The hypocretins (orexins) are recently described hypothalamic neuropeptides thought to have an important role in the regulation of sleep and arousal states1. Their discovery was reported independently by two groups using different techniques. de Lecea et al.2 identified the pro-hormone pre-prohypocretin, and its peptide products hypocretin-1 (Hcrt-1) and hypocretin-2 (Hcrt-2), by nucleotide sequencing. The discovery of the orexins, orexin-A (Orx-A) and orexin-B (Orx-B), was reported almost simultaneously by Sakurai et al.3 who used the technique of orphan receptor cloning. The terms orexin and hypocretin are synonymous and in this article we will use hypocretin (Hcrt). The finding that cerebrospinal fluid (CSF) levels of these peptides were abnormal in patients with narcolepsy has stimulated research on the potential role of these peptides in human disease. We present here an overview of the pertinent findings from animal studies and a review of the published data from human studies, with a particular emphasis on narcolepsy. Finally, we consider the possible roles of these peptides in neurological and psychiatric disorders.
BACKGROUND
Identification of the peptides
In 1996, a set of neuropeptides related to the hormone secretin were isolated from the rat lateral hypothalamus by the process of directional tag PCR subtraction cloning4. The cloning of the gene for these peptides from rat and mouse, the localization of the peptide-producing cell bodies and a description of some of their efferent projections were first presented in 19775,6.
The receptors
The receptors for these neuropeptides (Hcrtr1 [Orxr1] and Hcrtr2 [Orxr2]) have been identified as G-protein coupled receptors and shown in the rat brain, by analysis of their mRNA, to display a striking distribution7,8. The Hcrtr1 receptor has a much higher (100 to 1000-fold) affinity for Hcrt-1 than for Hcrt-2. The Hcrtr2 receptor seems to have equal affinities for both neuropeptides. The distinctive distribution of the receptors has led some authors to hypothesize a sleep-specific role for the Hcrtr1 receptor and a more general role for Hcrtr2 receptor. The receptors have been mapped on human chromosome 1p33 and 6cen, respectively5,7,8,9.
Projections of the hypocretin system
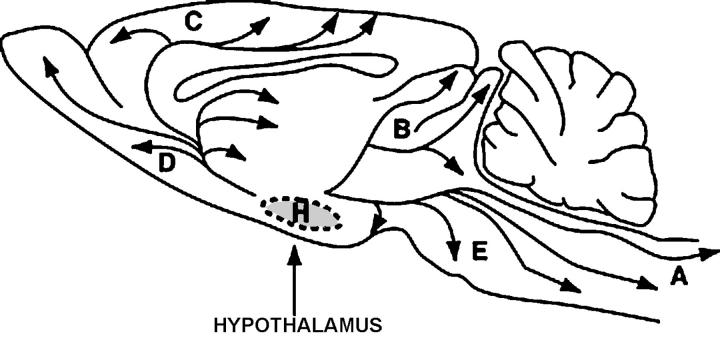
The hypocretin-producing cell bodies are specific to the hypothalamus and have widespread anatomical projections within the central nervous system of the rat with the densest extra-hypothalamic projection to the noradrenergic locus coeruleus (LC) and lesser projections to the basal ganglia, thalamic regions, the medullary reticular formation, and the nucleus of the solitary tract. There are minor projections to the cortical regions, central and anterior amygdaloid nuclei, and the olfactory bulb4,10,11. In humans, the localization of hypocretin-producing cell bodies is restricted to the dorso-lateral hypothalamus with extensive dense projections to the locus coeruleus (LC), dorsal raphe nuclei, amygdala, suprachiasmatic nucleus, basal forebrain, cholinergic brainstem12,13 and spinal cord (Figure 1)14.
Figure 1.
Projections of the hypocretin (orexin) system (A), to cholinergic neurons, reticular formation and spinal cord; (B), to thalamus and basal ganglia; (C), to basal forebrain; (D), to amygdala and dopaminergic neurons including suprachiasmatic nucleus; (E), to locus coeruleus.
Neurochemical actions of the hypocretins
The hypocretins are thought to act primarily as excitatory neurotransmitters1,2,7. Systemic and intracerebroventricular administration of hypocretins directly stimulates cells on the LC noradrenergic system in rats and monkeys, suggesting a role for the hypocretins in various central nervous functions related to noradrenergic innervation, including vigilance, attention, learning, and memory15. Their actions on serotonin, histamine, acetylcholine and dopamine neurotransmission is also thought to be excitatory and a facilitatory role on gamma-aminobutyric acid (GABA) and glutamate-mediated neurotransmission is suggested16,17. In particular, intravenous administration of Hcrt-1 in rats produces a differential release of GABA and glutamate in the hypocretin-dense amygdala compared with the cerebellum, suggesting that modulation of these neuro-transmitters is dependent on hypocretin innervation18.
Functions of hypocretin
Apart from their primary role in the control of sleep and arousal1,7, the hypocretins have been implicated in multiple functions including feeding and energy regulation3,16,19,20,21, neuroendocrine regulation17,22, gastrointestinal23 and cardiovascular system24 control, the regulation of water balance, and the modulation of pain1. A role in behaviour is also postulated25. The cell bodies responsible for hypocretin synthesis are localized to the tuberal part of the hypothalamus, the so-called feeding centre. The observation that Hcrt-1 increases metabolic rate and the demonstration that insulin-induced hypoglycaemia activates up to one-third of hypocretin containing neurons21 has led to the suggestion that the hypocretins are mediators of energy metabolism26. The neuroendocrine effects of the hypocretins include a lowering of plasma prolactin and growth hormone and an increase in the levels of corticotropin and cortisol, insulin and luteinizing hormone1,16,17. Central administration of the hypocretins increases water consumption, stimulates gastric acid secretion and increases gut motility1,23. The hypocretins increase mean arterial blood pressure and heart rate7. The localization of long descending axonal projections containing hypocretin at all levels of the spinal cord14 suggests a role in the modulation of sensation and pain. Strong innervation of the caudal region of the sacral cord suggests a role in the regulation of both sympathetic and parasympathetic functions.
HYPOCRETIN IN NARCOLEPSY
Narcolepsy is a primary disorder of alertness with an estimated prevalence of 0.03-0.05%. It may develop at any age but peak onset is in adolescence with a secondary peak in the fourth decade. The presenting symptom is usually excessive daytime sleepiness, with irresistible sleep attacks during the day. Other symptoms of this syndrome are cataplexy (brief episodes of muscle weakness or paralysis precipitated by strong emotion, such as laughter or surprise), sleep paralysis, which is a symptom due to the persistence of rapid-eye-movement (REM) sleep atonia on waking, and hypnogogic hallucinations or dream-like images, which characteristically occur at sleep onset. Short periods of automatic behaviour may also occur, a reflection of brief intrusions of sleep (‘micro-sleeps’) into the drowsy state28.
Animal studies
In 1999, Lin et al.29 demonstrated a mutation in the hypocretin receptor 2 gene in canine narcolepsy. The subsequent finding that mice lacking hypocretin receptors show behavioural arrests similar to symptoms of narcolepsy-cataplexy—i.e. direct transitions from wakefulness to REM sleep, gait disturbance preceding and rocking activity during behavioural arrest episodes30,31—led to a recognition of the potential importance of the hypocretins in sleep, arousal and activation. The animal models of narcolepsy show some variability in the defect causing the narcolepsy-like syndrome. In the mouse model, disruption of both types of hypocretin receptor pathways, Hcrtr1 and Hcrtr2, is necessary to produce the narcoleptic findings30,31,32,33 whereas in the canine model of narcolepsy the predominant defect is at the Hcrtr-2 receptor29. Intravenous administration of Hcrt-1 to narcoleptic dogs (dobermans) reduces cataplexy and normalizes their sleep and waking durations34.
Hypocretin in cerebrospinal fluid
There have been several studies of hypocretin in human CSF. The published work to date has tested for the presence of Hcrt-1 only and not Hcrt-2. CSF hcrt-1 levels in healthy adults are within a narrow range (250-280 pg/mL)35. A recent study indicated no significant difference in hypocretin levels with respect to gender or age, and concluded that very low or undetectable CSF hypocretin concentrations are an abnormal finding at any age36.
The initial study by Nishino et al.35 found that 7 of 9 patients with narcolepsy-cataplexy had undetectable levels of hypocretin in their CSF. Of the 2 patients with detectable hypocretin, one was within the control range and the other had raised levels. Both these patients were indistinguishable from the other patients with narcolepsy. The authors suggested that these patients might have a hypocretin receptor defect rather than a hypocretin production deficiency. Ripley et al.37 have reported undetectable levels of hypocretin in the CSF from 32 of 36 patients tested. In the remaining 4 the hypocretin levels were below the control range.
There have been two studies examining hypocretin cells post mortem in the brains of patients with narcolepsy12,13. Both found a striking reduction, to about 10% of the normal number of hypocretin neurons, in narcoleptic brains. In the initial study12 there was cell loss without gliosis or signs of inflammation. However, in the other study13 there was evidence of gliosis in the hypocretin cell region, implying that a degenerative process was the cause of hypocretin cell loss in narcolepsy. Further support for the degenerative hypothesis is their finding of a higher number of astrocytes in the hypothalamus of narcoleptic patients than in controls. The absence of hypocretin neurons can be explained by mechanisms including neurodegeneration, failure of development, reduction in synthesis or release of hypocretins or some mutation in the DNA sequence coding for hypocretin (though only 1 out of the 74 narcoleptic patients screened showed a mutation12).
HYPOCRETIN IN NEUROLOGICAL AND PSYCHIATRIC DISORDERS
The role of hypocretin in other neurological illnesses is yet to be established. A recent study38 found that CSF hypocretin levels did not differ significantly between two groups, one with neuroimmunological disease and the other with non-neuroimmunological disease, and normal controls. In a subgroup analysis the investigators found that 4 of 10 patients with Guillain—Barré syndrome had significantly lower Hcrt-1 levels than the controls. Another study37 has demonstrated low CSF hypocretin levels in patients with subarachnoid haemorrhage, acoustic schwannoma and head trauma—perhaps explained by damage to and/or dysfunction of the hypothalamus.
The dense hypocretin projections to the noradrenergic, serotonin, dopaminergic, cholinergic, and GABA/glutamate areas of the brain suggest a possible role in psychiatric and neuropsychiatric disorders39,40. The hypocretin system may be important in affective disorders such as major depression and bipolar affective disorder. The monoamine hypothesis (biogenic amine hypothesis) of depression suggests that dysfunctional or deficient neurotransmission of noradrenaline and/or serotonin underlies the symptoms of depression41,42,43. More recently, emphasis has shifted to the possible roles of neuropeptides in the aetiology and treatment of depression44,45,46,47,48,49. Involvement of the hypocretin system in depression is suggested on neuroanatomical and pharmacological grounds. The only substance known to innervate all the relevant areas of the brain implicated in the neurobiology of depression is hypocretin and the excitatory innervation of the LC and dorsal raphe region, the stimulation of dopamine and acetylcholine and the prohistaminergic actions all point to an antidepressant effect. These therapeutic possibilities remain to be clarified by appropriate studies.
CONCLUSION
There is strong evidence that narcolepsy is associated with abnormalities of the hypocretin neurotransmitter system. Low or undetectable levels of hypocretin are found in most patients but some have normal or raised levels. Thus it has been suggested that there are two variants of narcolepsy. In most patients there seems to be a hypocretin deficiency but there may also be a form with ‘hypocretin resistance’ due to abnormal hypocretin receptor/post-receptor dynamics leading to overproduction of hypocretin9,50. There may be involvement of the hypocretin/orexin system in other disorders of sleep such as primary hypersomnolence, insomnia, and the Kleine-Levin syndrome10, and a potential role in sleep disorders affecting the ageing population7,28,41,51,52. The role of these peptides in other neurological and psychiatric disorders remains putative.
References
- 1.Sutcliffe JG, de Lecea L. The hypocretins: excitatory neuromodulatory peptides for multiple homeostatic systems, including sleep and feeding. J Neurosci Res 2000;62: 161-8 [DOI] [PubMed] [Google Scholar]
- 2.de Lecea L, Kilduff TS, Peyron C, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA 1998;95: 322-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sakurai T, Amemiya A, Ishii M, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G-protein coupled receptors that regulate feeding behaviour. Cell 1998;92: 573-5 [DOI] [PubMed] [Google Scholar]
- 4.Gautvik KM, de Lecea L, Gautvik VT, et al. Overview of the most prevalent hypothalamus-specific mRNAs, as identified by directional tag PCR subtraction. Proc Natl Acad Sci USA 1996;93: 8733-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peyron C. Distribution of immunoreactive neurons and fibers for a hypothalamic neuropeptide precursor related to secretin. Soc Neurosci Abstr 1997;23: 2032 [Google Scholar]
- 6.Sutcliffe JG. Two novel hypothalamic peptides related to secretin derived from a single neuropeptide precursor. Soc Neurosci Abstr 1997;23: 2032 [Google Scholar]
- 7.Kilduff TS, Peyron C. The hypocretin/orexin ligand-receptor system: implications for sleep and sleep disorders. TINS 2000;23: 359-64 [DOI] [PubMed] [Google Scholar]
- 8.Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM. Distribution of orexin receptor mRNA in the rat brain. FEBS Lett 1998;438: 71-5 [DOI] [PubMed] [Google Scholar]
- 9.Chicurel M. The sandman's secrets. Nature 2000;407: 554-6 [DOI] [PubMed] [Google Scholar]
- 10.Mignot E. Perspectives in narcolepsy and hypocretin (orexin) research. Sleep Med 2000;1: 87-90 [DOI] [PubMed] [Google Scholar]
- 11.Peyron C, Tighe DK, Van den Pol AN, et al. Neurones containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci 1998;23: 9996-10015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peyron C, Faraco J, Rogers W, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med 2000;6: 991-7 [DOI] [PubMed] [Google Scholar]
- 13.Thannickal TC, Moore RY, Nienhuis R, et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron 2000;27: 469-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van den Pol AN. Hypothalamic hypocretin (orexin): robust innervation of the spinal cord. J Neurosci 1999;19: 3171-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horvath TL, Peyron C, Diane S, et al. Hypocretin (orexin) activation and synaptic innervation of the locus coeruleus noradrenergic system. J Comp Neurol 1999;415: 145-59 [PubMed] [Google Scholar]
- 16.Ida T, Nakahara K, Kuroiwa T, et al. Both corticotrophin releasing factor and neuropeptide Y are involved in the effect of orexin (hypocretin) on the food intake in rats. Neurosci Lett 2000;293: 119-22 [DOI] [PubMed] [Google Scholar]
- 17.van den Pol AN, Gao XB, Obrietan K, Kilduff TS, Belousov AB. Presynaptic and postsynaptic actions and modulation of neuroendocrine neurones by a new hypothalamic peptide, hypocretin/orexin. J Neurosci 1998;18: 7962-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.John J, Wu MF, Kodana T, Siegel JM. Hypocretin-1 (orexin-A) produced changes in glutamate and GABA release: an in vivo microdialysis study [Abstract]. Sleep 2001;24(suppl): A20 [Google Scholar]
- 19.Edwards CM, Abusnana S, Sunter D, Murphy KG, Ghaten MA, Bloom SR. The effect of orexins on food intake: comparison with neuropeptide Y, melanin-concentrating hormone and galanin. J Endocrinol 1999;160: R7-12 [DOI] [PubMed] [Google Scholar]
- 20.Haynes AC, Jackson B, Overend P, et al. Effects of single and chronic intracerebroventricular administration of the orexins on feeding in the rat. Peptides 1999;20: 1099-105 [DOI] [PubMed] [Google Scholar]
- 21.Moriguchi T, Sakurai T, Nambu T, Yanagisawa M, Goto K. Neurons containing orexin in the lateral hypothalamic area of the adult rat brain are activated by insulin-induced acute hypoglycemia. Neurosci Lett 1999;264: 101-4 [DOI] [PubMed] [Google Scholar]
- 22.Kuru M, Ueta Y, Serino R, et al. Centrally administered orexin-hypocretin activates HPA axis in rats. Neuroreport 2000;11: 1977-80 [DOI] [PubMed] [Google Scholar]
- 23.Kirchgessner AL, Liu M. Orexin synthesis and response in the gut. Neuron 1999;24: 941-51 [DOI] [PubMed] [Google Scholar]
- 24.Samson WK, Gosnell B, Chang JK, Resch ZT, Murphy TC. Cardiovascular regulatory actions of the hypocretins in brain. Brain Res 1999;831: 248-53 [DOI] [PubMed] [Google Scholar]
- 25.Ida T, Nakahara K, Katayama T, Murakami N, Nakazato M. Effect of lateral cerebroventricular injection of the appetite-stimulating neuropeptides, orexin and neuropeptide Y, on the various behavioural activities of rats. Brain Res 1999;821: 526-9 [DOI] [PubMed] [Google Scholar]
- 26.Chemelli RM, et al. Metabolic characterisation of orexin knockout mice [Abstract]. Sleep 2001;24(suppl): A21 [Google Scholar]
- 27.Samson WK, Resch ZT. The hypocretin/orexin story. Trends Endocrinol Metab 2000;11: 257-62 [DOI] [PubMed] [Google Scholar]
- 28.Krahn LE, Black JL, Silber MH. Narcolepsy: new understanding of irresistible sleep. Mayo Clin Proc 2001;76: 185-94 [DOI] [PubMed] [Google Scholar]
- 29.Lin L, Farace J, Li R, et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell 1999;98: 365-76 [DOI] [PubMed] [Google Scholar]
- 30.Chemelli RM, Willie JT, Sinton CM, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell 1999;98: 437-51 [DOI] [PubMed] [Google Scholar]
- 31.Kisanuki YY, Chemelli RM, Tokita S, et al. Behavioural and polysomnographic characterization of orexin-1 receptor and orexin-2 receptor double knockout mice [Abstract]. Sleep 2001;24(suppl): A22 [Google Scholar]
- 32.Kisanuki YY, Chemelli RM. The role of orexin receptor type-1 (OX1R) in the regulation of sleep [Abstract]. Sleep 2000;23(suppl): A91 [Google Scholar]
- 33.Takita S, Chemelli RM, Willie JT, Yanagisawa M. Behavioural characterisation of orexin-2 receptor (OX2R) knockout mice [Abstract]. Sleep 2001;24(suppl): A20 [Google Scholar]
- 34.John J, Wu MF, Siegel JM. Systemic administration of hypocretin-1 reduces cataplexy and normalizes sleep and waking durations in narcoleptic dogs. Sleep Res Online 2000;3: 23-8 [PMC free article] [PubMed] [Google Scholar]
- 35.Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet 2000;355: 39-40 [DOI] [PubMed] [Google Scholar]
- 36.Kanbayashi T, Yano T, Ishiguro H, et al. Hypocretin (orexin) levels in human lumbar CSF in different age groups [Abstract]. Sleep 2001;24(suppl): A330. [DOI] [PubMed] [Google Scholar]
- 37.Ripley B, Overeem S, Fujiki N, et al. CSF hypocretin levels in various neurological conditions: low levels in narcolepsy and Guillain Barré Syndrome [Abstract]. Sleep 2001;24(suppl): A322 [Google Scholar]
- 38.Kanbayashi T, Ishiguro H, Yano T, et al. Hypocretin/orexin concentrations are low in patients with Guillain Barré syndrome [Abstract]. Sleep 2001;24(suppl): A331-2 [Google Scholar]
- 39.Charney DS. Monoamine dysfunction and the pathophysiology and treatment of depression. J Clin Psychiatry 1998;59: 11-14 [PubMed] [Google Scholar]
- 40.van den Pol AN. Narcolepsy: a neurodegenerative disease of the hypocretin system? Neuron 2000;27: 415-18 [DOI] [PubMed] [Google Scholar]
- 41.Bunney WE, Davis JM. Noradrenaline in depressive reactions. A review. Arch Gen Psychiatry 1965;13: 483-94 [DOI] [PubMed] [Google Scholar]
- 42.Coppen A. The biochemistry of affective disorders. Br J Psychiatry 1967;113: 1237-64 [DOI] [PubMed] [Google Scholar]
- 43.Miller HL, Delgado PL, Salomon RM, et al. Clinical and biochemical effects of catecholamine depletion on antidepressant-induced remission of depression. Arch Gen Psychiatry 1996;53: 117-28 [DOI] [PubMed] [Google Scholar]
- 44.Gold PW, Chrousos G, Kellner C, et al. Psychiatric implications of basic and clinical studies with corticotropin-releasing factor. Am J Psychiatry 1984;141: 619-27 [DOI] [PubMed] [Google Scholar]
- 45.Gold PW, Loriaux DL, Roy A, et al. Responses to corticotropin-releasing hormone in the hypercorticolism of depression and Cushing's disease. Pathophysiologic and diagnostic implications. N Engl J Med 1986;314: 1329-35 [DOI] [PubMed] [Google Scholar]
- 46.Holsboer F, Van Bardeleben U, Gerken A, Stalla GR, Muller OA. Blunted corticotropin and normal cortisol response to human corticotropin-releasing factor in depression. N Engl J Med 1984;311: 1127. [DOI] [PubMed] [Google Scholar]
- 47.Kramer MS, Cutler N, Feighner J, et al. Distinct mechanism for antidepressant activity by blockade of central substance P receptors. Science 1998;281: 1640-5 [DOI] [PubMed] [Google Scholar]
- 48.Wong M-L, Licinio J. Research and treatment approaches to depression. Nat Rev Neurosci 2001;2: 343-51 [DOI] [PubMed] [Google Scholar]
- 49.Zobel AW, Nickel T, Kunzel ME, et al. Effects of high-affinity corticotropin releasing hormone receptor 1 antagonist R121919 in major depression: the first 20 patients treated. J Psychiatr Res 2000;34: 171-81 [DOI] [PubMed] [Google Scholar]
- 50.Melberg A, Ripley B, Lin L, Hetta J, Mignot E, Nishino S. Hypocretin deficiency in familial symptomatic narcolepsy. Ann Neurol 2001;49: 136-7 [PubMed] [Google Scholar]
- 51.Salin-Pascual RJ. The role of the hypothalamic neuropeptides hypocretin/orexin the sleep-wake cycle. Isr Med Assoc J 2001;3: 144-6 [PubMed] [Google Scholar]
- 52.Scammell TE, Estabrooke IV, McCarthy MT, et al. Hypothalamic arousal regions are activated during Modafinil-induced wakefulness. J Neurosci 2000;20: 8620-8 [DOI] [PMC free article] [PubMed] [Google Scholar]