Abstract
The NRAMP1 gene (Slc11a1) encodes an ion transporter protein involved in the control of intraphagosomal replication of parasites and in macrophage activation. It has been described in mice as the determinant of natural resistance or susceptibility to infection with antigenically unrelated pathogens, including Leishmania. Our aims were to sequence and map the canine Slc11a1 gene and to identify mutations that may be associated with resistance or susceptibility to Leishmania infection. The canine Slc11a1 gene has been mapped to dog chromosome CFA37 and covers 9 kb, including a 700-bp promoter region, 15 exons, and a polymorphic microsatellite in intron 1. It encodes a 547-amino-acid protein that has over 87% identity with the Slc11a1 proteins of different mammalian species. A case-control study with 33 resistant and 84 susceptible dogs showed an association between allele 145 of the microsatellite and susceptible dogs. Sequence variant analysis was performed by direct sequencing of the cDNA and the promoter region of four unrelated beagles experimentally infected with Leishmania infantum to search for possible functional mutations. Two of the dogs were classified as susceptible and the other two were classified as resistant based on their immune responses. Two important mutations were found in susceptible dogs: a G-rich region in the promoter that was common to both animals and a complete deletion of exon 11, which encodes the consensus transport motif of the protein, in the unique susceptible dog that needed an additional and prolonged treatment to avoid continuous relapses. A study with a larger dog population would be required to prove the association of these sequence variants with disease susceptibility.
Natural resistance or susceptibility to several antigenically unrelated pathogens, such as Mycobacterium, Salmonella, and Leishmania, has been associated with the locus Bcg/Ity/Lsh in mice (43). The resistant phenotype (Bcgr) results in macrophage activation producing the destruction of intracellular pathogens early during infection (43). Positional cloning revealed that the Bcg/Ity/Lsh locus corresponds to a gene encoding an ion transporter protein, designated natural resistance-associated macrophage protein (NRAMP1) (52) and referred to here with the new nomenclature, solute carrier family 11 member 1 (Slc11a1).
The Slc11a1 protein is composed of 12 putative transmembrane (TM) domains, an extracellular glycosylated loop, and a transport motif conserved among other transporter proteins in both eukaryotic and prokaryotic organisms (52, 21). A nonconservative amino acid substitution (G169A) in the TM4 domain has been associated with a susceptible phenotype in mice (52, 31). Within macrophages, Slc11a1 has been located in late endocytic compartments and is acquired by the phagosomal membrane after phagocytosis (22). The protein drives divalent metals out of the phagosome, controlling the replication of intracellular pathogens by altering the intravacuolar environment (18). This behavior could also provide an explanation for the observed pleiotropic effects on macrophage function that have been attributed to the Slc11a1 gene, suggesting a role in infectious and autoimmune disease susceptibility (5).
Slc11a1 has attracted a great deal of interest and has been characterized in different species in a search for polymorphisms involved in disease susceptibility. For instance, a functional repeat polymorphism in the promoter region of the human Slc11a1 gene was found to drive gene expression, conferring protection against or susceptibility to juvenile rheumatoid arthritis (42) or multiple sclerosis (25). Moreover, a single base change in intron 4 (14) and a 4-bp deletion in the 3′ untranslated region (UTR) (40) were significantly associated with tuberculosis in humans. Recently, a microsatellite in the 3′ UTR also was demonstrated to affect the expression of the bovine Slc11a1 gene and to control the in vitro replication of Brucella abortus (3). Finally, an amino acid substitution (G696A) in chicken Slc11a1 was found only in chicken cell lines susceptible to Salmonella enterica serovar Typhimurium (24).
In southern European countries, leishmaniasis is an endemic and zoonotic disease caused by the protozoan parasite Leishmania infantum. This parasitic infection in this area is important not only to veterinary medicine, because dogs are considered the main reservoir (4), but also because of its zoonotic aspects. Human leishmaniasis, which traditionally affected young children and infants, is now a common complicating factor in adults infected with human immunodeficiency virus or receiving immunosuppressive drugs (2, 15).
In dogs, leishmaniasis is a systemic disease with a variety of clinical signs, including nonpruritic skin lesions (such as exfoliative dermatitis and ulcerations), local or generalized lymphadenopathy, loss of weight, poor appetite, ocular lesions, epistaxis, lameness, renal failure, and diarrhea (45). In this parasitic process, infection is not synonymous with disease. The prevalence of Leishmania infection (65%) in an area of endemicity is higher than the seroprevalence (20%) and the prevalence of disease (10%) (11, 47). The immune response in dogs is humoral and cellular, resulting in a wide array of responses: the cellular immune response is protective against L. infantum, whereas a strong humoral immune response correlates with disease susceptibility (11, 35, 46).
Based on in vitro and in vivo studies, it is widely accepted that macrophages, where Slc11a1 is expressed, play a central role in the control of the parasite (1, 48). Moreover, as previously described for L. donovani infection, the early outcome of L. infantum infection in mice is under the control of the Slc11a1 gene (28). All of these issues suggest that Slc11a1 is a strong candidate gene for natural resistance or susceptibility to Leishmania infection in dogs. We have addressed this issue by mapping and sequencing the canine Slc11a1 gene and analyzing sequence variants that could be associated with disease susceptibility in four dogs experimentally infected with L. infantum. A microsatellite located in intron 1 of the gene was used in a case-control study to provide evidence of the association with Leishmania infection in dogs.
MATERIALS AND METHODS
Study animal.
We characterized the canine Slc11a1 gene in a healthy Rottweiler.
The case-control study was performed with 33 healthy dogs of different breeds that showed a Leishmania-specific cellular immune response by means of positive delayed-type hypersensitivity (DTH) to leishmanin (resistant dogs) and 84 dogs of different breeds with patent leishmaniasis diagnosed by a positive Leishmania-specific PCR result for bone marrow samples (39) collected from veterinary clinics (susceptible dogs).
Sequence variant analysis was done with samples from four beagles experimentally infected with L. infantum. These dogs were inoculated intravenously with 5 × 107 promastigotes of L. infantum (MCAN/ES/92/BCN-83/MON-1) diluted in 0.5 ml of saline solution and were kept in the Animalarium at the Facultat de Veterinària, Universitat Autònoma de Barcelona. They were monitored for 5 years, during which time clinical, hematological, biochemical, serological, and parasitological control analyses were performed under the auspices of the Universitat Autònoma de Barcelona animal care committee. Once infection was confirmed and serologic results were positive, dogs were treated when protein and gamma globulin concentrations reached abnormally high values (>7 g/dl). All dogs were subjected to treatment with meglumine antimoniate (20 mg of Sb5+/kg of body weight every 12 h for 20 days) (38, 50) and to a second treatment with liposome-encapsulated meglumine antimoniate (9.8 mg of Sb5+/kg every 24 h for 20 days) (51). One dog was treated again with liposome-encapsulated meglumine antimoniate and allopurinol (10 mg every 12 h for 8 months).
Specific L. infantum humoral and cellular immune responses.
Specific L. infantum humoral and cellular immune responses were assessed as described elsewhere (17) 5 years after experimental infection. Briefly, the humoral response was analyzed by an enzyme-linked immunosorbent assay for detecting specific anti-Leishmania immunoglobulin G1 (IgG1) and IgG2 antibodies. The cellular response was analyzed by determining DTH to leishmanin, by a lymphocyte proliferation assay (LPA), and by determining in vitro gamma interferon (IFN-γ) production. The LPA and the determination of IFN-γ production were carried out after incubation of samples with phytohemagglutinin (PHA) or leishmanial soluble antigen (LSA).
Sequencing and mapping of the canine Slc11a1 gene.
Genomic DNA was isolated from peripheral blood as described elsewhere (41). The canine Slc11a1 gene was PCR amplified from different overlapping fragments corresponding to the promoter (promoter 1), exon 2 to exon 7 (E2-E7), exon 7 to exon 10 (E7-E10), exon 9 to exon 11 (E9-E11), and exon 10 to the 3′ UTR (E10-E15). Mammalian orthologous gene-specific PCR primers are shown in Table 1. PCR conditions and thermocycling profiles are shown in Table 2. Amplification of the 3′ UTR from cDNA was accomplished with an internal primer from the oligo(dT) used in the reverse transcription (RT) reaction (see below). PCR products of the expected sizes were isolated from agarose gels by using a QIAquick gel extraction kit (Qiagen) and were cloned into the PCR 2.1-TOPO vector (TOPO TA cloning kit; Invitrogen). Two independent clones of each fragment were sequenced by using the dideoxy method (BigDye terminator cycle sequencing ready reaction kit, version 2.0; Applied Biosystems) with fluorescent terminators and an automated DNA sequencer (ABI-PRISM; Applied Biosystems).
TABLE 1.
Mammalian orthologous and dog-specific primers designed to amplify the canine Slc11a1 gene and cDNA
| Primera | Location | Sequence (5′ → 3′) |
|---|---|---|
| NRPROM-F | Promoter | CACTTCTGCCTTTCCAAAATGTTTCACA |
| NRPROM3-F | Promoter | TCTCATAGTTTATCTTACCTGC |
| NRPROMD-Fb | Promoter | CCTCTCAGCTAGTCTGAGCC |
| NRPROM2-Rb | Promoter | CAGCTGATCTCAGCTGTCCTC |
| NRPROM3-Rb | Promoter | AGCTACTCAGCACATGCTTC |
| NRMICRI1-Fb | Intron 1 | GAGTCTGCTTGAGATTCTCTC |
| NRMICRI1-Rb | Intron 1 | TATCACCTCCACCCTTCAAAC |
| NRE2-F | Exon 2 | CCTACCTRAGTGAGAAGATCC |
| NRI2E2-Rb | Exon 2-intron 2 boundary | GGTTTCCAGCATTCCTACCGGTTC |
| NRE6I6-Fb | Exon 6-intron 6 boundary | CCTTGACAACTACGGTGGGT |
| NRI6-Rb | Intron 6 | CGAAGAAACAGAAGCCCAAG |
| NRE7-R | Exon 7 | CTCATAGCCRAAGGTCAARGCCAT |
| NRE7-F | Exon 7 | GTTGCGGAAGCTGGAAGCCTT |
| NRE9-F | Exon 9 | GAGAAGCCAACATGTACTTCCT |
| NRE9-R | Exon 9 | GCTTGTAGAAGGCCTGCCCAA |
| NRE10-F | Exon 10 | TTCAACATCTGTGCCAACAGCA |
| NRE10-R | Exon 10 | TGCTGTTGGCACAGATGTTGAA |
| NRE11-R2b | Exon 11 | CAAACTGTCCCGCGTAGGTGCC |
| NR3′UTR-Rb | Exon 15 | CACATCAGGAAGCCCATACAG |
F, forward; R, reverse.
Dog specific.
TABLE 2.
PCR conditions and thermocycling profiles used for canine Slc11a1 sequencing and polymorphism analysis
| Region | Primers | MgCl2concn (mM) | Primer concn (μM) | Conditions for:
|
No. of cycles | Enzyme(s)a | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Denaturation
|
Annealing
|
Extension
|
|||||||||
| °C | s | °C | s | °C | s | ||||||
| Promoter 1 | NRPROM-F-NRI2E2-R | 1.50 | 0.2 | 94 | 30 | 55 | 30 | 72 | 60 | 35 | A |
| NRPROM3-F-NRPROM2-R | 1.50 | 0.2 | 94 | 30 | 54 | 30 | 72 | 60 | 35 | A | |
| Promoter 2 | NRPROMD-F-NRPROM3-R | 1.50 | 0.2 | 94 | 30 | 53 | 30 | 72 | 60 | 35 | A |
| NRPROMD-F-NRPROM2-R | 1.50 | 0.2 | 94 | 30 | 53 | 30 | 72 | 60 | 35 | A | |
| E2-E7 | NRE2-F-NRE7-R | 2.25 | 0.3 | 92 | 10 | 58 | 30 | 68 | 240 | 10 | B |
| 92 | 10 | 58 | 30 | 68 | 240b | 25 | |||||
| E2-E9 | NRE2-F-NRE9-R | 1.50 | 0.2 | 94 | 30 | 53 | 30 | 72 | 60 | 35 | A |
| E7-E15 | NRE7-F-NRE3′UTR-R | 1.50 | 0.2 | 94 | 30 | 55 | 30 | 72 | 60 | 35 | A |
| E6-I6 | NRE6I6-F-NRI6-R | 2.00 | 0.3 | 94 | 30 | 63c | 30 | 72 | 30 | 20 | D |
| 94 | 30 | 53 | 30 | 72 | 30 | 10 | |||||
| E7-E10 | NRE7-F-NRE10-R | 1.50 | 0.3 | 94 | 15 | 55 | 30 | 68 | 480 | 35 | C |
| E9-E11 | NRE9-F-NRE11-R2 | 1.50 | 0.3 | 94 | 15 | 55 | 30 | 68 | 480 | 35 | C |
| E10-E15 | NRE10-F-NR3′UTR-R | 1.50 | 0.3 | 94 | 15 | 55 | 30 | 68 | 480 | 35 | C |
| Microsatellite | NRMICRI1-F-NRMICR1-R | 1.50 | 0.2 | 94 | 30 | 55 | 30 | 72 | 30 | 25 | A |
| Intron 10 | NRE10-F-NRE11-R2 | 1.50 | 0.2 | 95 | 30 | 55 | 30 | 72 | 45 | 35 | E |
Enzymes and commercial kits: A, Taq polymerase (Invitrogen); B, Expand long-template PCR system (system 3; Taq and Pwo polymerases) (Roche); C, Platinum Pfx DNA polymerase (with enhancer solution ×1) (Invitrogen); D, AmpliTaq Gold (Applied Biosystems); and E, GC-Rich PCR amplification system (0.5 M GC rich resolution solution and Taq and Tgo polymerases).
Plus 20 s per cycle.
Minus 0.5°C per cycle.
We used canine Slc11a1-specific primers (NRE6I6-F and NRI6-R) to amplify a 179-bp fragment in the RHDF5000 dog whole-genome radiation hybrid panel (53, 54) as previously described (37). PCR conditions and thermocycling profiles are shown in Table 2 (E6-I6). Based on a pairwise analysis with a logarithm of odds ratio score threshold of >8.0, the typing data generated for the Slc11a1 marker were then incorporated into the integrated radiation hybrid (RH)-linkage map (34) by using the Multimap package (32). Distances (D), calculated as D = -ln(1 − Θ), where Θ is the breakage frequency, are expressed in centiRays 5000 (cR5000), where 5000 is the radiation dose used to generate the RH panel. In this panel, the correspondence between cR5000 and a physical distance has been estimated to be 1 cR5000 for 166 kb. A multipoint analysis was carried out to order the markers belonging to the group in a comprehensive manner, and the intermarker distances were calculated.
Sequence variant analysis.
Different tissues were used for RNA isolation. Blood and spleen or liver were extracted postmortem and stored at −80°C until RNA isolation. Peripheral blood mononuclear cells were isolated from heparinized venous blood samples by standard Ficoll-Hypaque (Histopaque 1.077; Sigma, St. Louis, Mo.) density gradient centrifugation as described elsewhere (7). Total RNA was isolated by using Trizol reagent (GibcoBRL-Life Technologies) as specified by the manufacturer. RT-PCR was carried out with a 20-μl final reaction mixture containing 1 μg of total RNA, 1 mM each deoxynucleoside triphosphate, 2 μM oligo(dT) primer, and 200 U of Moloney murine leukemia virus RT (Amersham Life Science). cDNA amplification was accomplished with two overlapping fragments: exon 2 to exon 9 (E2-E9) and exon 7 to exon 15 (E7-E15). PCR conditions and thermocycling profiles are shown in Table 2. PCR products were isolated, cloned, and sequenced as described above.
A seminested PCR was used to amplify the promoter region (promoter 2). A 1-μl sample of the first PCR product (NRPROMD-Fand NRPROM3-R) was used as a template to perform the second PCR (NRPROMD-F and NRPROM2-R). PCR products were isolated, cloned, and sequenced as described above.
The microsatellite located in intron 1 was amplified by using dog-specific primers NRMICRI1-F (fluorescence labeled) and NRMICRI1-R. PCR conditions and thermocycling profiles are shown in Table 2. PCR products were analyzed by capillary electrophoresis with an ABI 3100 genetic analyzer (Applied Biosystems), and labeled PCR products were automatically sized relative to the internal standard (PRISM GeneScan-350 TAMRA; Applied Biosystems) with GeneScan analysis software, version 3.5 (Applied Biosystems). Intron 10 amplification was performed as shown in Table 2 by using a GC-rich PCR amplification system (Roche) and a 50-μl final reaction mixture containing 0.5 M GC-rich resolution solution and 2 U of a Taq and Tgo DNA polymerase mix, under the manufacturer’s conditions.
Computation and statistical analysis.
Allele frequencies of the intron 1 microsatellite of the canine Slc11a1 gene were analyzed by using a two-by-two chi-square test with 1 df.
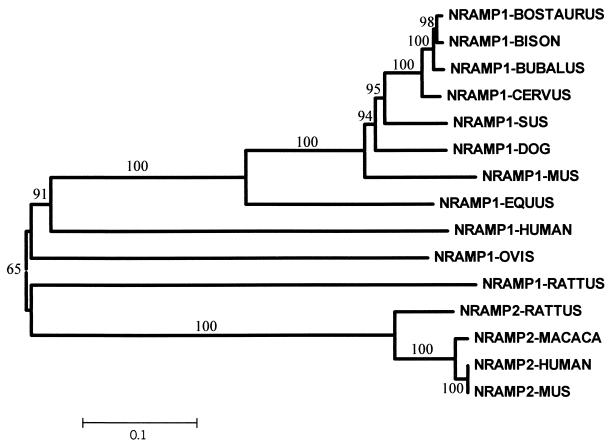
A neighbor-joining relationship tree for mammalian Slc11 cDNA sequences, both Slc11a1 and Slc11a2, was created with the assumption that distance is equal to the number of nucleotide differences and with a bootstrap value of 1,000 by using MEGA software, version 2.0 (26).
Nucleotide sequence accession number.
The canine Slc11a1 gene characterized has been deposited under GenBank accession number AF091049.
RESULTS
Sequencing and mapping of the canine Slc11a1 gene.
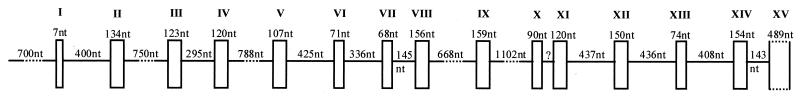
Mammalian orthologous gene-specific primers were designed to accomplish the complete sequencing of the canine Slc11a1 gene. The sequences of the primers were designed based on conserved coding regions from all Slc11a1 sequences from vertebrate species available up to that time: human (6, 12), sheep (9), mouse (21), cow (16, 23), rat (19), pig (49), deer (33), buffalo (M. Hashad et al., unpublished), and bison (J. Feng et al., unpublished). Primer sequences are shown in Table 1. The canine Slc11a1 gene includes 15 exons and encompasses 9 kb (Fig. 1). Both the sequence and the genomic organization of the coding regions are highly conserved in relation to those of the Slc11a1 genes from other mammals (Fig. 2). Intron-exon boundaries were determined by comparison of the entire genomic sequence with the beagle cDNA and the mouse gene (21). Intronic regions conform perfectly to the GT-AG donor-acceptor splice rule and vary in size from 143 to 1,102 bp. In general, they are shorter than those in mice and humans, especially introns 4, 8, and 11. A microsatellite, (TAAA)n, was identified in intron 1 (position 1253 of the sequence). Only a partial sequence was obtained for intron 10 due to the presence of GGGGC-CCCCG terminal repeats that confer a complex secondary structure to this region. The canine Slc11a1 gene encodes a 547-amino-acid protein with amino acid identity of up to 87% with Slc11a1 proteins of different vertebrate species and with the same structure as the other Slc11a1 proteins described up to this time. Slc11a1 is composed of 12 TM domains, three phosphorylation sites for protein kinase C, two N-linked glycosylation sites located in exon 10, and the consensus transport motif located between TM8 and TM9 (exon 11). The G169 residue in TM4 is conserved in the dog Slc11a1 protein, as it is in the human, cow, sheep, pig, buffalo, bison, and deer proteins.
FIG. 1.
Genomic structure of the canine Slc11a1 gene. nt, nucleotides.
FIG. 2.
Neighbor-joining relationship tree of mammalian Slc11 cDNA sequences. The distance indicates the number of nucleotide differences. Numbers on the nodes are percent bootstrap values from 1,000 replications, and a scale bar for branch lengths is shown.
The translational start codon is located at position 689 of the sequence, and the translational stop codon and the 3′ UTR are located in exon 15. The first 680 nucleotides of the sequence includes the 5′ UTR and the promoter of a gene that does not contain a classical TATA box. A sequence motif for a potential initiator element (Inr) which is often found in TATA-less gene promoters was found at position 510. This region also contains several sites for binding of transcription factors involved in tissue-specific transcription and expression or associated with the response to induction by differentiating agents specific to the macrophage (Fig. 3A). Briefly, we have found two potential AP-1 binding sites (TGASTMA), four potential AP-2 binding sites (CCCMNSSS), nine potential IFN-γ binding sites (CWKKANNY), two potential IFN-α/β binding sites (AARKGA), one potential granulocyte-macrophage colony-stimulating factor binding site (CATTW), three SP-1 binding motifs (GGGCG), a potential binding site for the transforming growth factor β-inducible NF-1 transcription factor (YGGMN5-6GCCAA), and a potential binding site for bacterial lipopolysaccharide (LPS) (NF-IL-6; TKNNGNAAK).
FIG. 3.
Sequence analysis. (A) Promoter and 5′ UTR of the canine Slc11a1 gene from nucleotide 1 to the translational start codon (position 689). Putative binding sites for transcription factors are indicated with a line above (sense strand) or below (antisense strand) the consensus sequence. (B) G-rich polymorphism of the promoter regions from beagles and a Rottweiler. GM-CSF, granulocyte-macrophage colony-stimulating factor.
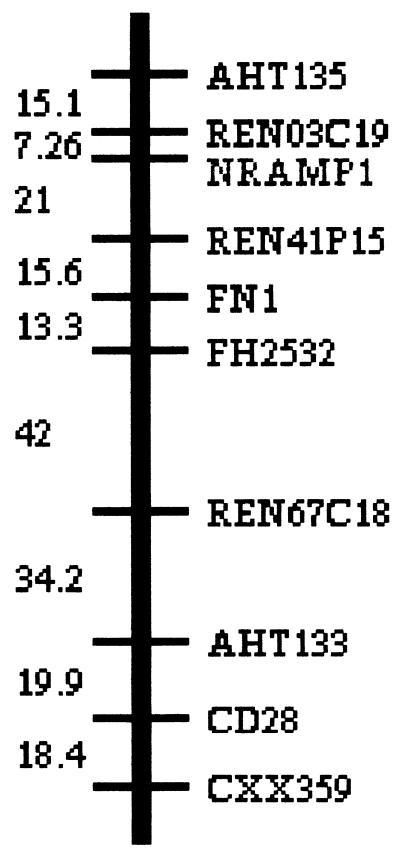
By use of the RHDF5000 dog whole-genome radiation hybrid panel (53, 54) and a pairwise analysis with the Multimap package (32), Slc11a1 was mapped to RH syntenic group 11 (SRH11) between the microsatellites Ren03C19 and Ren41P15 with Lod scores of 21.7 and 14.8 and distances of 7 cR5000 and 21 cR5000, respectively. RH syntenic group 11 is shown in Fig. 4.
FIG. 4.
Slc11a1 mapped to canine RH syntenic group 11.
Case-control study.
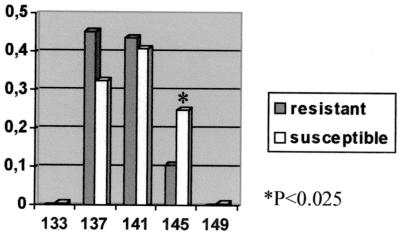
In order to assess the possible role of the Slc11a1 gene in determining Leishmania susceptibility, we performed a case-control study with 33 resistant and 84 susceptible dogs and the intron 1 microsatellite as a marker. The different alleles detected and the allelic distribution observed in the two populations analyzed are shown in Fig. 5. Significant differences between resistant and susceptible animals were observed in the frequency of allele 145 (P < 0.025). Furthermore, allele 145 has only been observed in a homozygous state in the susceptible population (0.32), with the exception of one dog in the resistant population (0.03).
FIG. 5.
Allelic frequencies of the intron 1 microsatellite. x axis, basepairs of alleles; y axis, allelic frequency.
Beagle immunoprofiles.
In order to search for mutations that could have functional implications, sequence variant analysis was performed with four unrelated beagle dogs experimentally infected with L. infantum. Dogs were divided into resistant (R1 and R2) and susceptible (S1 and S2) groups based on their predominant immune response (cellular or humoral, respectively). Resistant dogs did not have clinical relapses after the second treatment and were negative for detection of parasite DNA in bone marrow by PCR after 5 years. In susceptible dogs, the disease evolved gradually and became chronic after the second treatment, and a third long treatment was administered to dog S1 to avoid continuous relapses. The results of immunological tests are shown in Table 3.
TABLE 3.
Assessment of resistance or susceptibility to infection by a range of immunological testsa
| Dog | OD492 for:
|
DTH response (mm) | OD450 in LPA with:
|
IFN-γ (IU/ml) with:
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IgG | IgG2 | IgG1 | UPBMC | PHA | LSA | UPBMC | PHA | LSA | ||
| R1 | 0.929 | 1.138 | 0.123 | 22 | 0.133 | 0.916 | 1.266 | 80 | 160 | 320 |
| R2 | 0.922 | 1.294 | 0.140 | 15 | 0.152 | 1.330 | 1.600 | 80 | 160 | 640 |
| S1 | 2.285 | 3.000 | 0.513 | 0 | 0.100 | 1.090 | 0.098 | 20 | 40 | 0 |
| S2 | 1.560 | 2.296 | 0.124 | 0 | 0.112 | 0.512 | 0.178 | 0 | 20 | 0 |
OD450, optical density at 450 nm; UPBMC, unstimulated peripheral blood mononuclear cells.
Sequence variant analysis.
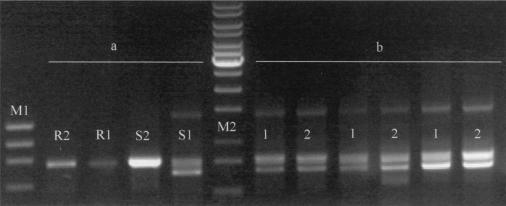
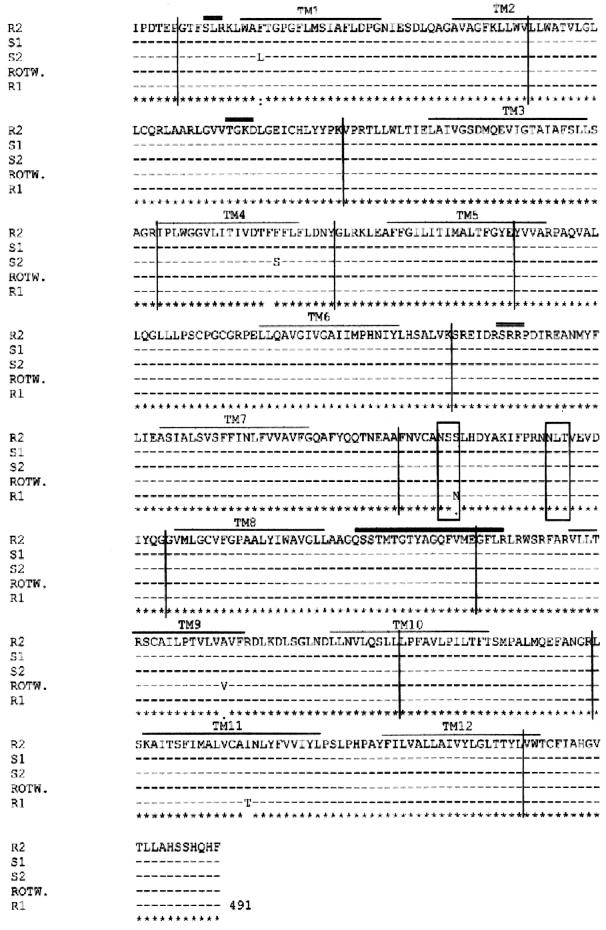
Slc11a1 sequence variants were analyzed at different levels: cDNA, promoter, and microsatellite. cDNA sequences obtained from the experimentally infected beagles were compared with the Rottweiler genomic sequence. Interestingly, two Slc11a1 cDNAs were coamplified in dog S1: one of the expected size and the other one shorter. The shorter fragment was explained by the complete deletion of exon 11, which includes TM8 and most of the consensus transport motif of the protein. We analyzed this sample at the genomic level (data not shown); after PCR amplification from exon 10 to the 3′ UTR, we found a single band and no evidence of genomic deletion. Once we sequenced the exon-intron boundaries for intron 10, we found no mutation at the GT-AG donor-acceptor splice sites. However, both bands were also coamplified in two independent RT-PCRs from liver and spleen RNAs from the same animal (Fig. 6). We also found five single-nucleotide polymorphisms that resulted in amino acid substitutions. Four of them were located within the TM domains, and the other one, located in exon 10, produced the loss of an N-glycosylation site. Alignment of the five proteins is shown in Fig. 7.
FIG. 6.
mRNA alternative splicing in dog S1. (a) PCR amplification from exon 9 to the 3′ UTR of cDNAs from the four beagles experimentally infected with L. infantum. R1 and R2 are resistant dogs, and S1 and S2 are susceptible dogs. (b) PCR amplification of the same region of dog S1 from three different RNA extractions. Two independent RT-PCRs of each RNA extraction are shown (lanes 1 and 2). Lane M1, φX174 HaeIII; M2, 1-kb ladder.
FIG. 7.
Multiple alignment of the Slc11a1 proteins from the Rottweiler (ROTW.) and the four beagles experimentally infected with L. infantum. For dog S1, the normal allele is shown. TM domains are indicated by thin overlining, and the consensus transport motif of the protein is indicated by thick overlining. Potential N-glycosylation sites are boxed, and potential phosphorylation sites for protein kinase C are indicated by double overlining. Exons are separated by vertical lines.
Differences were also observed between susceptible and resistant animals in the promoter region. There is a G-rich region (positions 310 to 330) in the susceptible animals that is disrupted by G-to-A substitutions in different places in the resistant animals or in the Rottweiler dog sequence (Fig. 3B). The loss of a potential SP-1 binding site in dog R1 and both the gain and the loss of an AP-2 binding site were observed in dog R2 (positions 226 and 328, respectively). The latter dog also had an A-to-G substitution at position 405; this substitution did not modify any transcriptional binding site.
We also analyzed the polymorphism of the (TAAA)9-11 microsatellite located in intron 1. Both susceptible animals were homozygous (allele 141), and dogs R1 and R2 were heterozygous (alleles 137/141 and 141/145, respectively).
DISCUSSION
In this study, we sequenced and mapped the canine Slc11a1 gene as a strong candidate gene for leishmaniasis on the basis of studies with its murine homologue. The canine Slc11a1 gene has been assigned to linkage group SRH11 located in chromosome CFA37 (8, 37), which includes at least two genes (FN1 and CD28) that have been mapped either to human chromosome 2q35 or mouse chromosome 1. The conserved syntenic group of the fragment delineated by Slc11a1 and CD28 can be assumed for dogs, humans, and mice.
In mice, the early outcome of L. infantum infection is under the control of the Slc11a1 gene, which regulates the intraphagosomal replication of parasites inside the macrophage (28). In addition, Slc11a1 regulates macrophage activation and has multiple pleiotropic effects involved in antigen processing and presentation (27). Slc11a1 has a relevant function in the modulation of the subsequent immune response that could be predominantly cellular or humoral and that is of great importance in resistance or susceptibility to leishmaniasis (11, 35, 46). To the best of our knowledge, there are no reports describing the genetic factors that could influence Leishmania infection in dogs. However, the high degree of conservation of amino acids and structural motifs between the dog and mouse Slc11a1 proteins suggests that they may have a similar role in the progression of the disease. As observed in humans (10), one should not expect an absolute association between Slc11a1 and Leishmania infection, as in the mouse model, but a significant association between canine Slc11a1 polymorphism and a susceptible phenotype in dogs.
In order to analyze the association between the canine Slc11a1 gene and leishmaniasis, we performed a case-control study with resistant and susceptible dogs. Significant differences were observed for allele 145 of the intron 1 microsatellite between the two populations. These results suggest an association between Slc11a1 and leishmaniasis, indicating a role of this gene in disease susceptibility. Sequence variants analyzed in four beagles experimentally infected with L. infantum could be associated with possible functional mutations related to their susceptible or protective immune responses. One of the susceptible beagles (S1) had a complete deletion of exon 11 in one of the Slc11a1 alleles which resulted in the elimination of TM8 and the consensus transport motif of the protein. Since no mutation was observed at the genomic level, including the GT-AG donor-acceptor intron 10 splicing sites, we suggest that the deletion of exon 11 could be caused by alternative mRNA splicing. Intron 10 has complementary terminal repeats (5′-GGGGCCCCCG-3′) which are canine specific and which could confer a complex secondary structure resulting in alternative mRNA splicing. Alternative splicing has been described for the human Slc11a1 and Slc11a2 genes, but in no case was the region of the consensus transport motif of the protein included (29, 30). The loss of the consensus transport motif caused by this deletion could produce a lack of function of the mutated protein, resulting in a higher level of proliferation of the parasite inside the macrophages of susceptible dogs. It is important to note that this dog was the only one that needed a third, sustained treatment to avoid continuous relapses.
We also described five amino acid substitutions, four located in the TM domains of the protein. The most significant mutations were F179S (TM4), I476T (TM11), and S323N (exon 10). The F179S amino acid substitution in TM4 of dog S2 implies a nonconservative change from a hydrophobic to a polar residue. Although this is a region that contains several polar residues (13), this substitution is located in the same TM domain in which the mouse mutation (G169A) was described to be located and resulted in the lack of function of the protein (21, 31). Moreover, this change has never been described for other mammals and has been observed only in Slc11a1 orthologues from plants and prokaryotic organisms. The second amino acid substitution, I476T in TM11 of dog R1, represents a polar change in one of the most hydrophobic regions of the protein (13). Dog R1 also had an S323N substitution in exon 10 that results in the loss of one of the N-glycosylation sites; this substitution has also been described for buffalo and cow Slc11a1 proteins.
The canine Slc11a1 promoter region has been found to posses nine consensus IFN-γ binding sites and one potential binding site for LPS (NF-IL-6), consistent with the regulation of expression previously described (20). Slc11a1 gene expression can be regulated during macrophage activation by cytokines and LPS. Furthermore, lymphocytes from resistant Leishmania-infected dogs have been found to produce IFN-γ upon parasite-specific stimulation and to lyse infected macrophages in a major histocompatibility complex-restricted manner (36), leading to a cellular protective immune response. Although no polymorphism has been found at the IFN-γ and NF-IL-6 binding sites, different immune responses have been described for resistant and susceptible animals analyzed. Resistant dogs demonstrated Leishmania-specific cellular immunity due to their ability to produce IFN-γ, lymphocyte proliferation, and positive DTH. The only polymorphism common to the susceptible animals was the G-rich region in the promoter, which is located close to the region where the human microsatellite has been described to be located. Different alleles in the human dinucleotide microsatellite have been associated with a different ability to drive gene expression and has been correlated with infectious versus autoimmune disease susceptibility (44). A microsatellite located in the 3′ UTR of the bovine Slc11a1 gene has also been associated with different gene expression controlling the in vitro replication of B. abortus (3, 23). All these data suggest that the G-rich region of the Slc11a1 promoter could also have an effect on gene expression.
In conclusion, the characterization of the canine Slc11a1 gene, the association between the canine Slc11a1 gene and disease susceptibility, and the description of new sequence variants will be useful in the elucidation of the genetic factors involved in canine leishmaniasis. However, in order to determine whether each sequence variant described is associated with Leishmania susceptibility in dogs, a larger population-based analysis would be required to prove the functional association of Slc11a1 with the disease.
Acknowledgments
This work was supported by a grant from the Real Sociedad Canina Española.
We are grateful to Marcel Amills for critical review of the manuscript, Atilio Aranguren for MEGA computation analysis, and Oscar Ramirez for helping us with the statistical analysis.
Editor: B. B. Finlay
REFERENCES
- 1.Alexander, J., and D. G. Russell. 1992. The interaction of Leishmania species with macrophages. Adv. Immunol. 31:175-254. [DOI] [PubMed] [Google Scholar]
- 2.Alvar, J., C. Cañavate, B. Gutierrez-Solar, M. Jiménez, F. Laguna, R. López-Vélez, R. Molina, and J. Moreno. 1997. Leishmania and human immunodeficiency virus coinfection: the first 10 years. Clin. Microbiol. Rev. 10:298-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barthel, R., J. Feng, J. A. Piedrahita, D. N. McMurray, J. W. Templeton, and L. G. Adamns. 2001. Stable transfection of the bovine NRAMP1 gene into murine RAW264.7 cells: effect on Brucella abortus survival. Infect. Immun. 69:3110-3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bettini, S., and L. Gradoni. 1986. Canine leishmaniasis in the Mediterranean area and its implications for human leishmaniasis. Insect Sci. Appl. 7:241-245. [Google Scholar]
- 5.Blackwell, J. M. 1996. Structure and function of the natural-resistance-associated macrophage protein (Nramp1), a candidate protein for infectious and autoimmune disease susceptibility. Mol. Med. Today 2:205-211. [DOI] [PubMed] [Google Scholar]
- 6.Blackwell, J. M., C. H. Barton, J. K. White, S. Searle, A. M. Baker, H. Williams, and M. A. Shaw. 1995. Genomic organization and sequence of human NRAMP gene: identification and mapping of a promoter region polymorphism. Mol. Med. 1:194-205. [PMC free article] [PubMed] [Google Scholar]
- 7.Böyum, A. 1968. Isolation of mononuclear cells and granulocytes from human blood. Scand. J. Clin. Lab. Investig. 21:77-89. [PubMed] [Google Scholar]
- 8.Breen, M., R. Thomas, M. M. Binns, N. P. Carter, and C. F. Langford. 1999. Reciprocal chromosome painting reveals detailed regions of conserved synteny between the karyotype of the domestic dog (Canis familiaris) and human. Genomics 61:145-155. [DOI] [PubMed] [Google Scholar]
- 9.Bruner, R. M., M. Henke, G. Guerin, T. Goldammer, H. M. Seyfert, and M. Schwerin. 1994. The macrophage expressed variant of the bovine lysozyme-encoding gene maps to cromosome 5q23. Mamm. Genome 5:834-835. [DOI] [PubMed] [Google Scholar]
- 10.Buu, N., F. Sánchez, and E. Schurr. 2000. The Bcg host-resistance gene. Clin. Infect. Dis. 31:S81-S85. [DOI] [PubMed] [Google Scholar]
- 11.Cabral, M., J. E. O'Grady, S. Gomes, J. C. Sousa, H. Thompson, and J. Alexander. 1998. The immunology of canine leishmaniosis: strong evidence for a developing disease spectrum from asymptomatic dogs. Vet. Parasitol. 76:173-180. [DOI] [PubMed] [Google Scholar]
- 12.Cellier, M., G. Govoni, S. Vidal, T. Kwan, N. Groulx, J. Liu, F. Sanchez, E. Skamene, E. Schurr, and P. Gros. 1994. Human natural resistance-associated macrophage protein: cDNA cloning, chromosomal mapping, genomic organisation, and tissue-specific expression. J. Exp. Med. 180:1741-1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cellier, M., G. Privé, A. Belouchi, T. Kwan, V. Rodrigues, W. Chia, and P. Gros. 1995. Nramp defines a family of membrane proteins. Proc. Natl. Acad. Sci. USA 92:10089-10093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cervino, A. C., S. Lakiss, O. Sow, and A. V. Hill. 2000. Allelic association between the NRAMP1 gene and susceptibility to tuberculosis in Guinea-Conakry. Ann. Hum. Genet. 64:507-512. [DOI] [PubMed] [Google Scholar]
- 15.Dedet, J. P., and F. Pratlong. 2000. Leishmania, Trypanosoma and monoxenous trypanosomatids as emerging opportunistic agents. J. Eukaryot. Microbiol. 47:37-39. [DOI] [PubMed] [Google Scholar]
- 16.Feng, J., Y. Li, M. Hashad, E. Schurr, P. Gros, L. G. Adams, and J. W. Templeton. 1996. Bovine natural resistance associated macrophage protein 1 (Nramp1) gene. Genome Res. 6:956-964. [DOI] [PubMed] [Google Scholar]
- 17.Ferrer, L., L. Solano-Gallego, M. Arboix, and J. Alberola. Evaluation of the specific immune response in dogs infected by Leishania infantum. Adv. Vet. Dermatol., in press.
- 18.Forbes, J. R., and P. Gros. 2001. Divalent-metal transport by NRAMP proteins at the interface of host-pathogen interactions. Trends Microbiol. 9:397-407. [DOI] [PubMed] [Google Scholar]
- 19.Ge, L., E. F. Remmers, Y. Du, and R. L. Wilder. 1996. Genomic cloning and genetic mapping of the rat Nramp1 (Bcg) gene on chromosome 9. Mamm. Genome 7:856-857. [DOI] [PubMed] [Google Scholar]
- 20.Govoni, G., S. Gauthier, F. Billia, N. N. Iscove, and P. Gros. 1997. Cell-specific and inducible Nramp1 gene expression in mouse macrophages in vitro and in vivo. J. Leukoc. Biol. 62:277-286. [DOI] [PubMed] [Google Scholar]
- 21.Govoni, G., S. Vidal, M. Cellier, P. Lepage, D. Malo, and P. Gros. 1995. Genomic structure, promoter sequence, and induction of expression of the mouse Nramp1 gene in macrophages. Genomics 27:9-19. [DOI] [PubMed] [Google Scholar]
- 22.Gruenheid, S., E. Pinner, M. Desjardins, and P. Gros. 1997. Natural resistance to infection with intracellular pathogens: the Nramp1 protein is recruited to the membrane of the phagosome. J. Exp. Med. 185:717-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horín, P., I. Rychlík, J. W. Templeton, and L. G. Adams. 1999. A complex pattern of microsatellite polymorphism within the bovine NRAMP1 gene. Eur. J. Immunogenet. 26:311-313. [DOI] [PubMed] [Google Scholar]
- 24.Hu, J., N. Bumstead, P. Barrow, G. Sebastiani, L. Olien, K. Morgan, and D. Malo. 1997. Resistance to salmonellosis in the chicken is linked to NRAMP1 and TNC. Genome Res. 7:693-704. [DOI] [PubMed] [Google Scholar]
- 25.Kotze, M. J., J. N. P. de Villiers, R. N. Rooney, J. J. Grobbelaar, E. P. G. Mansvelt, C. S. H. Bouwens, J. Carr, I. Stander, and L. du Plessis. 2001. Analysis of the NRAMP1 gene implicated in iron transport: association with multiple sclerosis and age effects. Blood Cells Mol. Dis. 27:44-53. [DOI] [PubMed] [Google Scholar]
- 26.Kumay, S., K. Tamura, I. Jakobsen, and M. Nei. 2001. MEGA 2: molecular evolutionary genetics analysis software. Arizona State University, Tempe.
- 27.Lang, T., E. Prina, D. Sibthorpe, and J. Blackwell. 1997. Nramp1 transfection transfers Ity/Lsh/Bcg-related pleiotropic effects on macrophage activation: influence on antigen processing and presentation. Infect. Immun. 65:380-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leclercq, V., M. Lebastard, Y. Belkaid, J. Louis, and G. Milton. 1996. The outcome of the parasitic process initiated by Leishmania infantum in laboratory mice: a tissue-dependent pattern controlled by the Lsh and MHC loci. J. Immunol. 157:4537-4545. [PubMed] [Google Scholar]
- 29.Lee, P. L., T. Gelbart, C. West, C. Halloran, and E. Beutler. 1998. The human Nramp2 gene: characterization of the gene structure, alternative splicing, promoter region and polymorphisms. Blood Cells Mol. Dis. 24:199-215. [DOI] [PubMed] [Google Scholar]
- 30.Liu, J., T. M. Fujiwara, N. T. Buu, F. O. Sánchez, M. Cellier, A. J. Paradis, D. Frappier, E. Skamene, P. Gros, K. Morgan, and E. Schurr. 1995. Identification of polymorphisms and sequence variants in the human homologue of the mouse natural resistance-associated macrophage protein gene. Am. J. Hum. Genet. 56:845-853. [PMC free article] [PubMed] [Google Scholar]
- 31.Malo, D., K. Vogan, S. Vidal, J. Hu, M. Cellier, E. Schurr, A. Fuks, N. Bumstead, K. Morgan, and P. Gros. 1994. Haplotype mapping and sequence analysis of the mouse Nramp gene predict susceptibility to infection with intracellular parasites. Genomics 23:51-61. [DOI] [PubMed] [Google Scholar]
- 32.Matise, T. C., M. Perlin, and A. Chakravarti. 1994. Automated construction of genetic linkage maps using an expert system (MultiMap): a human genome linkage map. Nat. Genet. 6:384-390. [DOI] [PubMed] [Google Scholar]
- 33.Matthews, G. D., and A. M. Crawford. 1998. Cloning, sequencing and linkage mapping of the NRAMP1 gene of sheep and deer. Anim. Genet. 29:1-6. [DOI] [PubMed] [Google Scholar]
- 34.Mellersh, C. S., C. Hitte, M. Richman, F. Vignaux, C. Priat, S. Jouquand, P. Werner, C. André, S. DeRose, D. F. Patterson, E. A. Ostrander, and F. Galibert. 2000. An integrated linkage-radiation hybrid map of the canine genome. Mamm. Genome 11:120-130. [DOI] [PubMed] [Google Scholar]
- 35.Pinelli, E., R. Killick-Kendrick, J. Wagenaar, W. Bernadina, G. Del Real, and J. Ruitenberg. 1994. Cellular and humoral immune responses in dogs experimentally and naturally infected with Leishmania infantum. Infect. Immun. 62:229-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pinelli, E., V. Rutten, and E. J. Ruitenberg. 1999. Cellular immune responses in canine leishmaniasis, p. 60-65. In R. Killick-Kendrick (ed.), Canine leishmaniasis: an update. Proceedings of the International Canine Leishmaniasis Forum.
- 37.Priat, C., C. Hitte, F. Vignaux, C. Renier, Z. Jiang, S. Jouquand, A. Chéron, C. André, and F. Galibert. 1998. A whole genome radiation hybrid map of the dog genome. Genomics 54:361-378. [DOI] [PubMed] [Google Scholar]
- 38.Riera, C., J. E. Valladares, M. Gállego, M. J. Aisa, S. Castillejo, R. Fisa, N. Ribas, J. Carrió, J. Alberola, and M. Arboix. 1999. Serological and parasitological follow-up in dogs experimentally infected with Leishmania infantum and treated with meglumine antimoniate. Vet. Parasitol. 84:33-47. [DOI] [PubMed] [Google Scholar]
- 39.Roura, X., A. Sánchez, and L. Ferrer. 1999. Diagnosis of canine leishmaniasis by a polymerase chain reaction technique. Vet. Rec. 144:262-264. [DOI] [PubMed] [Google Scholar]
- 40.Ryu, S., Y. K. Park, G. H. Bai, S. J. Kim, S. N. Park, and S. Kang. 2000. 3′ UTR polymorphisms in the NRAMP1 gene are associated with susceptibility to tuberculosis in Koreans. Int. J. Tuberc. Lung Dis. 4:577-580. [PubMed] [Google Scholar]
- 41.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 42.Sanjeevi, C. B., E. N. Miller, P. Dagadghao, I. Rumba, A. Shtauvere, A. Denisova, D. Clayton, and J. M. Blackwell. 2000. Polymorphism at NRAMP1 and D2S1471 loci associated with juvenile rheumatoid arthritis. Arthritis Rheum. 43:1397-1404. [DOI] [PubMed] [Google Scholar]
- 43.Schurr, E., E. Buschman, D. Malo, P. Gros, and E. Skamene. 1990. Immunogenetics of mycobacterial infections: mouse-human homologies. J. Infect. Dis. 161:634-639. [DOI] [PubMed] [Google Scholar]
- 44.Searle, S., and J. M. Blackwell. 1999. Evidence for a functional repeat polymorphism in the promoter of the human NRAMP1 gene that correlates with autoimmune versus infectious disease susceptibility. J. Med. Genet. 36:295-299. [PMC free article] [PubMed] [Google Scholar]
- 45.Slappendel, R. J., and L. Ferrer. 1998. Leishmaniasis, p. 450-458. In C. E. Greene (ed.), Infectious diseases of dog and cat. The W. B. Saunders Co., Philadelphia, Pa.
- 46.Solano-Gallego, L., J. Llull, G. Ramos, C. Riera, M. Arboix, J. Alberola, and L. Ferrer. 2000. The Ibizian hound presents a predominantly cellular immune response against natural Leishmania infection. Vet. Parasitol. 90:37-45. [DOI] [PubMed] [Google Scholar]
- 47.Solano-Gallego, L., P. Monell, M. Arboix, J. Alberola, and L. Ferrer. 2001. Prevalence of Leishmania infantum infection in dogs living in an area of canine leishmaniasis endemicity using PCR on several tissues and serology. J. Clin. Microbiol. 39:560-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Solbach, W., H. Moll, and M. Röllinghoff. 1991. Lymphocytes play the music but macrophage calls the tune. Immunol. Today 12:4-6. [DOI] [PubMed] [Google Scholar]
- 49.Tuggle, C. K., C. B. Schmitz, and D. Gingerich-feil. 1997. Cloning of a pig full-length natural resistance associated macrophage protein (NRAMP1) cDNA. J. Anim. Sci. 75:277.. [DOI] [PubMed] [Google Scholar]
- 50.Valladares, J. E., C. Riera, J. Alberola, M. Gállego, M. Portús, C. Cristòfol, C. Franquelo, and M. Arboix. 1998. Pharmacokinetics of meglumine antimoniate after administration of a multiple dose in dogs experimentally infected with Leishmania infantum. Vet. Parasitol. 75:33-40. [DOI] [PubMed] [Google Scholar]
- 51.Valladares, J. E., C. Riera, P. González-Ensenyat, A. Díez-Cascón, G. Ramos, L. Solano-Gallego, M. Gállego, M. Portús, M. Arboix, and J. Alberola. 2001. Long term improvement in the treatment of canine leishmaniosis using an antimony liposomal formulation. Vet. Parasitol. 97:15-21. [DOI] [PubMed] [Google Scholar]
- 52.Vidal, S. M., D. Malo, K. Voyan, E. Skamene, and P. Gros. 1993. Natural resistance to infection with intracellular parasites: isolation a candidate for Bcg. Cell 73:469-485. [DOI] [PubMed] [Google Scholar]
- 53.Vignaux, F., C. Hitte, C. Priat, J. C. Chuat, C. André, and F. Galibert. 1999. Construction and optimization of a dog whole genome radiation hybrid panel. Mamm. Genome 10:888-894. [DOI] [PubMed] [Google Scholar]
- 54.Vignaux, F., C. Priat, S. Jouquand, C. Hitte, Z. Jiang, A. Chéron, C. Renier, C. André, and F. Galibert. 1999. Toward a dog radiation hybrid map. J. Hered. 90:62-67. [DOI] [PubMed] [Google Scholar]