In health, there are three main control mechanisms for maintaining steady gaze—fixation; the vestibulo-ocular reflex; and a gaze-holding system (the neural integrator), which operates whenever the eyes are required to hold an eccentric gaze position1,2,3. Failure of any of these control systems will bring about a disruption of steady fixation. Two types of abnormal fixation can result—nystagmus and saccadic intrusions/oscillations. The essential difference between them lies in the initial movement that takes the line of sight off the object of regard. In the case of nystagmus, it is a slow drift or ‘slow phase’ often due to a disturbance of one of the three mechanisms for gaze stability. On the other hand, with either saccadic intrusions or saccadic oscillations, it is an inappropriate fast movement that moves the eyes off target4,5. This paper will concern itself only with the clinical aspects of nystagmus and cover the possible reasons for its presence.
CLINICAL FEATURES OF NYSTAGMUS
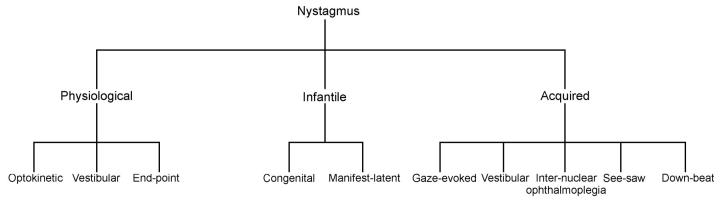
Nystagmus is an involuntary oscillation of one or both eyes about one or more axes. Broadly, nystagmus may be divided into one of three categories (Figure 1). First, it may be induced physiologically (e.g. optokinetic, vestibular and end-point). Secondly, it can be present at birth or soon after, when it is referred to as congenital or infantile nystagmus. And thirdly, it may be acquired (e.g. neurological disease or drug toxicity)2,3,4,5,6,7.
Figure 1.
Schematic guide illustrating some of the nystagmus types
Clinically, a nystagmus is characterized by the degree of conjugacy, the plane or planes of the oscillation, the direction or directions of gaze at which it is present, and the waveform, its amplitude and its frequency. Although a reasonable indication of the oculomotor behaviour may be obtained by just viewing the eyes, greater clarity and precision is gained when oculographic techniques are used. When the eyes oscillate like a sine wave, it is called pendular nystagmus (Figure 2a). If the nystagmus consists of drifts in one direction with corrective fast phases, it is called jerk nystagmus (Figure 2b-d).
Figure 2.
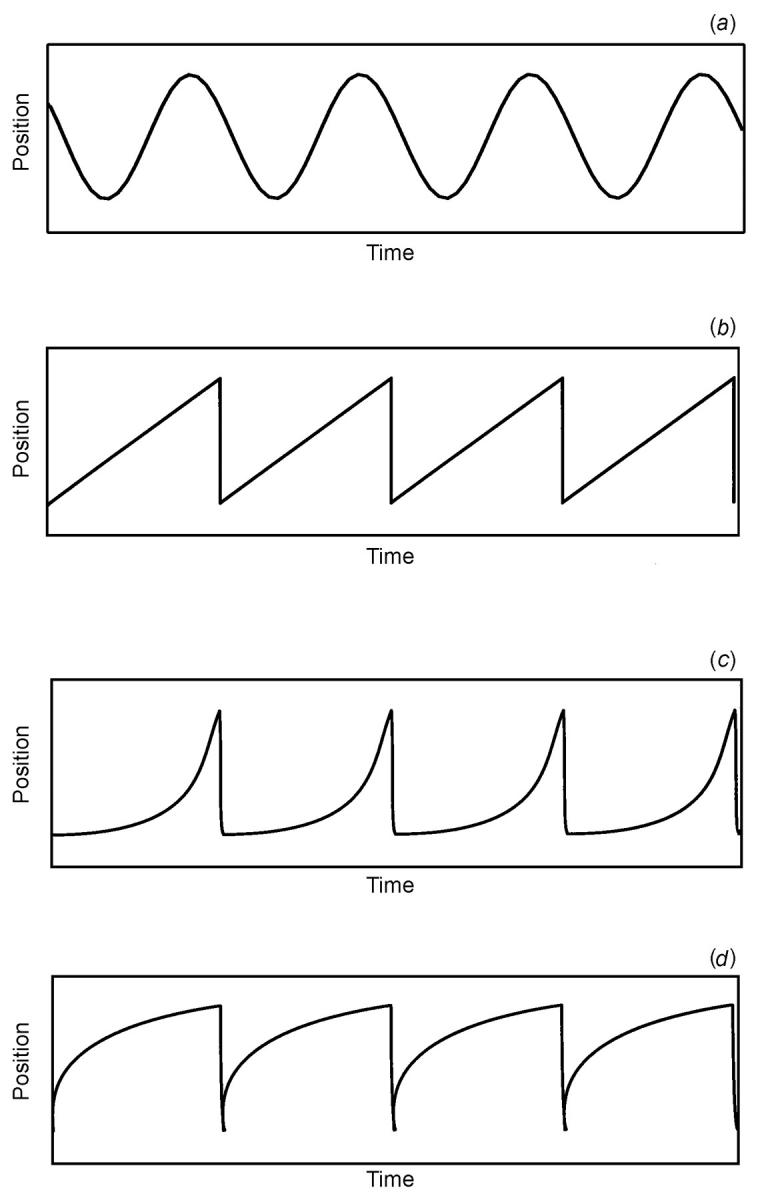
Schematic illustration of the most common nystagmus waveforms. (a) Pendular nystagmus. This oscillation is often seen in infants with congenital nystagmus, and in brainstem and cerebellar disease. (b) Linear or constant velocity slow phase is followed by a quick phase giving it a ‘saw tooth’ appearance. This oscillation is seen in optokinetic and vestibular nystagmus. (c) An accelerating velocity exponential slow phase. This is invariably seen in congenital nystagmus. (d) A decelerating velocity exponential slow phase. This is invariably seen in physiological end-point nystagmus, manifest latent nystagmus and pathological gaze-evoked nystagmus.
PHYSIOLOGICALLY INDUCED NYSTAGMUS
In health, nystagmus occurs during self-rotation in order to hold images of the visual world steady on the retina and maintain clear vision. Two forms of nystagmus are induced by self-rotation—optokinetic and vestibular. An optokinetic nystagmus is an involuntary, conjugate, jerk nystagmus that is seen when a person gazes into a large moving field (Figure 2b). The oscillations, which are in the plane of the moving field, are generally 3-4° in amplitude and 2-3 Hz in frequency. Both cortical and subcortical pathways contribute to the response, which is driven by the retinal image slip velocity. Smooth pursuit inputs are of particular importance1,2,8,9,10.
Vestibular nystagmus occurs during self-rotation even in darkness: the inner ear contains motion detectors (vestibular labyrinth) which project to the vestibular nuclei and cerebellum1,2,3. A vestibular nystagmus can also be induced by irrigating the ears with warm or cold water. With unilateral irrigation the conjugate nystagmus is horizontal, torsional or oblique, depending on the position of the head. Both a convection mechanism and a direct temperature effect on the canal's sensory apparatus have been proposed to account for the involuntary oscillations2.
Lastly, many healthy individuals show a small-amplitude (<2°) conjugate jerk nystagmus on far eccentric gaze (>40°). These oscillations are thought to reflect the time constant of the gaze-holding control system and in particular the cerebellar neural integrator1,2,3,8,11,12,13 (see Acquired Nsytagmus, below).
INFANTILE NYSTAGMUS
The two most common types of benign nystagmus seen in infancy are congenital nystagmus (CN) and manifest latent nystagmus (MLN)4,5. In both, the oscillations are typically conjugate, horizontal and jerky. Differential diagnosis is made on the basis that the CN slow phases are typically of an increasing exponential velocity form (Figure 2c), whereas in MLN the slow phases are decreasing or linear14,15,16,17,18 (Figures 2b and 2d). In addition to its distinguishing slow phase, the fast phase of MLN always beats toward the viewing eye. MLN is also closely associated with the presence of strabismus and dissociated vertical divergence, is strongly visually driven and is largely dependent on the attentional state of the patient18.
Both CN and MLN are associated with various disorders including albinism, optic nerve hypoplasia and congenital cataracts15,16,17,18,19. CN may also occur without ocular or central nervous system abnormalities (i.e. idiopathic CN).
Some patients with CN have near-normal vision, especially if they have developed ‘foveation periods’—brief epochs when the eye is still and pointed at the object of interest. CN is often suppressed during convergence and decreased at certain gaze angles—properties that can sometimes be used to provide optical or surgical therapies.
Over the years several mechanisms underlying CN and MLN have been proposed. These include anomalies of the smooth pursuit, fixation and optokinetic systems2,3,4,5,6,7. To date, five distinct models have been specifically constructed to account for CN. The first, proposed by Dell'Osso20 in 1967, suggested that CN resulted from a high gain instability in the slow eye movement control system. Some seventeen years later Optican and Zee21 provided a model in which the time constant of the neural integrator is lengthened by a velocity feedback signal and, when the sign of the feedback signal is reversed, the small post-saccadic drift velocities are amplified by the unstable velocity feedback loop, leading to exponentially growing slow phases. Tusa and his colleagues22 extended this model by proposing that the fixation system has both normal and abnormal feedback loops. Patients with CN who cannot suppress their nystagmus either have only the abnormal feedback loop or cannot voluntarily manipulate the normal feedback loop. Central to these two models is the need for a neuronal mis-wiring. This seems somewhat untenable given the range of visual disorders associated with CN in the absence of chiasmal misdirection, the absence of an abnormal visual evoked response in idiopathic CN and the finding of CN in achiasmic dogs23,24 and humans25.
Fourthly, in 1995 Harris26 suggested that CN was due to excessive gain in an internal efference copy loop in the smooth pursuit system around a leaky neural integrator. Finally, and most recently, Broomhead and his colleagues27, using a non-linear dynamics approach, proposed that the behaviour of burst cell firing, in the form of a saccadic termination abnormality, could account for the variety of CN waveforms that previous models were unable to create.
Since MLN occurs frequently in patients who have congenital or uniocular visual loss, or who have experienced visual deprivation, it has been proposed that disturbances of egocentric localization may be in part responsible for these oscillations28,29, as well as the possibility of an abnormality of extraocular proprioception30. In summary, we do not have well-accepted hypotheses to account for infantile forms of nystagmus, but certain properties of these oscillations present opportunities for specific therapies.
ACQUIRED NYSTAGMUS
Many forms of acquired nystagmus can be attributed to disturbances of the three mechanisms that normally ensure steady gaze—visual fixation, the vestibulo-ocular reflex, and the mechanism that makes it possible to hold the eyes at an eccentric eye position (e.g. far right gaze)1,2,3,4,5,6,7.
Diseases affecting the visual system, such as retinal disorders causing visual loss, commonly lead to nystagmus because visual fixation is no longer possible. Disease affecting the vestibular organ in the inner ear causes an imbalance that leads to a mixed horizontal—torsional nystagmus, usually associated with vertigo. Disease affecting the central connections of the vestibular system, including the cerebellum, may cause several forms of nystagmus. These include down-beat, torsional, periodic alternating and see-saw nystagmus. None of these nystagmus types are, in themselves, pathognomonic of central nervous system disease. Nonetheless, down-beat nystagmus is usually associated with lesions of the vestibulo-cerebellum (flocculus, paraflocculus, nodulus and uvula) and the underlying medulla; up-beat nystagmus is most commonly reported with lesions of the medulla, including the perihypoglossal nuclei and adjacent vestibular nucleus (both structures are important for gaze-holding), the ventral tegmentum and the anterior vermis of the cerebellum; periodic alternating nystagmus is often linked to cerebellar disease (note that in this case the horizontal jerk nystagmus spontaneously reverses direction of the quick phase every few seconds); see-saw nystagmus is linked to parasellar lesions of the optic chiasm (e.g. pituitary tumours) and achiasma (note that this is a rare form of pendular nystagmus in which the torsional components are conjugate and the vertical components are disjunctive—one eye rises and intorts while the other falls and extorts); and gazeevoked nystagmus is commonly seen as a side-effect of drugs, including sedatives, anticonvulsants and alcohol, as well as cerebellar disease2.
Lesions affecting the medial longitudinal fasciculus cause internuclear ophthalmoplegia. A unilateral internuclear ophthalmoplegia is commonly related to ischaemia, whilst bilateral internuclear ophthalmoplegias are associated with multiple sclerosis. An adduction weakness on conjugate movements and a jerk nystagmus of the abducting eye are the classic ocular motor signs (‘dissociated nystagmus’).
An acquired pendular nystagmus can occur in any plane; it can be monocular or have a greater intensity in one eye and typically remains pendular in all directions of gaze. It is associated with a wide range of brainstem and cerebellar disease including several disorders of myelin, with the syndrome of oculopalatal myoclonus, with Wipple's disease and with drug toxicities.
Recently Das and his colleagues31 have modified a neural-network model previously developed by Arnold and Robinson32 to account for the pendular oscillations (Figure 2a) caused by disease of central myelin. Their findings suggest that, in multiple sclerosis, the pendular nystagmus arises from an instability in the feedback control of the neural integrator.
One of the most studied and frequently seen of the acquired oscillations is gaze-evoked nystagmus. The nystagmus is elicited when the patient attempts to maintain an eccentric eye position. The oscillations are jerky with a centripetal decreasing velocity exponential slow phase taking the eyes away from the desired eye position, followed by a corrective fast phase (Figure 2d). It is seen in patients with cerebellar disease (particularly the flocculus), muscle palsy and drug toxicity. A failure of the step (or tonic) eye position command from the gaze-holding network (the neural integrator) is deemed to be the reason for the presence of the nystagmus2,6,12. After the eyes are returned to the primary position, a short-lived reflex nystagmus with quick phases opposite to the direction of the previous eccentric gaze oscillation can typically be seen in vestibulocerebellar diseases. It is very difficult to differentiate a physiological end-point nystagmus from an acquired gaze-evoked nystagmus by viewing the eye movements alone11,12,13.
Finally, no review of acquired nystagmus could be complete without mention of the vestibular apparatus and pathway. Diseases affecting the vestibular labyrinth or nerve (including the root entry zone) cause a jerk nystagmus with linear or constant velocity slow phase drifts (Figure 2b). Characteristically the nystagmus increases when the eyes are turned in the direction of the quick phases (Alexander's law), and can be markedly suppressed by visual fixation. The direction of the unidirectional nystagmus is related to the geometrical relationship of the semicircular canals with the fast phase opposite to the side of the lesion. A change in head position often exacerbates the nystagmus. On the other hand, a central vestibular nystagmus, which is caused by disease of the brainstem and/or cerebellum, is not attenuated by fixation and invariably exhibits bidirectionality to the nystagmus (i.e. left-beating on left gaze and right-beating on right gaze).
Clearly, the mechanisms that give rise to congenital nystagmus remain to be fully understood. Nonetheless, knowledge of the nystagmus characteristics can often give clues to the location of the lesion, pathogenesis, and underlying mechanisms. For example, a periodic alternating nystagmus which is seen frequently both in albinos33 and in patients with cerebellar disease suggests that multiple mechanisms are at work. The recent use of a systems approach to analyse and understand congenital nystagmus has suggested that the dynamics in the region of foveation are low-dimensional and deterministic34. Furthermore, on the basis of recent studies13,22,27, future control and dynamic systems models of nystagmus will almost certainly need to incorporate attentional and adaptive loops in order to better describe and simulate the oscillations. In parallel with these, advances in the surgical and medical treatment of nystagmus have yielded promising results.
Clinicians often regard nystagmus as a perplexing subject—oscillations of the eyes that can only be understood by neuro-ophthalmologists. Research over the past three decades has led to better understanding of the pathophysiology of acquired forms of nystagmus, suggesting drug therapies. Congenital nystagmus remains unexplained, but better documentation of several factors that reduce these oscillations has led to the development of surgical therapies. This symposium begins with a summary of current concepts of the pathophysiology of nystagmus by RV Abadi, which provides the background to current medical treatments (JS Stahl and colleagues) and surgical therapies (J Lee).
References
- 1.Cuiffreda KJ, Tannen B. Eye Movements for the Clinician. St Louis: Mosby, 1995
- 2.Leigh RJ, Zee DS. The Neurology of Eye Movements. Philadelphia: FA Davis, 1999
- 3.Sharpe JA. Neural control of ocular motor systems. In: Miller NR, Newman NJ, eds. Walsh and Hoyt's Clinical Neuro-ophthalmology, Vol. 1. Baltimore: Williams & Wilkins, 1998: 1101-67 [Google Scholar]
- 4.Harris C. Nystagmus and eye movement disorders. In: Taylor D, ed. Paediatric Ophthalmology. Oxford: Blackwell, 1997: 869-96
- 5.Dell'Osso LF, Daroff RB. Nystagmus and saccadic intrusions and oscillations. In: Glaser JS, ed. Neuro-ophthalmology. Baltimore Lippincott, Williams & Wilkins, 1999: 369-401
- 6.Abel LA. Ocular oscillations. Congenital and acquired. In: Daroff RB, Neetens A, eds. Neurological Organisation of Ocular Movements. Amsterdam: Kluger & Ghedini, 1990: 163-89
- 7.Leigh RJ, Averbuch-Heller L. Nystagmus and related ocular motility disorders. In: Miller NR, Newman NJ, eds. Walsh and Hoyt's Clinical Neuro-ophthalmology, Vol. 1. Baltimore: Williams & Wilkins, 1998: 1461-1505 [Google Scholar]
- 8.Carpenter RHS. Movements of the Eyes. London: Pion Press, 1988
- 9.Abadi RV, Pascal E. The effects of simultaneous central and peripheral field motion on the optokinetic response. Vision Res 1991;31: 2219-25 [DOI] [PubMed] [Google Scholar]
- 10.Howard IP. The optokinetic system. In: Sharpe JA, Barber HO, eds. The Vestibular-ocular Reflex and Vertigo. New York: Raven, 1993: 163-84
- 11.Eizenman M, Cheng P, Sharpe JA, Frecker RC. Endpoint nystagmus and ocular drift: an experimental and theoretical study. Vision Res 1990;30: 863-77 [DOI] [PubMed] [Google Scholar]
- 12.Dell'Osso LF. Neural integration in ocular motility. In: Daroff RB, Neetens A, eds. Neurological Organisation of Ocular Movements. Amsterdam: Kluger & Ghedini, 1990: 19-33
- 13.Abadi RV, Scallan CJ. Ocular oscillations on eccentric gaze. Vision Res 2001;41: 2895-907 [DOI] [PubMed] [Google Scholar]
- 14.Dell'Osso LF, Daroff RB. Congenital nystagmus waveforms and foveation strategy. Doc Ophthalmol 1975;39: 155-82 [DOI] [PubMed] [Google Scholar]
- 15.Abadi RV, Dickinson CM. Waveform characteristics in congenital nystagmus. Doc Ophthalmol 1986;64: 153-67 [DOI] [PubMed] [Google Scholar]
- 16.Abadi RV, Dickinson CM, Pascal E, Whittle J, Worfolk R. Sensory and motor aspects of congenital nystagmus. In: Schmid R, Zambarbieri D, eds. Oculomotor Control and Cognitive Processes. Amsterdam: Elsevier, 1991: 249-62
- 17.Dell'Osso LF. Congenital latent and manifest latent nystagmus: similarities, differences and relation to strabismus. Jpn J Ophthalmol 1985;29: 351-63 [PubMed] [Google Scholar]
- 18.Abadi RV, Scallan CJ. Waveform characteristics of manifest latent nystagmus. Invest Ophthalmol Vis Sci 2000;41: 3805-17 [PubMed] [Google Scholar]
- 19.Gage JE, Abadi RV, Lloyd IC, Thompson CM. Fixation stability and infantile cataract. Invest Ophthalmol Vis Sci 2001;42: 4S164 [Google Scholar]
- 20.Dell'Osso LF. A model for the horizontal tracking system of a subject with nystagmus. Visual and vestibular responses. 20th Annual Conference on Engineering and Medicine. USA: 1967
- 21.Optican LM, Zee DS. A hypothetical explanation of congenital nystagmus. Biol Cybern 1984;50: 119-34 [DOI] [PubMed] [Google Scholar]
- 22.Tusa RJ, Zee DS, Hain TC, Simonsz HJ. Voluntary control of congenital nystagmus. Clin Vis Sci 1992;7: 195-210 [Google Scholar]
- 23.Brodsky MC, Baker RS, Hamed LM. Nystagmus in infancy and childhood. In: Brodsky MC, Baker RS, Hamed LM, eds. Pediatric Neuro-Ophthalmology. New York: Springer, 1996: 303-49
- 24.Dell'Osso LF, Williams RW. Ocular motor abnormalities in achiasmatic mutant Belgium sheepdogs: unyoked eye movements in a mammal. Vision Res 1995;35: 109-16 [DOI] [PubMed] [Google Scholar]
- 25.Dell'Osso LF. See-saw nystagmus in dogs and humans: an international, across discipline, serendipitous collaboration. Neurology 1996;47: 1372-4 [DOI] [PubMed] [Google Scholar]
- 26.Harris CM. Problems modelling congenital nystagmus: towards a new model. In: Findlay JM, Walker R, Kentridge RW, eds. Eye Movement Research: Processes, Mechanisms and Applications. Amsterdam: Elsevier, 1995: 239-53
- 27.Broomhead DS, Clement RA, Muldoon MR, Whittle JP, Scallan C, Abadi RV. Modelling of congenital nystagmus waveforms produced by saccadic system abnormalities. Biol Cybern 2000;82: 391-9 [DOI] [PubMed] [Google Scholar]
- 28.Abadi RV. Pattern contrast thresholds in latent nystagmus. Acta Ophthalmol 1980;58: 210-20 [DOI] [PubMed] [Google Scholar]
- 29.Dell'Osso LF, Schmidt D, Daroff RB. Latent, manifest latent and congenital nystagmus. Arch Ophthalmol 1977;97: 1877-85 [DOI] [PubMed] [Google Scholar]
- 30.Ishikawa S. Latent nystagmus and its etiology. In: Reineke RD, ed Third Meeting of the International Stratismological Association, Kyoto, Japan New York: Grune & Stratton, 1979: 203-14
- 31.Das VE, Oruganti P, Kramer PD, Leigh RJ. Experimental tests of a neural-network model for oscillations caused by disease of central myelin. Exp Brain Res 2000;133: 189-97 [DOI] [PubMed] [Google Scholar]
- 32.Arnold DB, Robinson DA. The oculomotor integrator: testing of a neural network model. Exp Brain Res 1997;113: 57-74 [DOI] [PubMed] [Google Scholar]
- 33.Abadi RV, Pascal E. Periodic alternating nystagmus in humans with albinism. Invest Ophthalmol Vis Sci 1994;35: 4080-6 [PubMed] [Google Scholar]
- 34.Abadi RV, Broomhead DS, Clement RA, Whittle JP, Worfolk R. Dynamical systems analysis: a new method of analysing congenital nystagmus waveforms. Exp Brain Res 1997;117: 355-61 [DOI] [PubMed] [Google Scholar]