Abstract
Neisseria gonorrhoeae releases soluble fragments of peptidoglycan during growth. These molecules are implicated in the pathogenesis of various forms of gonococcal infection. A major peptidoglycan fragment released by gonococci is identical to the tracheal cytotoxin of Bordetella pertussis and has been shown to kill ciliated fallopian tube cells in organ culture. Previous studies indicated that a unique lytic peptidoglycan transglycosylase (AtlA) was responsible for some, but not all, of the peptidoglycan-derived cytotoxin (PGCT) production in certain gonococcal strains. To examine the role of other putative lytic transglycosylases in PGCT production, we made a deletion mutation in a gonococcal gene exhibiting similarity with genes encoding lytic transglycosylases from other bacterial species. The gonococcal mutant was viable and grew normally, but it was less autolytic than the wild-type strain in stationary-phase culture and under nongrowth conditions. The gonococcal mutant was reduced in peptidoglycan turnover, and the profile of the released products showed a reduction in monomeric peptidoglycan. Proportionally more multimeric fragments were released. These results suggest that this gonococcal gene (ltgA) encodes a lytic peptidoglycan transglycosylase and that it is responsible for a significant proportion of the PGCT released by N. gonorrhoeae.
Neisseria gonorrhoeae is a gram-negative bacterium and the causative agent of the sexually transmitted disease gonorrhea. Gonococcal infection usually results in easily treatable urethritis or cervicitis. However, untreated gonorrhea can cause pelvic inflammatory disease, disseminated gonococcal infection, arthritis, or neonatal blindness. Peptidoglycan (PG) is an important virulence factor in gonococcal infections, and both multimeric and monomeric PG fragments have been shown to have potent biological effects (8, 24, 27). Gonococci are unusual among gram-negative bacteria in that soluble PG fragments generated during growth are released into the surrounding milieu (28). Sinha and Rosenthal characterized the PG fragments released by growing gonococci and determined that the most abundant fragments released were the 1,6-anhydro-disaccharide PG monomers (33). The 1,6-anhydro-disaccharide tetrapeptide monomer is a 921-Da molecule identical to the tracheal cytotoxin of Bordetella pertussis and is referred to as PG-derived cytotoxin (PGCT) (22). PGCT induces the production of the inflammatory cytokines interleukin-1 (IL-1) and IL-6 in cultured cells and induces arthritis in a rat model (6, 8, 15). Arthropathic effects are characteristic of disseminated gonococcal infection (23). The sloughing of the majority of ciliated cells and the disruption of the mucosal integrity by application of purified PG monomers mimic the effects of gonococcal infection in the fallopian tube organ culture model of pelvic inflammatory disease (24). Thus, PG fragments may be involved in the pathogenic processes of disseminated infection and pelvic inflammatory disease and, through the production of IL-1 and IL-6, may be involved in the inflammatory response characteristic of uncomplicated gonorrhea.
Lytic transglycosylases cleave the N-acetylmuramic acid-β-1,4-N-acetylglucosamine linkage in PG and catalyze the formation of a 1,6-anhydro bond on the N-acetylmuramic acid. Several lytic PG transglycosylase mutants have been characterized in Escherichia coli (16). These mutants show no growth defects (21). The only growth phenotype seen for E. coli soluble lytic transglycosylase 70 (Slt70) (sltY) mutants is increased sensitivity to β-lactam antibiotics, suggesting that Slt70 may act in conjunction with PG biosynthetic enzymes (35).
Gonococcal mutants with a deletion in atlA, encoding a putative lytic PG transglycosylase, show a reduction in PGCT production but still produce about 40% as much PGCT as the wild type (our unpublished observations). E. coli produces multiple lytic transglycosylases with similar biochemical functions (16). Therefore, it seemed likely that N. gonorrhoeae produces one or more additional lytic transglycosylases and that these enzymes act in the production of PGCT. Analysis of the gonococcal genome sequence yielded an open reading frame with significant similarity to the open reading frames of E. coli lytic transglycosylases. Here we describe generation and characterization of a gonococcal mutant carrying a deletion in this gene (designated ltgA), which encodes a putative lytic transglycosylase.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Gonococcal strains used in this study are listed in Table 1. All experiments except transformations were performed with nonpiliated variants. Gonococci were grown with aeration in GC base liquid (GCBL) medium (1.5% proteose peptone no. 3, 0.4% K2HPO4, 0.1% KH2PO4, 0.1% NaCl; pH 7.2) containing Kellogg's supplements and 0.042% NaHCO3 or on GCB agar plates (Difco) in the presence of 5% CO2 at 37°C (19, 25). E. coli was grown in Luria broth or on Luria agar plates (30). Antibiotics were used at the following concentrations: for N. gonorrhoeae, 10 μg of erythromycin per ml and 100 μg of streptomycin per ml; and for E. coli, 250 μg of erythromycin per ml and 40 μg of kanamycin per ml.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Properties | Reference |
|---|---|---|
| pIDN1 | N. gonorrhoeae insertion-duplication plasmid (Ermr) | (12) |
| pHSS6 | E. coli cloning vector (Kanr) | (32) |
| pKC1 | pIDN1 with F62 rpsL inserted at XhoI and KpnI sites (Ermr Strs) | This study |
| pKC3 | pHSS6 with ltgA inserted at EcoRI and XbaI sites (Kanr) | This study |
| pKC4 | pKC3 with rpsL/ermC cassette from pKC1 replacing 872-bp ltgA coding sequence (Ermr/Kanr) | This study |
| pKC5 | pKC3 with 872-bp SpeI-HincII deletion (Kanr) | This study |
| MS11A | Wild-type N. gonorrhoeae (Strr) | (31) |
| F62 | Wild-type N. gonorrhoeae (Strs) | (34) |
| FA1090 | Wild-type N. gonorrhoeae | (17) |
| KC99 | MS11A transformed with pKC4 (Ermr/Strs) | This study |
| KC100 | MS11A ltgA | This study |
| KC103 | FA1090 ltgA | This study |
Plasmid construction.
Plasmids used in this study are listed in Table 1. For cloning of ltgA, the following specific primers, which included restriction enzyme recognition sites (underlined), were designed based on the Gonococcal Genome Sequencing Project sequence: 5′ GCATCTAGAGGGCAACCATTTCGGACAA 3′ and 5′ TTAGAATTCGCCGTCAATGCCGTT 3′. ltgA was amplified by PCR from MS11A chromosomal DNA in a hot-start reaction with an annealing temperature of 64°C. The PCR product was digested with EcoRI and XbaI and ligated into pHSS6 (32). The ligation product was transformed into chemically competent E. coli, and Kanr transformants were screened for a plasmid of the expected size. For construction of the ermC/rpsL cassette, rpsL was amplified by PCR using chromosomal DNA from streptomycin-sensitive N. gonorrhoeae strain F62 as the template and primers 5′ CAGCTCGAGTGATTGTGAGGGATGTCGG 3′ and 5′ TTAGGTACCGGCAGTACGTCGCGCTTGG 3′ with an annealing temperature of 62°C. The PCR product was digested with XhoI and KpnI and ligated into pIDN1, forming pKC1. To insert the ermC/rpsL cassette into ltgA, pKC1 was digested with NheI and Ecl136II and ligated into pKC3 digested with SpeI and HincII and then transformed into E. coli, resulting in pKC4 (see Fig. 2). To construct a deletion in the cloned ltgA, pKC3 was digested with SpeI and HincII, blunted with T4 polymerase, ligated, and transformed into E. coli, creating an 872-bp internal deletion. Plasmid construction was confirmed by PCR, restriction digest mapping, and DNA sequencing. Sequencing reactions were performed by using a Big Dye cycle sequencing kit (PE Biosystems) and ABI sequencers (models 377XL and 377-96).
FIG. 2.
Generation of the ltgA deletion strain (KC100) by the positive-negative selection method. ermC (erythromycin resistance), rpsL (streptomycin sensitivity), and two gonococcal uptake sequences were inserted into a clone of ltgA containing an 872-bp internal deletion. This construct (pKC4) was transformed into MS11A, and Ermr colonies were selected. These colonies were transformed with a plasmid containing ltgA with an internal deletion (pKC5), and Strr colonies were selected.
ltgA mutants of N. gonorrhoeae.
Approximately 2 μg of pKC4 was linearized with NcoI, purified by using Geneclean (Bio 101), and used to transform N. gonorrhoeae strain MS11A by the method of Gunn and Stein (11). Individual erythromycin-resistant colonies were chosen, and insertion of the plasmid in the expected location was confirmed by PCR. Mutants were retransformed with pKC5 and then swabbed onto GCB medium containing streptomycin. The deletion in ltgA was detected by PCR. The presence of the mutation in KC100 was confirmed by Southern blotting by using standard procedures (30). MS11A and KC100 chromosomal DNA were digested with ClaI, Sau3AI, or NdeI. DNA was transferred from the agarose gel by vacuum blotting to a Duralon-UV membrane and UV cross-linked. The blot was probed with ltgA coding sequence DNA produced by PCR and labeled with digoxigenin. The blot was washed at high stringency and developed by the chemiluminescent method according to the manufacturer's instructions (Boehringer Mannheim). The ltgA mutation was introduced into strain FA1090 by transformation with KC99 chromosomal DNA, followed by transformation with pKC5 as described above.
Lysis in buffer.
Autolysis in buffer was measured essentially as described by Hebeler and Young (13). Gonococci were inoculated into liquid cultures at a density of approximately 108 CFU/ml and were grown to the stationary phase (17 h). Optical densities at 540 nm (OD540) were determined, and approximately 3 × 108 CFU was centrifuged (8 min, 750 × g), suspended in 3 ml of 50 mM Tris HCl (pH 8 or 6), and monitored for a decrease in density at room temperature by measuring the OD540 at different times.
PG purification.
Gonococcal PG was labeled and purified essentially as described by Rosenthal and Dziarski (29). Log-phase gonococci (OD540, 0.6 to 1.4) were centrifuged (1 min, 8,400 × g) and washed in GCBL medium containing 0.4% pyruvate. Cells were resuspended in GCBL-pyruvate medium at an OD540 of 0.2, [6-3H]glucosamine was added at a concentration of 2 μCi/ml, and the cells were grown for 2 h. Cells were then washed with GCBL medium and resuspended at an OD540 of 0.2. After 2.5 h of growth in GCBL medium without label, cultures were centrifuged (5 min, 1,700 × g), and each supernatant was passed through a 0.22-μm-pore-size filter and stored at −20°C. Two 350-ml size exclusion columns (Bio-Gel P6 and Bio-Gel P30; Bio-Rad) were connected in tandem, and 10 ml of filtered gonococcal supernatant was applied. The columns were eluted with 0.1 M LiCl, and 3-ml fractions were collected. Three hundred microliters of each fraction was added to 3 ml of LS cocktail (Research Products International), and samples were counted with a Packard Tri-Carb 2100TR liquid scintillation counter.
PG turnover.
PG turnover was measured by monitoring the loss of 3H-labeled PG from gonococci growing in culture. The cells were labeled as described above and diluted to an OD540 of 0.2. For each time point, 1 ml of gonococcal culture was centrifuged (754 × g, 6 min, 4°C) and then suspended in 165 μl of 50 mM sodium acetate (pH 5.0). Then 165 μl of 8% sodium dodecyl sulfate was added, and the samples were boiled for 30 min. Samples were centrifuged (14,000 × g, 30 min) and then suspended in 200 μl of sterile water and counted as described above. Eight hundred microliters of unlabeled carrier cells was added to each sample up through the 4-h time point to facilitate efficient recovery of bacteria.
Computer-based searches and alignments.
To identify potential lytic PG transglycosylases, the N. gonorrhoeae genome sequence of strain FA1090 was searched by using the TBLASTN program and a consensus lytic transglycosylase motif based on the sequence identified by Koonin and Rudd (IPQSYAMAIARQESAWNPKVKSPVGASGLMQIMPGTA-IFSSAAYNAG) (20). Alignment was performed by using the GAP program of the GCG Wisconsin package.
RESULTS
Identification of PG transglycosylase homologues in the gonococcal genome.
Previously, we characterized the PG hydrolase AtlA. Mutations in atlA resulted in altered PG turnover and cell lysis. Furthermore, AtlA was found to be highly similar to PG transglycosylases, suggesting that AtlA is involved in cell wall hydrolysis and production of toxic PG fragments (4). The atlA gene was found to be located in a genetic island present in most, but not all, gonococcal strains (5). Characterization of PG fragments released by an atlA insertion-deletion mutant showed a reduction in release of PGCT to 40% of the wild-type level (Dillard, unpublished data). Additionally, we found that FA1090, a strain that does not carry atlA or the genetic island, still produced PG monomers (unpublished observations). These results suggested that another PG transglycosylase was encoded in the gonococcal chromosome and was responsible for a significant portion of the PGCT made by atlA+ strains and possibly all the PGCT made by atlA strains. In order to identify other gonococcal PG transglycosylases, we searched the N. gonorrhoeae strain FA1090 genome sequence using a consensus lytic PG transglycosylase motif. This search identified two contigs in the then-uncompleted genome sequence. One of the putative PG transglycosylases was found to show similarity to the well-characterized soluble lytic transglycosylase Slt70 of E. coli. The second was similar to the E. coli membrane-bound lytic transglycosylase MltC.
We pursued characterization of the Slt70 homologue, which we designated lytic transglycosylase A (LtgA). ltgA encodes a 616-amino-acid predicted protein with a molecular mass of 67.5 kDa. A potential signal sequence cleavage site is found at residue 20 of the predicted protein. LtgA has a nearly consensus lipoprotein processing site, suggesting that like some known PG hydrolases, LtgA may be a lipoprotein (16). Comparison with the E. coli lytic PG transglycosylase Slt70 amino acid sequence revealed 26.1% identity and 32.9% similarity to N. gonorrhoeae LtgA. Although similarity is found over the entire length of the proteins, the greatest degree of similarity is found in the C-terminal portion of the proteins, which contains the catalytic residue and peptide and substrate binding sites of Slt70 (Fig. 1A). The E. coli Slt70 catalytic site at Glu478 is conserved in LtgA. By cocrystallization of E. coli Slt70 with 1,6-anhydromuropeptide, van Asselt et al. identified nine residues that line a peptide binding site (37). Four of these residues are identical in LtgA, and four more positions have similar amino acids substituted. The substrate binding motif and conserved tyrosine are also found in LtgA (36).
FIG. 1.
(A) Alignment of the deduced amino acid sequences of N. gonorrhoeae LtgA and E. coli Slt70. LtgA shows significant similarity to Slt70 in the region surrounding the Slt70 catalytic residue (triangle), the substrate binding motif and conserved tyrosine (boxes), and the residues in the peptide binding cleft (boldface type). (B) Physical and genetic map of the ltgA region of the gonococcal chromosome. ltgA is adjacent to a divergently transcribed hypothetical ABC transporter. Restriction sites were determined by DNA sequencing or Southern blotting. GCU, gonococcal uptake sequence.
Adjacent to ltgA and divergently transcribed is a hypothetical open reading frame similar to ABC transporters. Directly downstream of ltgA are a small open reading frame encoding a hypothetical 30-amino-acid polypeptide and rpsU, predicted to encode ribosomal subunit protein S21 (Fig. 1B). ltgA is closely followed by an inverted repeat sequence that likely serves as a transcriptional terminator. The inverted repeat contains the gonococcal uptake sequence, a 10-bp sequence that is necessary for efficient DNA uptake in gonococcal natural transformation and is commonly found in gonococcal transcription terminators (9). The small open reading frame directly downstream of ltgA is preceded by a putative ribosome binding site and promoter; therefore, it is unlikely that its expression, or that of rpsU, is affected by transcription and translation of ltgA.
Creation of an ltgA deletion mutation in N. gonorrhoeae.
To study the function of LtgA, we created a deletion in ltgA in the gonococcal chromosome. We created the mutation by using a positive-negative selection cassette and the method of Johnston and Cannon (18). This method allows for selection of an insertion in the gene of interest and for selection of recombinants that lose the insertion when they are transformed with a mutated construct. Although we used the same markers as Johnston and Cannon, we created our own construct for this purpose in the insertion-duplication plasmid pIDN1, which we previously described (12). The resulting plasmid, pKC1, contains rpsL, ermC, and the gonococcal uptake sequences that can be excised as a cassette and ligated into the cloned gene of interest.
Construction of the ltgA deletion mutation by use of the positive-negative selection cassette is illustrated in Fig. 2. A 2.1-kb fragment containing ltgA was amplified from N. gonorrhoeae strain MS11A. This fragment was cloned into pHSS6, generating plasmid pKC3. A mutation was constructed by cloning the positive-negative selection cassette in place of 872 bp (approximately one-half) of the ltgA coding sequence. This construct was transformed into N. gonorrhoeae MS11A, and erythromycin-resistant transformants were selected. Ten transformants were tested by PCR, and all were confirmed to have the insertion in ltgA. A second mutated construct of ltgA was created by removing the same region of the ltgA coding sequence, creating an 872-bp deletion. The deletion construct (pKC5) was then used to transform the gonococcal mutant that carried the ermC/rpsL insertion. The transformation mixture was plated on streptomycin to select for those transformants in which the ermC/rpsL insertion was replaced with the simple deletion. Fifteen streptomycin-resistant transformants were tested for the presence of the deletion by PCR. All 15 were found to have incorporated the deletion. In two of these transformants, the presence of the deletion was then confirmed by Southern blotting (data not shown).
ltgA mutants have normal growth characteristics and exhibit increased survival under autolytic conditions.
Since PG hydrolases are involved in cell wall maintenance, growth, and septation, mutations in PG hydrolases have the potential to be lethal or detrimental to growth. However, the deletion mutation in ltgA had no obvious effects on growth in culture. When cells were grown in liquid culture, the doubling time in the log phase was the same as that of the wild type. The colonies of the ltgA mutant (KC100) were the same size as those of the wild-type strain and showed no obvious morphological changes when viewed under a dissecting microscope (data not shown). When viewed by light microscopy, KC100 gonococci showed normal morphology and did not differ from the wild type in terms of number of cells per group, indicating that cell separation was not affected (data not shown).
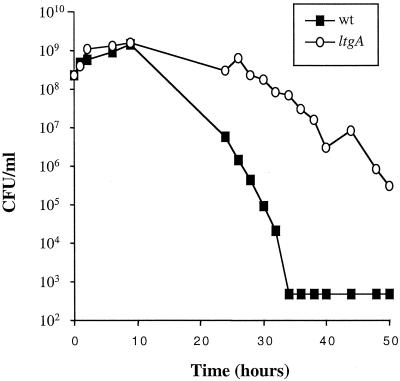
In addition to functioning in growth and separation, PG hydrolases are thought to be involved in autolysis. The only mutation that has been shown to affect gonococcal cell lysis is in atlA, the gene encoding a PG transglycosylase (4). Since LtgA is similar to known PG transglycosylases, we tested the effects of the ltgA mutation on autolysis. When grown in liquid culture, gonococci lyse and die in the stationary phase after carbon sources in the medium are depleted (25). The wild-type and ltgA mutant strains were grown in liquid culture, and cell viability was monitored (Fig. 3). The wild-type strain reached a density of more than 109 CFU/ml. By 24 h, the viable counts had dropped 100-fold to less than 107 CFU/ml. The number of viable gonococci continued to decrease and by 32 h had dropped below the limit of detection. By contrast, after the ltgA mutant grew normally in the log phase, the density dropped only 10-fold by 24 h and still was more than 108 CFU/ml. The viable counts decreased slowly, and at 50 h more than 105 CFU/ml survived. This phenotype is highly similar to that of an atlA mutant of N. gonorrhoeae MS11A (4).
FIG. 3.
Survival in stationary-phase culture. The ltgA mutant KC100 and wild-type strain MS11A (wt) were grown in liquid culture, and aliquots were removed and plated to determine viable CFU at different times. The values for times at which no colonies were obtained were recorded as 500 CFU/ml, the lower limit of detection. The results shown are representative of six trials.
As a further test of the effects of the ltgA deletion on autolysis, lysis of the mutant and wild-type strains was measured in buffer. It was previously shown that gonococci lyse rapidly in pH 8 buffer and more slowly in pH 6 buffer, conditions that are permissive and nonpermissive for the major autolysin, respectively (13, 14). At pH 6, there was no significant difference in the rates of lysis between the wild type and the ltgA mutant (data not shown). However, at pH 8, autolysis was significantly reduced for the ltgA mutant (Table 2). This result suggests that either LtgA acts directly in cell lysis and is responsible for producing some of the lesions in the cell wall or it acts indirectly in autolysis by facilitating the action of other PG hydrolases.
TABLE 2.
Autolysis in buffer
Autolysis was measured as the loss of optical density following transfer to 50 mM Tris HCl (pH 8) as determined at time zero and 2 h. The values are arithmetic means ± standard deviations based on three separate trials.
The mean was significantly different from the wild-type mean, as determined by Student's t test.
PG fragment production.
To determine if LtgA acts in the release of PG fragments, we measured PG turnover in a pulse-chase experiment. The gonococcal PG was metabolically labeled in the mutant and wild-type strains by growing the cells in medium containing [6-3H]glucosamine and lacking glucose. Following the labeling, the cells were washed and diluted to a concentration of 108 CFU/ml in medium containing glucose. At various points during the chase period, the macromolecular PG was purified and the amount of original PG remaining in the cells was quantified. The ltgA mutant KC100 showed a decreased rate of PG turnover during log-phase growth and during entry into the stationary phase compared to the MS11A rate (Fig. 4). These results show that LtgA is involved in PG turnover during growth.
FIG. 4.
PG turnover. MS11A (wild type [wt]) and KC100 (ltgA) were each pulse-labeled with [6-3H]glucosamine and grown in liquid culture. Aliquots were taken, the macromolecular PG was harvested, and the 3H content was measured at different times. The values are the values from three trials and are the arithmetic means of the percentages of the original radioactivity remaining. The error bars indicate standard deviations. An asterisk indicates a statistically significant difference, as determined by Student's t test (P < 0.01).
To determine how the ltgA mutation affected the release of PG fragments, we analyzed PG fragments in culture supernatants by using size exclusion chromatography. It was previously shown that gonococci release three major types of glycan-containing PG fragments which can be separated by size. These are free disaccharide, PG monomers, and PG multimers (33). The PG monomers are 1,6-anhydro-disaccharides with either tripeptide or tetrapeptide side chains. The 1,6-anhydro-disaccharide tetrapeptide is PGCT. Comparison of the released PG fragment profiles for wild-type and ltgA gonococci showed increased release of PG multimer fragments and decreased release of PG monomers by the mutant strain (Fig. 5). The ltgA mutant also showed a reduction in the free disaccharide content. Similar results were obtained when the released PG profile for an ltgA mutant made from gonococcal strain FA1090 was analyzed. The PG monomer fraction was greatly reduced, and the PG multimers comprised a larger proportion of the released fragments (data not shown). The decrease in monomer release by the ltgA mutants supports the hypothesis that LtgA functions in PGCT production.
FIG. 5.
Characterization of released PG fragments by gel filtration chromatography. Supernatants containing released 3H-labeled fragments from each strain were separated by passage over sizing columns. Fractions were collected and counted, and the data are presented as percentages of the total counts per minute versus the volume passed through the columns. The identities of the PG fragments were determined by comparison to known standard chromatographs. wt, wild type.
DISCUSSION
The evidence presented here suggests that LtgA is a lytic PG transglycosylase. An ltgA mutant was reduced in PG turnover and release of PG monomers. Furthermore, the sequence of LtgA shows similarity to the sequences of lytic transglycosylases of E. coli, including conserved residues for PG binding. The reduced death of the ltgA mutant in stationary-phase culture and the reduced lysis in buffer are consistent with the hypothesis that LtgA functions as a cell wall hydrolase. We have not been able to produce a strain complemented for ltgA and therefore cannot conclude definitively that all the phenotypes of the ltgA mutants are due to the ltgA deletion. However, since the mutation is an internal deletion in ltgA and the adjacent genes appear to be transcribed separately from ltgA, the mutation is unlikely to have significant polar effects.
The ltgA mutant showed no obvious defect in growth or cell separation, suggesting that LtgA is not required for these functions. This result is consistent with the phenotype of E. coli lytic transglycosylase mutants. When the first lytic transglycosylase mutants of E. coli were found to have no growth phenotype, it was hypothesized that this was due to the presence of other PG transglycosylases (7, 35). However, now that double and triple mutants have been found to grow normally, it is beginning to appear that the PG transglycosylase reaction may not be necessary for normal growth (21). Lommatzsch et al. detected no growth defect and no morphological changes in E. coli lytic transglycosylase triple mutants when they were viewed by phase-contrast microscopy (21). Thus, it may be that other PG hydrolases (e.g., endopeptidases or muramidases) are sufficient to remove PG strands during growth and separation. The E. coli lytic transglycosylase triple mutant of Lommatzsch et al. showed an increase in the average length of glycan strands and a decrease in 1,6-anhydromuramyl residues, indicating that these characteristics do not have an impact on normal growth (21). These results suggest that the function of PG turnover may be something other than a physical requirement for the removal of PG. It has been hypothesized that PG turnover and recycling are involved in the cell sensing its own growth state (26). Transcriptional regulators that respond to the level of PG fragments in the cytoplasm have been identified in some bacterial species (reviewed in reference 26). The N. gonorrhoeae genome contains homologues of enzymes involved in PG recycling, and the levels of turnover are consistent with a certain level of recycling occurring in gonococci (28). We do not know whether N. gonorrhoeae has cytoplasmic proteins for sensing PG fragments; however, this would be an attractive mechanism for controlling cell processes, including autolysis.
The ltgA mutant released less PG monomer fragments but showed increased release of multimeric fragments. One possible explanation for this result is that the PG multimers are the normal substrate for LtgA. Without LtgA, the multimers accumulate and make up a larger proportion of the released PG. An alternative explanation is that in the absence of LtgA, other PG hydrolases with a different specificity degrade the PG strands. Amidase, endopeptidase, and a different type of hexosaminidase activity have been described in gonococci (2, 10, 14). Structural analysis of the fragments released by ltgA mutants may shed light on this process and identify the enzymes involved in the multimer fragment production.
The phenotypes of the ltgA mutant are highly similar to those of an atlA mutant (4). Both mutants are reduced in cell death in stationary-phase culture, they are both reduced in autolysis in buffer, and they are both reduced in PG turnover. However, the accumulation of PG multimers seen in an ltgA mutant does not occur in the atlA mutant (Dillard, unpublished). Another interesting difference is that the effect on autolysis in buffer could be observed only at pH 6 for the atlA mutant, conditions under which the major autolysin (N-acetylmuramyl-l-alanine amidase) is not active (14). The difference in autolysis is seen at pH 8 for the ltgA mutant, conditions under which the major autolysin should be active. These findings may simply reflect different pH optima for the two transglycosylases. However, the results may indicate that LtgA activity is required for optimal PG hydrolysis by the major autolysin. Dillard and Seifert suggested that another PG hydrolase might substitute for AtlA during growth if lytic PG transglycosylase activity is important for growth, but would not do so at an acidic pH or in the stationary phase (4). LtgA appears to be such an enzyme, since it appears to act in at least some of the same processes as AtlA but does not show a phenotype at an acidic pH. It is still possible that lytic transglycosylase activity is not important for growth or that other lytic transglycosylases, such as the MltC homologue which we identified, may substitute for the activity of AtlA or LtgA. The presence of two (and possibly more) enzymes with potentially redundant functions either indicates that gonococci have an elaborate backup system for cell wall processes or may suggest that the enzymes have different functions or are differently regulated or localized. AtlA is encoded in a group of type IV secretion genes in the gonococcal genetic island, and recent evidence suggests that AtlA may have a dedicated role in assembly of the type IV secretion system (5).
Due to the extensive turnover and release of PG fragments in vitro, PGCT is expected to be released during gonococcal infection. Although several lytic transglycosylases have been characterized in E. coli, the genes for PGCT production have not been previously characterized in bacteria in which PGCT is thought to act in infection (i.e., B. pertussis, Haemophilus influenzae, and N. gonorrhoeae) (1, 3, 24). Characterization of lytic transglycosylases in gonococci should increase our understanding of how PGCT is generated and released during infection, and creation of mutant strains deficient in these enzymes should aid investigation of the role of PG in gonococcal virulence-related processes.
Acknowledgments
We thank the Cremer Fellowship in the Basic Sciences for financial support of Karen A. Cloud. This work was supported in part from a grant to the University of Wisconsin Medical School under the Howard Hughes Medical Institute Research Resources Program for Medical Schools.
We thank R. S. Rosenthal for the kind gift of PG fragment standards. We acknowledge the Gonococcal Genome Sequencing Project supported by USPHS/NIH grant AI38399 and B. A. Roe, L. Song, S. P. Lin, X. Yuan, S. Clifton, T. Ducey, L. Lewis, and D. W. Dyer of the University of Oklahoma.
Editor: J. T. Barbieri
REFERENCES
- 1.Burroughs, M., S. Prasad, C. Cabellos, P. M. Mendelman, and E. Tuomanen. 1993. The biologic activities of peptidoglycan in experimental Haemophilus influenzae meningitis. J. Infect. Dis. 167:464-468. [DOI] [PubMed] [Google Scholar]
- 2.Chapman, S. J., and H. R. Perkins. 1983. Peptidoglycan-degrading enzymes in ether-treated cells of Neisseria gonorrhoeae. J. Gen. Microbiol. 129:877-883. [DOI] [PubMed] [Google Scholar]
- 3.Cookson, B. T., H.-L. Cho, L. A. Herwaldt, and W. E. Goldman. 1989. Biological activities and chemical composition of purified tracheal cytotoxin of Bordetella pertussis. Infect. Immun. 57:2223-2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dillard, J. P., and H. S. Seifert. 1997. A peptidoglycan hydrolase similar to bacteriophage endolysins acts as an autolysin in Neisseria gonorrhoeae. Mol. Microbiol. 25:893-901. [DOI] [PubMed] [Google Scholar]
- 5.Dillard, J. P., and H. S. Seifert. 2001. A variable genetic island specific for Neisseria gonorrhoeae is involved in providing DNA for natural transformation and is found more often in disseminated infection isolates. Mol. Microbiol. 41:263-277. [DOI] [PubMed] [Google Scholar]
- 6.Dokter, W. H. A., A. J. Dijkstra, S. B. Koopmans, B. K. Stulp, W. Keck, M. R. Halie, and E. Vellenga. 1994. G(Anh)MTetra, a natural bacterial cell wall breakdown product, induces interleukin-1β and interleukin-6 expression in human monocytes. J. Biol. Chem. 269:4201-4206. [PubMed] [Google Scholar]
- 7.Ehlert, K., J.-V. Höltje, and M. F. Templin. 1995. Cloning and expression of a murein hydrolase lipoprotein from Escherichia coli. Mol. Microbiol. 16:761-768. [DOI] [PubMed] [Google Scholar]
- 8.Fleming, T. J., D. E. Wallsmith, and R. S. Rosenthal. 1986. Arthropathic properties of gonococcal peptidoglycan fragments: implications for the pathogenesis of disseminated gonococcal disease. Infect. Immun. 52:600-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodman, S. D., and J. J. Scocca. 1988. Identification and arrangement of the DNA sequence recognized in specific transformation of Neisseria gonorrhoeae. Proc. Natl. Acad. Sci. USA 85:6982-6986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gubish, E. R., Jr., K. C. S. Chen, and T. M. Buchanan. 1982. Detection of a gonococcal endo-β-N-acetyl-d-glucosaminidase and its peptidoglycan cleavage site. J. Bacteriol. 151:172-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gunn, J. S., and D. C. Stein. 1996. Use of a nonselective transformation technique to construct a multiply restriction/modification-deficient mutant of Neisseria gonorrhoeae. Mol. Gen. Genet. 251:509-517. [DOI] [PubMed] [Google Scholar]
- 12.Hamilton, H. L., K. J. Schwartz, and J. P. Dillard. 2001. Insertion-duplication mutagenesis of Neisseria: use in characterization of DNA transfer genes in the gonococcal genetic island. J. Bacteriol. 183:4718-4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hebeler, B. H., and F. E. Young. 1975. Autolysis of Neisseria gonorrhoeae. J. Bacteriol. 122:385-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hebeler, B. H., and F. E. Young. 1976. Mechanism of autolysis of Neisseria gonorrhoeae. J. Bacteriol. 126:1186-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heiss, L. N., S. A. Moser, E. R. Unanue, and W. E. Goldman. 1993. Interleukin-1 is linked to the respiratory epithelial cytopathology of pertussis. Infect. Immun. 61:3123-3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Höltje, J.-V. 1998. Growth of the stress-bearing and shape-maintaining murein sacculus of Escherichia coli. Microbiol. Mol. Biol. Rev. 62:181-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jerse, A. E., M. S. Cohen, P. M. Drown, L. G. Whicker, S. F. Isbey, H. S. Seifert, and J. G. Cannon. 1994. Multiple gonococcal opacity proteins are expressed during experimental urethral infection in the male. J. Exp. Med. 179:911-920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnston, D. M., and J. G. Cannon. 1999. Construction of mutant strains of Neisseria gonorrhoeae lacking new antibiotic resistance markers using a two gene cassette with positive and negative selection. Gene 236:179-184. [DOI] [PubMed] [Google Scholar]
- 19.Kellogg, D. S., Jr., W. L. Peacock, W. E. Deacon, L. Brown, and C. I. Pirkle. 1963. Neisseria gonorrhoeae. Virulence genetically linked to clonal variation. J. Bacteriol. 85:1274-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koonin, E. V., and K. E. Rudd. 1994. A conserved domain in putative bacterial and bacteriophage transglycosylases. Trends Biochem. Sci. 19:106-107. [DOI] [PubMed] [Google Scholar]
- 21.Lommatzsch, J., M. F. Templin, A. R. Kraft, W. Vollmer, and J.-V. Höltje. 1997. Outer membrane localization of murein hydrolases: MltA, a third lipoprotein lytic transglycosylase in Escherichia coli. J. Bacteriol. 179:5465-5470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin, S. A., R. S. Rosenthal, and K. Biemann. 1987. Fast atom bombardment mass spectrometry and tandem mass spectrometry of biologically active peptidoglycan monomers from Neisseria gonorrhoeae. J. Biol. Chem. 262:7514-7522. [PubMed] [Google Scholar]
- 23.Masi, A. T., and B. I. Eisenstein. 1981. Disseminated gonococcal infection (DGI) and gonococcal arthritis (GCA). II. Clinical manifestations, diagnosis, complications, treatment and prevention. Semin. Arthritis Rheum. 10:173-197. [DOI] [PubMed] [Google Scholar]
- 24.Melly, M. A., Z. A. McGee, and R. S. Rosenthal. 1984. Ability of monomeric peptidoglycan fragments from Neisseria gonorrhoeae to damage human Fallopian-tube mucosa. J. Infect. Dis. 149:378-386. [DOI] [PubMed] [Google Scholar]
- 25.Morse, S. A., and L. Bartenstein. 1974. Factors affecting autolysis of Neisseria gonorrhoeae. Proc. Soc. Exp. Biol. Med. 145:1418-1421. [DOI] [PubMed] [Google Scholar]
- 26.Park, J. T. 1995. Why does Escherichia coli recycle its cell wall peptides? Mol. Microbiol. 17:421-426. [DOI] [PubMed] [Google Scholar]
- 27.Petersen, B. H., and R. S. Rosenthal. 1982. Complement consumption by gonococcal peptidoglycan. Infect. Immun. 35:442-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenthal, R. S. 1979. Release of soluble peptidoglycan from growing gonococci: hexaminidase and amidase activities. Infect. Immun. 24:869-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenthal, R. S., and R. Dziarski. 1994. Isolation of peptidoglycan and soluble peptidoglycan fragments. Methods Enzymol. 235:253-285. [DOI] [PubMed] [Google Scholar]
- 30.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 31.Segal, E., E. Billyard, M. So, and F. Heffron. 1985. Role of chromosomal rearrangement in N. gonorrhoeae pilus phase variation. Cell 40:293-300. [DOI] [PubMed] [Google Scholar]
- 32.Seifert, H. S., E. Y. Chen, M. So, and F. Heffron. 1986. Shuttle mutagenesis: a method of transposon mutagenesis for Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 83:735-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sinha, R. K., and R. S. Rosenthal. 1980. Release of soluble peptidoglycan from growing gonococci: demonstration of anhydro-muramyl-containing fragments. Infect. Immun. 29:914-925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sparling, P. F. 1966. Genetic transformation of Neisseria gonorrhoeae to streptomycin resistance. J. Bacteriol. 92:1364-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Templin, M. F., D. H. Edwards, and J.-V. Höltje. 1992. A murein hydrolase is the specific target of bulgecin in Escherichia coli. J. Biol. Chem. 267:20039-20043. [PubMed] [Google Scholar]
- 36.Thunnissen, A.-M. W. H., H. J. Rozeboom, K. H. Kalk, and B. W. Dijkstra. 1995. Structure of the 70-kDa soluble lytic transglycosylase complexed with bulgecin A. Implications for the enzymatic mechanism. Biochemistry 34:12729-12737. [DOI] [PubMed] [Google Scholar]
- 37.van Asselt, E. J., A.-M. W. H. Thunnissen, and B. W. Dijkstra. 1999. High resolution crystal structures of the Escherichia coli lytic transglycosylase Slt70 and its complex with a peptidoglycan fragment. J. Mol. Biol. 291:877-898. [DOI] [PubMed] [Google Scholar]