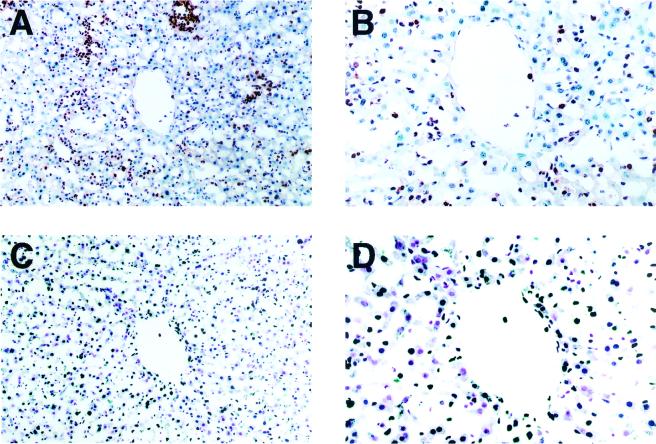
Neutrophil infiltration, iNOS expression, and nitrotyrosine formation in livers of wild-type mice were examined immunohistochemically by using serial liver sections obtained on day 3 after infection (Fig. 6). Staining for iNOS, neutrophils, and nitrotyrosine was localized mainly in the confined areas of the microabscesses that consisted mostly of neutrophils, some of which appeared to express iNOS. Nitrotyrosine was formed in some of the infiltrating cells and in the hepatocytes surrounding the septic areas as well. In addition, as shown in Fig. 7, we found that mice deficient in iNOS had extensive apoptotic changes in the hepatic cells rather than in neutrophils infiltrated in Salmonella-infected tissues, as identified by localization and morphological characteristics of TUNEL-positive cells that are apparently different from those of neutrophils. Also, apoptotic changes were not only localized intensely in the microabscesses but also distributed diffusely in the infected livers (Fig. 7).
FIG. 6.
Immunohistochemical analysis of livers of wild-type mice on day 3 after infection for neutrophil infiltration (A and B), nitrotyrosine formation (C and D), and iNOS expression (E and F). Three sequential sections of the liver (A→C→E; B→D→F) were immunostained using specific antibodies to neutrophils, nitrotyrosine, and iNOS. The area with the most intensive stain with each antibody, indicated with arrows in panels A, C, and E, is shown at higher magnification in panels B, D, and F. Mice were infected in the same manner as described for Fig. 3. Magnifications, ×36 (A, C, and E) and ×180 (B, D, and F).
FIG. 7.
Infiltration of neutrophils (A and B) and apoptotic change (C and D) in livers of iNOS-deficient mice during salmonellosis. iNOS−/− mice were infected with the serovar Typhimurium LT2 strain in the same manner as described for Fig. 3. Sequential sections of liver tissue obtained at 22 days after infection were examined for apoptosis by the TUNEL method and for neutrophil infiltration by immunohistochemical analysis as described for Fig. 6. The apoptotic cells are stained deep blue (C and D). Magnifications, ×80 (A and C) and ×160 (B and D).
Figure 8 shows the results of quantitative morphometric analyses for each pathological parameter: microabscess formation, neutrophil infiltration, and apoptotic change in the livers of wild-type and iNOS−/− mice. These results indicate again that serovar Typhimurium causes more severe liver damage in iNOS−/− mice than in wild-type mice. These data revealed that lack of iNOS expression, preventing excessive production of NO, exacerbated the inflammatory responses and hepatocyte injury induced by Salmonella infection, as evidenced by the increase in microabscess formation, neutrophil exudate, and apoptotic cell death in the liver.
FIG. 8.
Quantitative morphometric analyses for the size of the lesion (microabscess) (A) and the numbers of neutrophils infiltrated (B) and of apoptotic cells (C) in livers of wild-type and iNOS-deficient mice infected with serovar Typhimurium LT2 (i.p.; 4 × 102 CFU/mouse). Columns and error bars indicate means ± SEM (n = 6). ∗, P < 0.05, and ∗∗, P < 0.01, versus wild-type controls.
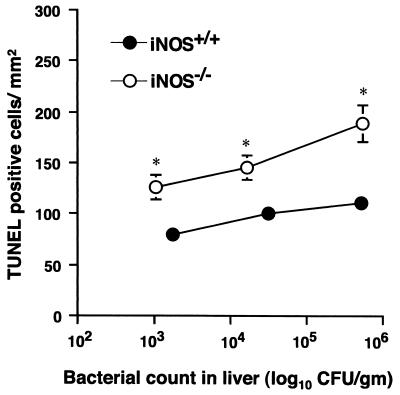
For Fig. 8C, we observed more extensive apoptotic changes in the iNOS−/− mouse livers than those in wild-type mice. One obvious reason for this great difference in apoptotic changes is that the wild-type mice were not severely infected, because these mice are protected by NO from bacterial injury, whereas the iNOS−/−mice have a progressive bacterial infection leading to intensive tissue damage. To more clearly illustrate the cytoprotective effect of NO, we performed TUNEL analyses with liver tissues from both wild-type and iNOS−/− mice having similar severity of infection (i.e., with similar bacterial burden) (Fig. 9). The result showed that the TUNEL-positive apoptotic cells in iNOS−/− mice were greater in number than in wild-type mice. As the bacterial number increased, ranging from 103 to more than 105 in both liver tissues, the apoptotic damage was also elevated, but iNOS−/− mice showed a significantly larger number of TUNEL-positive cells than did wild-type mice. It is thus hypothesized that NO may have a cytoprotective function by minimizing apoptotic damage in the liver.
FIG. 9.
TUNEL analysis in liver tissues from both wild-type and iNOS−/− mice with similar levels of bacterial growth in the liver. After wild-type and iNOS−/−mice were infected i.p. with different numbers of CFU of serovar Typhimurium LT2, the infected mice were sacrificed and viable bacterial counts were done with the livers. The mice (n = 3) with a similar range of bacterial growth in liver tissues were then analyzed for TUNEL experiments. Columns and error bars indicate means ± SEM (n = 3). ∗, P < 0.05, and ∗∗, P < 0.01, versus wild-type controls.
Susceptibility of Salmonella to various oxidants and peroxynitrite.
Because the present in vivo study demonstrated a significant difference in pathogenicity between the two serovar Typhimurium strains, it was thought that Salmonella virulence would depend on resistance to reactive nitrogen species derived from NO. To test this idea, stationary-phase serovar Typhimurium strains were exposed to peroxynitrite, and survival was monitored after specified time intervals (Fig. 10).
FIG. 10.
Salmonella killing by H2O2, t-BuOOH, and peroxynitrite. H2O2 (1, 5, and 10 mM) (A) or t-BuOOH (25 and 50 mM) (B) was added to suspensions of LT2 or Gifu 12142 at stationary-phase growth in BHI broth. (C) Peroxynitrite at 10, 25, and 50 mM in 10 mM NaOH was infused into 1.9 ml of bacterial suspension (M9 medium) at a flow rate of 3.3 μl/min. The concentrations of peroxynitrite in the reaction mixture during peroxynitrite infusion were assumed to be maintained at a constant 9.7, 15.34, and 21.7 μM, respectively. Decomposed peroxynitrite in M9 medium was infused in the same manner. At different intervals during the treatment with these oxidants, aliquots were removed from the reaction mixture and the number of viable bacteria was determined by means of the colony-forming assay. Data are means ± SEM from three independent experiments. ∗, P < 0.05, and ∗∗, P < 0.01, versus the LT2 strain.
H2O2 and t-BuOOH were used as standard oxidants. After exposure to various concentration of (1, 5, and 10 mM) H2O2 for 60 min, the virulent Gifu 12142 strain survived more than did the LT2 strain (Fig. 10A). Similarly increased survival of the virulent strain was observed after exposure to 25 and 50 mM t-BuOOH (Fig. 10B). In both cases bacterial killing is dose and time dependent (Fig. 10A and B). More important, viability of the Gifu 12142 strain remained high even after treatment with a constant concentration of peroxynitrite (21.7 μM) for 30 min: the initial inoculum of 108 CFU/ml of the LT2 strain was completely eliminated, but the Gifu strain showed appreciable survival (Fig. 10C). With peroxynitrite infused at as low as 9.7 and 15.34 μM, the LT2 strain was much more susceptible to peroxynitrite than the Gifu 12142 strain. The bacterial killing by peroxynitrite was observed in a dose- and time-dependent manner, and with all doses the viability of Gifu 12142 strain remained higher than that of the LT2 strain (Fig. 10C). Decomposed peroxynitrite did not affect bacterial viability. It is thus suggested that in vivo pathogenicity may be attributable to the resistance of Salmonella to NO-derived reactive nitrogen oxides such as peroxynitrite.
DISCUSSION
Previous work suggests that both NO and superoxide contribute in critical ways to host defense during serovar Typhimurium infection in mice (58). This interpretation is based on an in vivo study showing that pharmacological interventions aimed at inhibiting biosynthesis of either NO or superoxide markedly exacerbated septicemia and the lethal potential of Salmonella infection in mice (57, 58). In our studies reported here, we observed similar consequences of Salmonella infection in mice, related to the lack of the iNOS gene. We clearly demonstrated that iNOS-deficient mice infected with serovar Typhimurium showed increased bacterial load, mortality, and liver damage with extensive hepatocellular apoptosis, as well as development of severe septicemia.
In addition, we addressed the beneficial role of NO in murine salmonellosis produced by oral challenge with serovar Typhimurium. Almost all earlier studies of NO function in murine salmonellosis were performed with mice infected with serovar Typhimurium by i.p. inoculation; the p.o. route was rarely employed. Natural Salmonella infections occur initially via the oral route and then via bacterial invasion of the intestinal mucosa, where the Salmonella organisms traverse the epithelial barrier through M cells overlying the lymphoid follicles of Peyer's patches, followed by proliferation and spread to deeper tissues via lymphatics and the bloodstream (11, 24). It is important to explore the effect of NO in the first stage of infection, including Salmonella invasion of the intestinal mucosa. In the present study, therefore, we produced murine salmonellosis by p.o. administration of two serovar Typhimurium strains to wild-type and iNOS-deficient mice. Results showed that, as with i.p. infection, the pathological consequences of the infection were remarkably potentiated in iNOS-deficient mice compared with infection in the wild-type mice. This suggests that NO may have critical antimicrobial actions at any stage of salmonellosis.
NO, particularly NO produced in excess by activated phagocytes expressing iNOS, has been shown to function as a cytotoxic or cytostatic molecule and to inhibit the growth of pathogenic bacteria and protozoa (9, 10, 14, 18, 22, 36, 41, 42). Overproduction of NO has also been implicated in the pathogenesis of sepsis, cerebral malaria, and some viral infections (2, 3, 22, 62). It has been suggested that the mutagenic potential of NO is involved in carcinogenesis induced by parasites, viruses, and bacteria (Helicobacter pylori) (45). The pathological consequences of NO production in microbial pathogenesis seem to be determined by delicate and complicated interactions of the hosts and the pathogens. In this context, the potent host defense function of NO appears to be most clearly observed in murine salmonellosis, as revealed in our present study.
It is now well known that NO per se is not a bactericidal molecular species (1, 6, 12, 13, 61). Its cytotoxic effect is realized by its derivative reactive nitrogen oxides (51). For example, NO reacts with superoxide in a diffusion-limited reaction to yield peroxynitrite (4, 5, 49, 53), which is a strong oxidant and nitrating agent, to produce potent cytotoxic actions against various microbes through disintegration and chemical modification of various biomolecules, such as membrane lipids (51), nucleic acids (25, 45), and proteins (5, 47). The pathogenic intruders of the host organisms thus suffer from oxidative stress caused by NO-meditated host defense (2). Resistance of Salmonella to oxidative and nitrative stress may allow this pathogen to withstand NO- and oxygen radical-dependent killing mechanisms in phagocytic cells (12, 13, 16).
Our present data indicate that a virulent serovar Typhimurium strain, which is resistant to the bactericidal effect of peroxynitrite, was more invasive and pathogenetic in iNOS-competent wild-type mice than was the peroxynitrite-susceptible avirulent strain: the Gifu 12142 strain showed greater toxicity in both wild-type and iNOS-deficient mice than did the LT2 strain, regardless of inoculation route. In addition, the Gifu 12142 strain demonstrated a significantly higher resistance to peroxynitrite than did the LT2 strain. Thus, the greater in vivo pathogenicity of serovar Typhimurium Gifu strains may be attributable to higher resistance to peroxynitrite or other oxidants. This result may indirectly suggest a pivotal role of NO in host defense against Salmonella infections through formation of NO-derived oxidants such as peroxynitrite.
It has been demonstrated that the loss of virulence of the serovar Typhimurium LT2 strain results from a defect in the stationary-phase sigma factor S (RpoS) caused by an altered rpoS allele (60). Because RpoS is known to regulate the stationary-phase expression of a wide variety of genes in response to environmental stresses, such as starvation, oxidation, and low pH, the genetic defect in RpoS function may account for the high susceptibility of the LT2 strain to various oxidants and peroxynitrite (Fig. 10). Protective functions of serovar Typhimurium RpoS against oxidants and NO have been well characterized by Fang's group (16). Although the expression and function of RpoS in the serovar Typhimurium Gifu 12142 strain remain to be clarified, it is likely that tolerance of NO-dependent antimicrobial actions may confer the in vivo pathogenicity of the Gifu 12142 strain.
Our recent attempt to produce a RpoS-null mutant from the Gifu 12142 strain was unsuccessful, because the Gifu 12142 strain was entirely resistant to most commonly used antibiotics for the selection of the transformed mutants. However, a similar study using S. enterica serovar Typhi RpoS-null mutant worked well. An isogenic rpoS-deficient strain was successfully generated from a wild-type serovar Typhi 3P91 strain, named Gifu 3P330, and the wild-type Typhi 3P91 and Gifu 3P330 strains were subjected to the peroxynitrite susceptibility assay. It was thus found that the rpoS-deficient strain was much more susceptible, by 30-fold, to 21.7 μM constant flux of peroxynitrite than was the wild-type (Typhi 3P91) strain, suggesting the possible contribution of the rpoS gene to peroxynitrite tolerance (data not shown).
Peroxynitrite production can be indirectly identified by immunohistochemical detection of nitrotyrosine formed in cells and tissues (3, 29, 55). Although nitrotyrosine can be formed in a peroxynitrite-independent chemical reaction, such as the NO2−-H2O2-peroxidase pathway (15), peroxynitrite appears to be one of the major contributors to nitrotyrosine formation in vivo, for example, when excessive amounts and similar concentrations of both NO and superoxide are produced simultaneously (53). Intensive staining for nitrotyrosine was observed in the local area of microabscesses formed in the liver of Salmonella-infected wild-type mice; this staining was colocalized with iNOS immunostaining (Fig. 6). Furthermore, we previously showed that pharmacological inhibition of either NO or superoxide resulted in enhanced Salmonella growth in the liver and blood (57, 58). It is therefore quite reasonable to expect that peroxynitrite generated in the liver has an antibacterial effect during Salmonella infection in mice.
This idea is further supported by earlier findings that time profiles of NO production, as assessed by ESR spectroscopy (Fig. 4) (57), and of the superoxide-generating enzyme xanthine oxidase (57) were consistent with the time profile of bacterial yield in mouse liver after Salmonella infection (Fig. 3). Moreover, the lack of NO production led to a remarkable augmentation of bacterial growth in the liver and systemic blood circulation of the infected iNOS−/− animals (Fig. 3), strongly suggesting the importance of peroxynitrite in bacterial clearance in Salmonella infection.
Salmonella infection caused much more extensive liver damage (microabscess formation and induction of apoptosis) in NOS-deficient mice than in wild-type mice. The enhancement of apoptotic change was clearly demonstrated in the Salmonella-infected iNOS−/−mice, even when they were similarly affected by the bacteria in terms of bacterial growth in the liver (Fig. 9). These results suggest that NO potentially mediates its cytoprotective effect through its antiapoptotic activity.
In this context, it is interesting that Salmonella spp. were recently shown to induce apoptosis of host cells via activation of a cascade of intracellular proteases known as caspases (17, 23, 27, 38, 39). Serovar Typhimurium-derived SipB (for Salmonella invasion) protein, which is translocated into the infected cells after synthesis by Salmonella, appears to be directly involved in apoptosis induction by binding and activating caspases 1 and 2 (19, 23, 38). Such apoptotic processes are suggested as essential for Salmonella to penetrate the epithelial barrier and spread into the systemic circulation, subsequently causing typhoid-like diseases in mice.
NO is now known to exhibit potent antiapoptotic activity by affecting the caspase cascade (28, 33, 44). For example, NO can directly block caspase activity by S nitrosylation of the active site cysteine in caspases (33). If Salmonella activates proapoptotic molecules and if NO counteracts the apoptosis during Salmonella infections, a decrease or eventual absence of NO production may result in an increase in apoptosis. Indeed, our TUNEL assay of infected liver of iNOS-deficient mice showed a widespread distribution of TUNEL-positive hepatic cells. Salmonella organisms can infect and grow intracellularly, not only in macrophages but also in hepatocytes (34), and the extensive hepatic apoptosis observed in infected iNOS-deficient mice may have resulted from the loss of potent antiapoptotic effects of NO on the hepatocytes. The antiapoptotic effect of NO may thus contribute to host defense by preventing host cell apoptosis and subsequent Salmonella invasion and dissemination into deeper tissues from the primary septic foci.
It is intriguing that mice lacking iNOS undergo impaired liver regeneration (52), suggesting that hepatic iNOS expression is involved in an adaptive response for minimizing inflammatory injury. Thus, NO formed during salmonellosis may play an important role in hepatocellular regeneration or healing from damage caused by the bacteria, thus helping infected cells and organs maintain an effective defense against and recovery from salmonellosis.
Very recently, Mastroeni et al. reported that iNOS-deficient mice showed a much higher susceptibility to Salmonella infections than wild-type mice (35). They also suggested that iNOS and phox both have effective antimicrobial activity against Salmonella but work separately, at different stages of the infection. In addition, Vazquez-Torres et al. demonstrated by using macrophages from iNOS- or phox-deficient mice that macrophage killing of Salmonella requires both phox and iNOS and involves temporally coordinated actions of reactive oxygen intermediates and NO (59). Like Mastroeni et al., they suggested that phox is involved in the very early stage of bacterial clearance by macrophages, followed in the later and prolonged period of bacterial killing by sustained inhibition by NO of intracellular growth of Salmonella. In the macrophage-dependent antimicrobial effects that they reported, however, peroxynitrite did not contribute to bacterial killing but rather impaired the intracellular killing by the infected macrophages. Such NO-dependent suppressive effects on host defense have been reported not only for Salmonella (32) but also for other facultative, intracellular pathogens, such as mycobacteria (14).
This finding apparently conflicts with our interpretation of the antimicrobial activity of peroxynitrite in murine salmonellosis. We found that not only phox but also xanthine oxidase caused a potent anti-Salmonella effect as a superoxide-generating system occurring during murine salmonellosis, as described above. Because the level of xanthine oxidase is elevated in parallel with NO production (cf. reference 57 and Fig. 4), superoxide and reactive oxygen species derived from xanthine oxidase seem to work in a concerted manner with NO from iNOS for the effective antimicrobial activities of the host. More important, xanthine oxidase, which is normally localized in the cytoplasm of various cells, including hepatocytes and endothelial and epithelial cells, is released extracellularly under inflammatory conditions to function as an enzyme-producing superoxide (2, 3). Superoxide thus formed may produce peroxynitrite from NO (53), thereby helping to eliminate Salmonella organisms that have escaped from phagocytic cells within local septic foci and to effectively prevent their systemic dissemination in the late phase of infection. Thus, peroxynitrite formed from NO and superoxide generated by xanthine oxidase could conceivably be responsible for antimicrobial effects, particularly during the prolonged phase of salmonellosis.
In contrast to these findings, Shiloh et al. (54) showed that iNOS-deficient mice infected by the i.p. route were fully resistant to the serovar Typhimurium recBC mutant, which is an attenuated strain caused by a lack of DNA repair, but mice deficient in phox or deficient in both phox and iNOS were highly susceptible to the serovar Typhimurium recBC mutant. This result suggests that reactive oxygen species contribute to controlling murine salmonellosis to a greater extent than does NO. This is not consistent with our findings with the avirulent LT2 strain, which showed much higher pathogenicity in iNOS-deficient mice than in wild-type mice. We do not know the exact reason for these discrepant results, but they may be due to different contributions of recBC and rpoS (LT2 strain) genes to the tolerance of Salmonella to NO and its reactive intermediates.
The beneficial qualities of the cell-mediated immune effector mechanism involving cytokines are well known (56). It is now also evident that hosts respond to Salmonella infection by rapidly expressing iNOS, which in turn produces an excessive amount of NO, resulting in potent salmonellocidal activity, possibly through the formation of cytotoxic molecular species, such as peroxynitrite. As a whole, the present results clearly illustrate the beneficial effects of NO formed from iNOS as part of a primary immune response that helps the host to survive salmonellosis.
Acknowledgments
We thank Judith B. Gandy for excellent editorial work on the manuscript.
This work was supported by grants-in-aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology and from the Ministry of Health, Labor and Welfare of Japan.
Editor: A. D. O'Brien
REFERENCES
- 1.Akaike, T. 2000. Mechanisms of biological S-nitrosation and its measurement. Free Radic. Res. 33:461-469. [DOI] [PubMed] [Google Scholar]
- 2.Akaike, T. 2001. Role of free radicals in viral pathogenesis and mutation. Rev. Med. Virol. 11:87-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akaike, T., Y. Noguchi, S. Ijiri, K. Setoguchi, M. Suga, Y. M. Zheng, B. Dietzschold, and H. Maeda. 1996. Pathogenesis of influenza virus-induced pneumonia: involvement of both nitric oxide and oxygen radicals. Proc. Natl. Acad. Sci. USA 93:2448-2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beckman, J. S., T. W. Beckman, J. Chen, P. A. Marshall, and B. A. Freeman. 1990. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. USA 87:1620-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beckman, J. S., and W. H. Koppenol. 1996. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am. J. Physiol. 271:C1424-C1437. [DOI] [PubMed] [Google Scholar]
- 6.Brunelli, L., J. P. Crow, and J. S. Beckman. 1995. The comparative toxicity of nitric oxide and peroxynitrite to Escherichia coli. Arch. Biochem. Biophys. 316:327-334. [DOI] [PubMed] [Google Scholar]
- 7.Buchmeier, N. A., and F. Heffron. 1989. Intracellular survival of wild-type Salmonella typhimurium and macrophage-sensitive mutants in diverse populations of macrophages. Infect. Immun. 57:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butler, T., A. Islam, I. Kabir, and P. K. Jones. 1991. Patterns of morbidity and mortality in typhoid fever dependent on age and gender: review of 552 hospitalized patients with diarrhea. Rev. Infect. Dis. 13:85-90. [DOI] [PubMed] [Google Scholar]
- 9.Chan, J., Y. Xing, R. S. Magliozzo, and B. R. Bloom. 1992. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J. Exp. Med. 175:1111-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darrah, P. A., M. K. Hondalus, Q. Chen, H. Ischiropoulos, and D. M. Mosser. 2000. Cooperation between reactive oxygen and nitrogen intermediates in killing of Rhodococcus equi by activated macrophages. Infect. Immun. 68:3587-3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Darwin, K. H., and V. L. Miller. 1995. Molecular basis of the interaction of Salmonella with the intestinal mucosa. Clin. Microbiol. Rev. 12:405-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeGroote, M. A., and F. C. Fang. 2000. Antimicrobial properties of nitric oxide, p. 231-261. In F. C. Fang (ed.), Nitric oxide and infection. Kluwer Academic/Plenum, New York, N.Y.
- 13.DeGroote, M. A., D. Granger, Y. Xu, G. Campbell, R. Prince, and F. C. Fang. 1995. Genetic and redox determinants of nitric oxide cytotoxicity in a Salmonella typhimurium model. Proc. Natl. Acad. Sci. USA 92:6399-6403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doi, T., M. Ando, T. Akaike, M. Suga, K. Sato, and H. Maeda. 1993. Resistance to nitric oxide in Mycobacterium avium complex and its implication in pathogenesis. Infect. Immun. 61:1980-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eiserich, J. P., M. Hristova, C. E. Cross, A. D. Jones, B. A. Freeman, B. Halliwell, and A. van der Vliet. 1998. Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature (London) 391:393-397. [DOI] [PubMed] [Google Scholar]
- 16.Fang, F. C., S. J. Libby, N. A. Buchmeier, P. C. Loewen, J. Switala, J. Harwood, and D. G. Guiney. 1992. The alternative sigma factor katF (rpoS) regulates Salmonella virulence. Proc. Natl. Acad. Sci. USA 89:11978-11982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao, L. Y., and Y. A. Kwaik. 2000. The modulation of host cell apoptosis by intracellular bacterial pathogens. Trends Microbiol. 8:306-313. [DOI] [PubMed] [Google Scholar]
- 18.Granger, D. L., J. B. Hibbs, Jr., J. R. Perfect, and D. T. Durack. 1988. Specific amino acid (L-arginine) requirement for the microbiostatic activity of murine macrophages. J. Clin. Investig. 81:1129-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hersh, D., D. M. Monack, M. R. Smith, N. Ghori, S. Falkow, and A. Zychlinsky. 1999. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc. Natl. Acad. Sci. USA 96:2396-2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsu, H. S. 1989. Pathogenesis and immunity in murine salmonellosis. Microbiol. Rev. 53:390-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ignarro, L. J. 2000. Introduction and overview, p. 3-19. In L. J. Ignarro (ed.), Nitric oxide: biology and pathobiology. Academic Press, San Diego, Calif.
- 22.James, S. L. 1995. Role of nitric oxide in parasitic infections. Microbiol. Rev. 59:533-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jesenberger, V., K. J. Procyk, J. Yuan, S. Reipert, and M. Baccarini. 2000. Salmonella-induced caspase-2 activation in macrophages: a novel mechanism in pathogen-mediated apoptosis. J. Exp. Med. 192:1035-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones, B. D., and S. Falkow. 1996. Salmonellosis: host immune responses and bacterial virulence determinants. Annu. Rev. Immunol. 14:533-561. [DOI] [PubMed] [Google Scholar]
- 25.Juedes, M. J., and G. N. Wogan. 1996. Peroxynitrite-induced mutation spectra of pSP189 following replication in bacteria and in human cells. Mutat. Res. 349:51-61. [DOI] [PubMed] [Google Scholar]
- 26.Keusch, G. T. 1986. Typhoid fever, p. 1189-1195. In A. I. Braude, E. D. Charles, and J. Fierer (ed.), Infectious diseases and medical microbiology, 2nd ed. W. B. Saunders Co., Philadelphia, Pa.
- 27.Kim, J. M., L. Eckmann, T. C. Savidge, D. C. Lowe, T. Witthoft, and M. F. Kagnoff. 1998. Apoptosis of human intestinal epithelial cells after bacterial invasion. J. Clin. Investig. 102:1815-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim, Y. M., R. V. Talanian, J. Li, and T. R. Billiar. 1998. Nitric oxide prevents IL-1 and IFN-γ-inducing factor (IL-18) release from macrophages by inhibiting caspase-1 (IL-1β converting enzyme). J. Immunol. 161:4122-4128. [PubMed] [Google Scholar]
- 29.Kooy, N. W., J. A. Royall, Y. Z. Ye, D. R. Kelly, and J. S. Beckman. 1995. Evidence for in vivo peroxynitrite production in human acute lung injury. Am. J. Respir. Crit. Care Med. 151:1250-1254. [DOI] [PubMed] [Google Scholar]
- 30.Kuwahara, H., Y. Miyamoto, T. Akaike, T. Kubota, T. Sawa, T. Okamoto, and H. Maeda. 2000. Helicobacter pylori urease suppresses bactericidal activity of peroxynitrite via carbon dioxide production. Infect. Immun. 68:4378-4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laubach, V. E., E. G. Shesely, O. Smithies, and P. A. Sherman. 1995. Mice lacking inducible nitric oxide synthase are not resistant to lipopolysaccharide-induced death. Proc. Natl. Acad. Sci. USA 92:10688-10692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MacFarlane, A. S., M. G. Schwacha, and T. K. Eisenstein. 1997. In vivo blockage of nitric oxide with aminoguanidine inhibits immunosuppression induced by an attenuated strain of Salmonella typhimurium, potentiates Salmonella infection, and inhibits macrophage and polymorphonuclear leukocyte influx into the spleen. Infect. Immun. 67:891-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mannick, J. B., X. Q. Miao, and J. S. Stamler. 1997. Nitric oxide inhibits Fas-induced apoptosis. J. Biol. Chem. 272:24125-24128. [DOI] [PubMed] [Google Scholar]
- 34.Mastroeni, P., J. N. Skepper, and C. E. Hormaeche. 1995. Effect of anti-tumor necrosis factor alpha antibodies on histopathology of primary Salmonella infections. Infect. Immun. 63:3674-3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mastroeni, P., A. Vazquez-Torres, F. C. Fang, Y. Xu, S. Khan, C. E. Hormaeche, and G. Dougan. 2000. Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. II. Effects of microbial proliferation and host survival in vivo. J. Exp. Med. 192:237-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McInnes, I. B., B. Leung, X. Q. Wei, C. C. Gemmell, and F. Y. Liew. 1998. Septic arthritis following Staphylococcus aureus infection in mice lacking inducible nitric oxide synthase. J. Immunol. 160:308-315. [PubMed] [Google Scholar]
- 37.Miyamoto, Y., T. Akaike, M. S. Alam, K. Inoue, T. Hamamoto, N. Ikebe, J. Yoshitake, T. Okamoto, and H. Maeda. 2000. Novel functions of human α1-protease inhibitor after S-nitrosylation: inhibition of cysteine protease and antibacterial activity. Biochem. Biophys. Res. Commun. 267:918-923. [DOI] [PubMed] [Google Scholar]
- 38.Monack, D. M., D. Hersh, N. Ghori, D. Bouley, A. Zychlinsky, and S. Falkow. 2000. Salmonella exploits caspase-1 to colonize Peyer's patches in a murine typhoid model. J. Exp. Med. 192:249-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Monack, D. M., B. Raupach, A. E. Hromockyj, and S. Falkow. 1996. Salmonella typhimurium invasion induces apoptosis in infected macrophages. Proc. Natl. Acad. Sci. USA 93:9833-9838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moncada, S., and A. Higgs. 1993. The L-arginine-nitric oxide pathway. N. Engl. J. Med. 329:2002-2012. [DOI] [PubMed] [Google Scholar]
- 41.Nathan, C. 1997. Inducible nitric oxide synthase: what difference does it make? J. Clin. Investig. 100:2417-2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nathan, C., and M. U. Shiloh. 2000. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc. Natl. Acad. Sci. USA 97: 8841-8848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Negoescu, A., P. Lorimier, F. Labat-Moleur, C. Drouet, C. Robert, C. Guillermet, C. Brambilla, and E. Brambilla. 1996. In situ apoptotic cell labeling by the TUNEL method: improvement and evaluation on cell preparations. J. Histochem. Cytochem. 44:959-968. [DOI] [PubMed] [Google Scholar]
- 44.Ogura, T., M. Tatemichi, and H. Esumi. 1997. Nitric oxide inhibits CPP32-like activity under redox regulation. Biochem. Biophys. Res. Commun. 236:365-369. [DOI] [PubMed] [Google Scholar]
- 45.Ohshima, H., and H. Bartsch. 1994. Chronic infections and inflammatory processes as cancer risk factors: possible role of nitric oxide in carcinogenesis. Mutat. Res. 305:253-264. [DOI] [PubMed] [Google Scholar]
- 46.Okamoto, T ., T. Akaike, T. Nagano, S. Miyajima, M. Suga, M. Ando, K. Ichimori, and H. Maeda. 1997. Activation of human neutrophil procollagenase by nitrogen dioxide and peroxynitrite: a novel mechanism for procollagenase activation involving nitric oxide. Arch. Biochem. Biophys. 342:261-274. [DOI] [PubMed] [Google Scholar]
- 47.Radi, R., J. S. Beckman, K. M. Bush, and B. A. Freeman. 1991. Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J. Biol. Chem. 266:4244-4250. [PubMed] [Google Scholar]
- 48.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 49.Reiter, C. D., R. J. Teng, and J. S. Beckman. 2000. Superoxide reacts with nitric oxide to nitrate tyrosine at physiological pH via peroxynitrite. J. Biol. Chem. 275:32460-32466. [DOI] [PubMed] [Google Scholar]
- 50.Richter-Dahlfors, A., A. M. J. Buchan, and B. B. Finlay. 1997. Murine salmonellosis studied by confocal microscopy: Salmonella typhimurium resides intracellularly inside macrophages and exerts a cytotoxic effect on phagocytes in vivo. J. Exp. Med. 186:569-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rubbo, H., V. Darley-Usmar, and B. A. Freeman. 1996. Nitric oxide regulation of tissue free radical injury. Chem. Res. Toxicol. 9:809-820. [DOI] [PubMed] [Google Scholar]
- 52.Rudra, M. R., F. Y. J. Lee, A. Rosen, S. Q. Yang, H. Z. Lin, A. Koteish, F. Y. Liew, C. Zaragoza, C. Lowenstein, and A. M. Diehl. 1998. Impaired liver regeneration in inducible nitric oxide synthase-deficient mice. Proc. Natl. Acad. Sci. USA 95:13829-13834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sawa, T., T. Akaike, and H. Maeda. 2000. Tyrosine nitration by peroxynitrite formed from nitric oxide and superoxide generated by xanthine oxidase. J. Biol. Chem. 275:32467-32474. [DOI] [PubMed] [Google Scholar]
- 54.Shiloh, M. U., J. D. MacMicking, S. Nicholson, J. E. Brause, S. Potter, M. Marino, F. Fang, M. Dinauer, and C. Nathan. 1999. Phenotype of mice and macrophages deficient in both phagocyte oxidase and inducible nitric oxide synthase. Immunity 10:29-38. [DOI] [PubMed] [Google Scholar]
- 55.Szabó, C., A. L. Salzman, and H. Ischiropoulos. 1995. Endotoxin triggers the expression of an inducible isoform of nitric oxide synthase and the formation of peroxynitrite in the rat aorta in vivo. FEBS Lett. 363:235-238. [DOI] [PubMed] [Google Scholar]
- 56.Sztein, M. B., S. S. Wasserman, C. O. Tacket, R. Edelman, D. Hone, A. A. Lindberg, and M. M. Levine. 1994. Cytokine production patterns and lymphoproliferative responses in volunteers orally immunized with attenuated vaccine strains of Salmonella typhi. J. Infect. Dis. 170:1508-1517. [DOI] [PubMed] [Google Scholar]
- 57.Umezawa, K., T. Akaike, S. Fujii, M. Suga, K. Setoguchi, A. Ozawa, and H. Maeda. 1997. Induction of nitric oxide synthesis and xanthine oxidase and their roles in the antimicrobial mechanism against Salmonella typhimurium infection in mice. Infect. Immun. 65:2932-2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Umezawa, K., N. Ohnishi, K. Tanaka, S. Kamiya, Y. Koga, H. Nakazawa, and A. Ozawa. 1995. Granulation in livers of mice infected with Salmonella typhimurium is caused by superoxide released from host phagocytes. Infect. Immun. 63:4402-4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vazquez-Torres, A., J. Jones-Carson, P. Mastroeni, H. Ischiropoulos, and F. C. Fang. 2000. Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. I. Effects of microbial killing by activated peritoneal macrophages in vitro. J. Exp. Med. 192:227-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wilmes-Riesenberg, M. R., J. W. Foster, and R. Curtiss III. 1997. An altered rpoS allele contributes to the avirulence of Salmonella typhimurium LT2. Infect. Immun. 65:203-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yoshida, K., T. Akaike, T. Doi, K. Sato, S. Ijiri, M. Suga, M. Ando, and H. Maeda. 1993. Pronounced enhancement of NO-dependent antimicrobial action by an NO-oxidizing agent, imidazolineoxyl N-oxide. Infect. Immun. 61:3552-3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yoshida, M., T. Akaike, Y. Wada, K. Sato, K. Ikeda, S. Ueda, and H. Maeda. 1994. Therapeutic effects of imidazolineoxyl N-oxide against endotoxin shock through its direct nitric oxide-scavenging activity. Biochem. Biophys. Res. Commun. 202:923-930. [DOI] [PubMed] [Google Scholar]