Abstract
Bacteria or their products may cause chronic inflammation and subsequent bone loss. This inflammation and bone loss may be associated with significant morbidity in chronic otitis media, periodontitis, endodontic lesions, and loosening of orthopedic implants caused by lipopolysaccharide (LPS)-contaminated implant particles. Currently, it is not clear how bacteria or endotoxin-induced bone resorption occurs and what cell types are involved. Here we report that Porphyromonas gingivalis, a periodontal pathogen, and Escherichia coli LPS induce osteoclastic cell formation from murine leukocytes in the absence of osteoblasts. In contrast, stimulation with parathyroid hormone had no effect. These multinucleated, tartrate-resistant acid phosphatase-positive cells were positive for receptor activator of NF-κB (RANK), the receptor for osteoprotegerin ligand (OPGL), also known as RANK ligand (RANKL). Blocking antibodies demonstrated that their formation was dependent upon expression of OPGL and, to a lesser extent, on tumor necrosis factor alpha. Mononuclear cells represented a significant source of OPGL production. In vivo, P. gingivalis injection stimulated OPGL expression in both mononuclear leukocytes and osteoblastic cells. Thus, these findings describe a pathway by which bacteria could enhance osteolysis independently of osteoblasts and suggest that the mix of cells that participate in inflammatory and physiologic bone resorption may be different. This may give insight into new targets of therapeutic intervention.
Osteotrophic hormones and inflammatory cytokines may stimulate osteoclastic bone resorption by either enhancing the proliferation of osteoclastic precursors or promoting differentiation or maturation of multinucleated cells to resorb bone, or both (20). Osteotrophic hormones, such as parathyroid hormone (PTH), stimulate osteoclastic bone resorption through osteoblasts or other bone-lining cells (17). This is consistent with the observation that osteoclasts and their presumed precursors lack PTH receptors, while these receptors are present on osteoblasts (26).
Inflammatory cytokines, such as interleukin-1 (IL-1) or tumor necrosis factor alpha (TNF-α), have been reported to induce osteoclast formation indirectly by stimulating osteoblasts (4, 27, 28). Likewise, lipopolysaccharide (LPS), an inflammatory component of gram-negative bacteria, has been reported to induce osteoclastogenesis indirectly (21). Therefore, it is generally accepted that both osteotrophic hormones and factors that stimulate inflammation-induced osteoclast formation act through osteoblasts or stromal bone-lining cells (23).
The mechanism by which osteoblasts send a second signal to osteoclast precursors in response to primary osteolytic signals has been the subject of intense investigation. It has now been demonstrated that osteoblastic cells regulate osteoclastogenesis by expressing osteoprotegerin ligand (OPGL) (25, 30). Osteoclast precursors, which express the receptor activator of NF-κB (RANK), recognize OPGL expressed by osteoblasts. In the presence of other costimulators such as macrophage colony-stimulating factor (M-CSF) (29), OPGL stimulates the fusion of osteoclast precursors into multinucleated cells capable of resorbing bone (22, 24). Osteoclast formation is a critical process in normal development, since it allows for the formation of the marrow spaces within bone and the eruption of teeth. Mice with targeted deletion of OPGL have severe osteopetrosis and lack osteoclasts, due to the inability of osteoblasts to support osteoclastogenesis (15). Mice that fail to produce M-CSF also have osteopetrotic bone. Both types of genetic lesions result in death of the animals after weaning because of malnutrition secondary to a failure of tooth eruption (26). While OPGL has been considered to be a key regulator of osteoclastogenesis, there are reports demonstrating that TNF-α induces formation of osteoclast-like cells independent of OPGL activity (13).
Bacteria or their products cause inflammatory bone loss in a number of different infections, including chronic otitis media, periodontitis, endodontic lesions, and loosening of orthopedic implants, which may result in significant morbidity (8, 19). In most cases of chronic inflammation associated with infection, gram-negative bacteria and their products (such as LPS) have been implicated as causative factors. In these inflammatory conditions, a mononuclear cell infiltrate is typically present. That OPGL is expressed by lymphocytes raises the possibility that leukocytes play a prominent role in generating primary signals that induce osteoclastogenesis (14). Another prominent cell type found in mononuclear infiltrates is the monocyte. It plays a central role in orchestrating the response to LPS and gram-negative bacteria. Although monocytes have been implicated in osteolysis, the specific mechanisms by which they promote bone resorption have not been conclusively established (3, 5).
The present study examined bacterium-induced formation of osteoclast-like cells and the role of OPGL and TNF-α production by leukocytes. The results indicate that bacteria or LPS can induce formation of osteoclastic cells without osteoblastic cells present and that both TNF-α and OPGL may play a significant role.
MATERIALS AND METHODS
Bacterial culture and preparation.
Porphyromonas gingivalis (A7436) was grown in a commercially formulated complex medium, TSBY (Trypticase soy agar and brain heart infusion agar with yeast extract) plus Hemin and vitamin K (Northeast Laboratories, Winslow, Maine) in an anaerobic atmosphere of 10% CO2, 10% H2, and 80% N2 at 37°C. Bacteria were used at early- to middle-log-phase growth and heat killed by boiling for 10 min, followed by washing five times with phosphate-buffered saline (PBS). The amount of bacteria was quantified with a spectrophotometer (560 nm) based on a standard curve established by colony formation on bacterial plates.
Isolation of murine spleen cells.
Spleens were obtained from 8- to 10-week-old mice (C57BL6; Jackson Labs, Bar Harbor, Maine). After sacrifice in a CO2 chamber, the spleen was removed under sterile conditions and homogenized, and cells were collected by centrifugation. Red blood cells were lysed with ammonium chloride (0.8% [wt/vol]), and mononuclear cells were isolated by centrifugation in Histopaque 1083 (Sigma Diagnostics, St. Louis, Mo.). The procedures conducted were approved by the Institutional Animal Care and Use Committee at Boston University.
Formation of TRAP-positive multinucleated cells.
Freshly isolated splenocytes (1.5 × 106 per well [1 ml]) were plated in LAB-Tek chambers and stimulated with bacteria or LPS in α-minimal essential medium supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin for 7 to 11 days. M-CSF (24 ng/ml) was added unless stated otherwise and the medium was changed every 3 days. Cells were then stained for tartrate-resistant acid phosphatase (TRAP) (9, 18). TRAP-positive multinucleated cells containing over three nuclei were identified as osteoclast-like cells.
Immunostaining of RANK expression by multinucleated osteoclast-like cells.
Splenocytes were stimulated with bacteria in LAB-Tek chambers as described above for 11 days. Cells were fixed with 1% paraformaldehyde and incubated with antibodies to RANK or matched control immunoglobulin G (IgG; Santa Cruz Biotechnology, Santa Cruz, Calif.). Primary antibody was localized using an Elite-ABC kit from Vector Laboratories (Burlingame, Calif.) with diaminobenzidine (DAB) as a chromogen, following the manufacturer's instructions.
Flow cytometry.
Murine splenocytes (3 × 106/ml) were cultured with or without P. gingivalis (105 cells/ml) in six-well plates for 15 to 72 h. Nonadherent cells were collected and the attached cells were removed by an enzyme-free, PBS-based cell dissociation solution (Life Technologies, Rockville, Md.). The cells were centrifuged and transferred to a polypropylene round-bottom 96-well plate for incubation with antibodies. A goat anti-mouse OPGL antibody was purchased from R&D Systems (Minneapolis, Minn.) and used to identify OPGL-positive cells, followed by incubation with a fluorescein isothiocyanate-conjugated donkey anti-goat secondary antibody. Phycoerythrin-conjugated rat anti-mouse CD3 and CD19 were purchased from Pharmingen (San Diego, Calif.). Matched goat IgG and phycoerythrin-labeled rat IgG were used as controls for OPGL and CD3 or CD19, respectively.
Immunohistochemistry staining of OPGL expression in vivo.
Bacteria were applied by supraperiosteal injection in the midcalvarial region after removal of hair using a microshaver. Fifty microliters of P. gingivalis (1010/ml) or vehicle alone (PBS) was injected. Mice were sacrificed 7 days after injection and specimens were embedded in paraffin and cut at a thickness of 5 μm. The calvarial samples with attached scalp were harvested, fixed in 4% paraformaldehyde for 24 h at 4°C, and decalcified in Immunocal (Decal Chemical Corporation, Congers, N.Y.). The sections were incubated with antibodies to OPGL or matched control IgG. Primary antibodies were localized using an Elite kit from Vector Laboratories with DAB as chromogen, following the manufacturer's instructions.
RESULTS
Bacteria stimulate formation of multinucleated osteoclast-like cells.
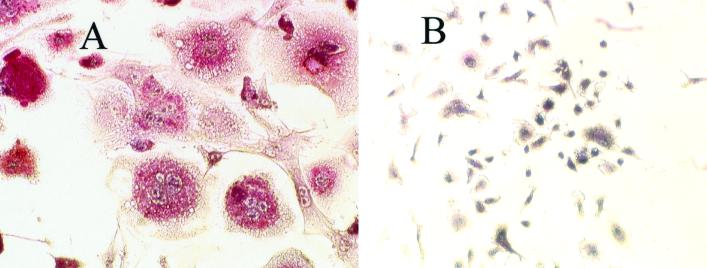
The first set of experiments was carried out to establish whether bacteria could directly induce osteoclastic cell formation from leukocytes. Osteoclastic cell formation was determined by the presence of multinucleated TRAP-positive cells. P. gingivalis cells incubated with leukocytes stimulated formation of a large number of TRAP-positive multinucleated cells (Fig. 1A). In the absence of bacterial stimulation, virtually no TRAP-positive cells were identified (Fig. 1B).
FIG. 1.
P. gingivalis stimulates formation of multinucleated TRAP-positive cells. Murine splenocytes were incubated with or without bacteria (105/ml) for 11 days. Cell cultures were fixed and stained for TRAP activity and counterstained with hematoxylin. (A) Stimulated splenocytes; (B) nonstimulated splenocytes. Magnification, ×20. The assay was carried out six times with similar results. The photomicrograph was taken from a representative experiment.
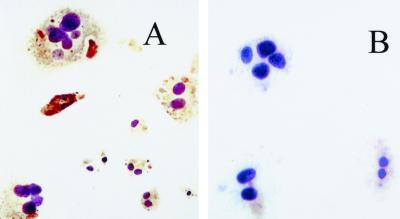
To further characterize the phenotype of the multinucleated TRAP-positive cells, immunohistochemistry was carried out to determine whether these cells express RANK, the receptor for OPGL, which is produced by osteoclast precursors and mature osteoclasts. Figure 2A demonstrates that the majority of cells with two or more nuclei were stained positive for RANK following bacterial stimulation, while there was no immunostaining with isotype-matched control IgG (Fig. 2B). Thus, these multinucleated cells are osteoclast-like.
FIG. 2.
Immunostaining of RANK expression by multinucleated osteoclast-like cells. Splenocytes were stimulated with P. gingivalis in LAB-Tek chambers for 11 days as described in the text. Cells were fixed and incubated with antibody to RANK (A) or matched control IgG (B). Photomicrograph magnification, ×20. The immunostaining was performed twice with the same result.
Bacteria and LPS induce a dose-dependent increase in osteoclastogenesis.
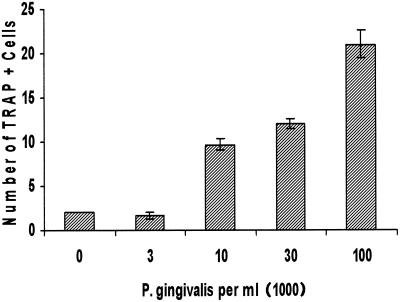
The number of osteoclastic cells formed in response to P. gingivalis stimulation was dose dependent. As shown in Fig. 3, few if any multinucleated osteoclastic cells formed after 11 days in culture medium that was supplemented with serum alone. In many experiments, no osteoclast-like cells were detected in the absence of bacterial stimulation. At a relatively low concentration of bacteria, 104/ml, large numbers of osteoclastic cells formed, which were further increased by the addition of higher concentrations of bacteria. However, at concentrations of P. gingivalis of 106/ml or greater, the response was variable, since high doses began to negatively affect the viability of leukocytes over the 11-day period in culture (data not shown). It should be noted that based on our previous animal study and those of Zubery et al., the dose of bacteria that we used in vitro was similar to or less than the dose required to create osteolytic lesions in vivo (12, 31).
FIG. 3.
Dose response of P. gingivalis-stimulated formation of osteoclastic cells. Murine splenocytes were stimulated with various doses of P. gingivalis in culture medium not supplemented with M-CSF. Cells were fixed and assayed for TRAP activity on day 11. TRAP-positive osteoclastic cells were counted if they had three or more nuclei per cell. One-way analysis of variance demonstrated that 104 cells/ml or higher concentrations of P. gingivalis significantly stimulated formation of osteoclastic cells in a dose-dependent manner (P < 0.01). The experiment was performed three times with similar results.
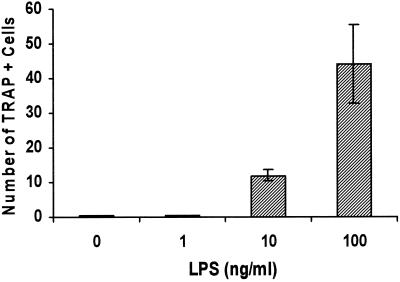
LPS has been found to play an important role in eliciting a host response to gram-negative bacteria and to cause pathological consequences, including bone resorption. Recent studies indicate that IL-1 and TNF induced by LPS contribute to osteoclastogenesis (1, 6). However, an understanding of the mechanisms by which LPS induces bone resorption remains incomplete. Therefore, experiments were carried out to examine whether LPS purified from enteric bacteria could induce formation of osteoclast-like cells in the absence of osteoblasts. Figure 4 demonstrates that Escherichia coli LPS stimulated formation of multinucleated TRAP-positive cells in a dose-dependent manner. The stimulatory activity leading to the formation of osteoclastic cells was observed at a relatively low concentration, 10 ng/ml, and peaked at 100 ng/ml. Higher concentrations of LPS did not further enhance the formation of osteoclastic cells (data not shown).
FIG. 4.
Dose response of LPS-stimulated multinucleated osteoclastic cells. Murine splenocytes were stimulated with various doses of LPS in culture medium not supplemented with M-CSF. Cells were fixed, assayed for TRAP activity, and counted on day 11 as described in the legend to Fig. 3. One-way analysis of variance demonstrated that the 10-ng/ml or higher concentrations of LPS significantly stimulated formation of osteoclastic cells in a dose-dependent manner (P < 0.01). The experiment was performed twice with similar results.
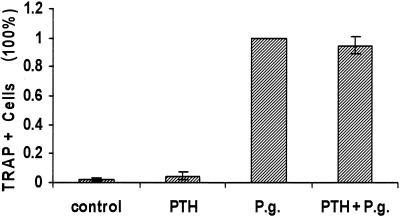
PTH does not directly stimulate osteoclastic cell formation from leukocyte cultures.
It has been reported by various laboratories that calcitropic hormones such as PTH induce osteoclast formation via an osteoblast-dependent pathway (2, 7, 16). In order to compare the induction of osteoclastic cells by bacteria with that stimulated by PTH, we measured osteoclastogenesis under the same conditions described above (Fig. 5). The results demonstrate that PTH does not directly stimulate formation of osteoclastic cells, while P. gingivalis-stimulated cultures formed large numbers of these cells in the same assay. Moreover, PTH did not further enhance the stimulatory capacity of P. gingivalis. This result indicates that the requirement of accessory cells in stimulating osteoclast formation differs between PTH and gram-negative bacteria.
FIG. 5.
Effect of PTH on murine splenocytes. Murine splenocytes were stimulated with PTH (5 × 10−8 M) and P. gingivalis (105 cells/ml). Cells were fixed, assayed for TRAP activity, and counted on day 11 as described in the legend to Fig. 3. The assay was performed three times with similar results.
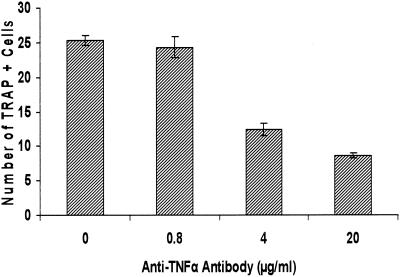
TNF-α plays a significant role in bacterium-stimulated osteoclastic cell formation.
To examine the mechanisms by which bacteria induce osteoclast-like cell formation, blocking antibodies to TNF-α were added to the leukocyte culture during bacterial stimulation (Fig. 6). The results demonstrated that the number of multinucleated TRAP-positive cells was decreased by a dose-dependent incubation of anti-TNF antibody. However, a plateau was reached, with the maximum inhibition typically around 40 to 60%. This suggests that TNF contributes to the formation of osteoclast-like cells but that other mediators are as important.
FIG. 6.
Effect of antibody to TNF-α on formation of osteoclastic cells. Murine splenocytes were stimulated with bacteria (105/ml) and culture medium not supplemented with M-CSF. Various concentrations of anti-murine TNF-α antibody or matched control IgG were added during the entire time of the culture. Cells were fixed, assayed for TRAP activity, and counted on day 11 as described in the legend to Fig. 3. One-way analysis of variance demonstrated that the 4-μg/ml or higher concentrations of anti-TNF-α antibody significantly inhibited formation of osteoclastic cells in a dose-dependent manner (P < 0.01). The experiments were carried out three times with similar results.
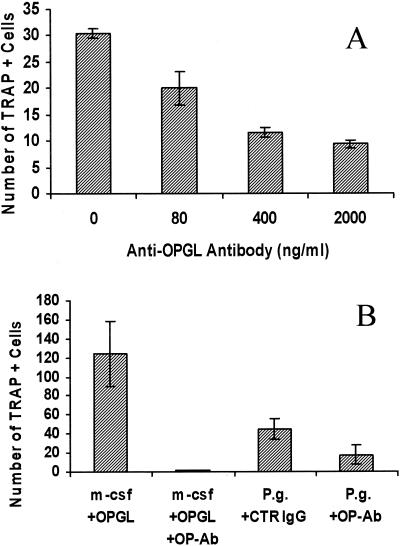
OPGL expressed by mononuclear leukocytes plays a significant role in bacterium-stimulated osteoclastic cell formation.
To examine the role of OPGL in bacterium-induced osteoclast formation, blocking antibodies to OPGL were incubated with leukocytes during bacterial stimulation. The results demonstrated that antibodies to OPGL decrease the number of TRAP-positive cells in a dose-dependent manner (Fig. 7). The formation of the osteoclastic cells was significantly inhibited by the antibody by approximately 20% at 80 ng/ml and by 60% at 400 ng/ml (Fig. 7A). When additional antibody was added, the degree of inhibition was somewhat higher, reaching 70%, a degree of inhibition that was consistently observed but not exceeded. We also tested the capacity of the OPGL antibody to inhibit osteoclastic cell formation induced by recombinant OPGL. When a moderate concentration of antibody was utilized (200 ng/ml), osteoclast formation induced by recombinant OPGL (30 ng/ml) was completely inhibited, demonstrating the effectiveness of the antibody (Fig. 7B). The observation that a 10-times-higher concentration of this antibody did not inhibit 100% of P. gingivalis-stimulated osteoclastogenesis would suggest that other mediators can also stimulate formation of osteoclastic cells even in the relative absence of OPGL, albeit less effectively.
FIG. 7.
Effect of antibody to OPGL on formation of osteoclastic cells. (A) Murine splenocytes were stimulated with bacteria (105/ml) with various concentrations of anti-murine OPGL in standard culture medium. One-way analysis of variance demonstrated that the 400-ng/ml or higher concentrations of antibody to OPGL significantly inhibited formation of osteoclastic cells in a dose-dependent manner (P < 0.01). (B) Murine splenocytes were stimulated with recombinant OPGL (30 ng/ml) or P. gingivalis (105/ml). Cells stimulated with recombinant OPGL were incubated in standard medium and cells incubated with P. gingivalis were incubated in culture medium not supplemented with M-CSF. Antibody to murine OPGL or matched control IgG was added during the entire time of the culture as indicated. Cells were fixed, assayed for TRAP activity, and counted on day 11 as described in the legend to Fig. 3. Student's t test demonstrated that antibody to OPGL (200 ng/ml) significantly decreased either OPGL- or P. gingivalis-induced osteoclastic cell formation compared to that in their respective controls (P < 0.05). The experiments were carried out three times with similar results.
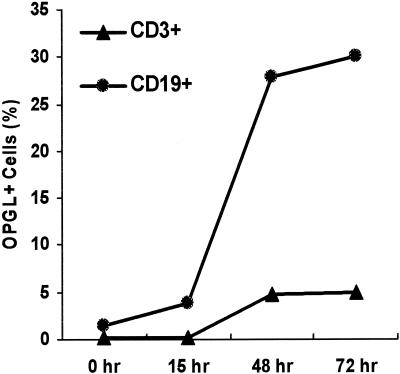
P. gingivalis stimulates mononuclear cells to express OPGL in vitro.
In order to identify the cell source of OPGL in the experimental system, we carried out double-immunofluorescence flow cytometry experiments using antibody to OPGL with antibody to either CD19 or CD3. In time course experiments, P. gingivalis maximally stimulated expression by B and T lymphocytes at 48 to 72 h (Fig. 8). In P. gingivalis-stimulated cells, approximately 30% were double positive for OPGL and CD19 expression, while only 5% were double positive for OPGL and CD3 (Table 1). The low contribution of CD3 T lymphocytes was due to the lower numbers present in the total cell population (Table 1). Previous investigators have shown that concanavalin A (ConA) induces OPGL expression in T lymphocytes (10). For comparison, we also examined OPGL expression by B and T lymphocytes when stimulated by ConA. ConA induced proliferation of the T lymphocytes so that they increased from 12.4 to 42.6% of the total cell population, a percentage that now was similar to that for B lymphocytes. Even under these conditions, B lymphocytes were the predominant contributor to the OPGL-positive cell population.
FIG. 8.
Time course of OPGL expression by B and T lymphocytes. Murine splenocytes were stimulated with P. gingivalis for the indicated time and double immunostained with antibodies to OPGL and CD3 or CD19 to identify T and B lymphocytes, respectively. Immunofluorescent flow cytometry was carried out as described in Materials and Methods. The assay was repeated three times with similar results.
TABLE 1.
B and T lymphocytes express OPGLa
| Stimulus | No. of cells OPGL+ (% of total)
|
No. of cells with marker (% of total)
|
||
|---|---|---|---|---|
| CD19+ | CD3+ | CD19 | CD3 | |
| None | 6.9 | 2.4 | 77.7 | 12.4 |
| P. gingivalis | 30.4 | 5.4 | 79.8 | 12.8 |
| ConA | 23.2 | 12.2 | 43.7 | 42.6 |
Murine splenocytes were stimulated with P. gingivalis for 72 h and double immunostained with antibodies to OPGL and CD3 or CD19 to identify T and B lymphocytes, respectively. Immunofluorescent flow cytometry was carried out as described in Materials and Methods. The assay was performed three times with similar results.
Bacterial infection can induce OPGL production by infiltrating mononuclear cells in vivo.
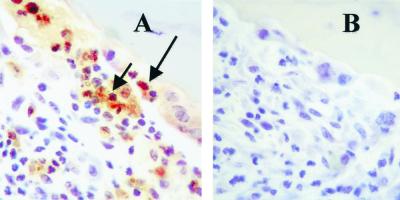
We have established that bacteria can induce OPGL expression and formation of osteoclastic cells from leukocytes in vitro and that OPGL plays an important role in this process. Moreover, we have recently demonstrated that Porphyromonas endodontalis, another Porphyromonas species found in oral bone lesions, when injected into the scalp of mice induces osteoclastogenesis in vivo (12). Figure 9 establishes that P. gingivalis induces OPGL expression by inflammatory cells in the same animal model. Seven days after injection of bacteria, a large number of inflammatory cells were recruited to the injection site, some of which were OPGL positive and in close proximity to bone (Fig. 9A). These were mononuclear in appearance. In addition, OPGL-positive bone-lining osteoblastic cells were also present. Tissue sections incubated with control IgG (Fig. 9B) were negative. In addition, tissue sections from PBS-injected animals had no bone resorption or positive immunostaining when incubated with OPGL antibody (data not shown). Thus, bacteria can induce OPGL in mononuclear cells, which could contribute to bone loss associated with chronic inflammation.
FIG. 9.
Bacterial infection can induce OPGL production by infiltrating mononuclear cells in vivo. P. gingivalis (5 × 108 cells) was injected into the mouse scalp. Mice were sacrificed 7 days after injection. The calvarial samples with attached scalp were harvested and prepared for immunohistochemistry staining as described in Materials and Methods. The tissue sections were immunostained with antibodies to OPGL (A) or matched control IgG (B). Photomicrograph magnification, ×50. The long arrow indicates an OPGL-positive bone-lining cell, and the short arrow indicates an OPGL-positive inflammatory cell. The experiment was carried out twice with similar results.
DISCUSSION
Until recently it was thought that bacteria or their products cause bone resorption via a pathway that requires the participation of osteoblasts or other bone-lining cells (21). Our results suggest that there is another pathway by which bacteria may stimulate osteolysis that is independent of osteoblasts. This conclusion is based upon evidence that bacteria or LPS directly stimulate formation of TRAP-positive multinucleated cells that express RANK. In addition, these cells also formed resorption pits in vitro on bone slices (data not shown).
Under the same conditions, the calciotropic hormone PTH had no osteoclast-promoting activity. This result is consistent with previous reports that hormones such as PTH require the participation of osteoblastic cells to induce osteoclastogenesis (17). Thus, the specific mediators and cell types that participate in bone resorption may depend upon the nature of the stimulus. Furthermore, inflammatory bone resorption may include both osteoblastic and nonosteoblastic pathways, which may interact and lead to vigorous stimulation of osteoclastogenesis. This may explain the fact that relatively large numbers of osteoclasts may be present at sites of inflammatory bone resorption.
The importance of either TNF-α or OPGL in bone resorption has been established in a different experimental system (13). Data presented here demonstrate that either TNF-α or OPGL plays a significant role in bacterium-induced osteoclast-like cell formation, with OPGL playing a relatively more prominent role. At maximum concentrations, TNF-α antibody blocked approximately 40 to 60% of bacterium-induced osteoclastogenic activity, while OPGL antibody inhibited it by 60 to 70%. Adding both antibodies to the culture did not consistently block the formation of osteoclastic cells to a greater extent than OPGL antibody alone (data not shown). It is possible that the residual osteoclastogenic activity that could not be blocked by antibody to OPGL or TNF is due to the production of other mediators. Alternatively, it could result from a direct effect of bacteria or LPS on formation of osteoclast-like cells. For example, the activation of toll-like receptors by LPS could potentially enhance formation of osteoclast-like cells (11).
Activated T lymphocytes have been shown to express OPGL and contribute significantly to the regulation of systemic and local bone loss (14, 15). The results extend these findings to indicate that under bacterial stimulation OPGL is also expressed by B lymphocytes. It should be noted that our in vitro assay system examined short-term responses, which would favor stimulation of B cells. However, when ConA was used as a stimulus, B lymphocytes still contributed more to OPGL-positive cell populations than did T lymphocytes. Thus, both B lymphocytes and T lymphocytes can contribute to OPGL expression.
Bacteria or their products such as LPS bind to receptors on monocytes or B lymphocytes and stimulate a number of inflammatory mediators. The production of these factors may enhance the capacity of leukocyte-produced OPGL to induce osteolysis. In contrast, calciotropic hormones stimulate osteolysis by binding to cognate receptors on osteoblasts rather than mononuclear cells. Although both processes appear to ultimately stimulate formation of osteoclast-like cells through the production of OPGL, the cascade of mediators as well as the cell types that participate may be different. Thus, under bacterial stimulation both osteoblast-dependent and -independent pathways may be simultaneously induced, while with osteotrophic hormones only osteoblast-dependent pathways may be operative.
Acknowledgments
The study was supported by the Joan and Herbert Schilder Endodontic Research Fund and the American Association of Endodontists Foundation.
We thank Joseph Lorenzo and Philip Osdoby for helpful discussions and Renee Anderson for assistance with the in vivo immunohistochemistry experiments.
Editor: R. N. Moore
REFERENCES
- 1.Abu-Amer, Y., F. Ross, J. Edwards, and S. Teitelbaum. 1997. Lipopolysaccharide-stimulated osteoclastogenesis is mediated by tumor necrosis factor via its P55 receptor. J. Clin. Investig. 100:1557-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ali, N., V. Gilston, and P. Winyard. 1999. Activation of NF-κB in human osteoblasts by stimulators of bone resorption. FEBS Lett. 460:315-320. [DOI] [PubMed] [Google Scholar]
- 3.Athanasou, N., and A. Sabokbar. 1999. Human osteoclast ontogeny and pathological bone resorption. Histol. Histopathol. 14:635-647. [DOI] [PubMed] [Google Scholar]
- 4.Bertolini, D., G. Nedwin, T. Bringman, and G. Mundy. 1986. Stimulation of bone resorption and inhibition of bone formation in vitro by human tumour necrosis factors. Nature 319:516-518. [DOI] [PubMed] [Google Scholar]
- 5.Burger, E., J. van der Meer, and P. Nijweide. 1984. Osteoclast formation from mononuclear phagocytes: role of bone-forming cells. J. Cell Biol. 99:1901-1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiang, C., G. Kyritsis, D. Graves, and S. Amar. 1999. Interleukin-1 and tumor necrosis factor activities partially account for calvarial bone resorption induced by local injection of lipopolysaccharide. Infect. Immun. 67:4231-4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grey, A., M. Mitnick, U. Masiukiewicz, B. Sun, S. Rudikoff, R. Jilka, S. Manolagas, and K. Insogna. 1999. A role for interleukin-6 in parathyroid hormone-induced bone resorption in vivo. Endocrinology 140:4683-4690. [DOI] [PubMed] [Google Scholar]
- 8.Harris, W. 1995. The problem is osteolysis. Clin. Orthoped. 311:46-53. [PubMed] [Google Scholar]
- 9.Helfrich, M., and R. Mieremet. 1988. A morphologic study of osteoclasts isolated from osteopetrotic microphthalmic (mi/mi) mouse and human fetal long bones using an instrument permitting combination of light and scanning electron microscopy. Bone 9:113-119. [DOI] [PubMed] [Google Scholar]
- 10.Horwood, N., V. Kartsogiannis, J. Quinn, E. Romas, T. Martin, and M. Gillespie. 1999. Activated T lymphocytes support osteoclast formation in vitro. Biochem. Biophys. Res. Commun. 265:144-150. [DOI] [PubMed] [Google Scholar]
- 11.Hou, L., H. Sasaki, and P. Stashenko. 2000. Toll-like receptor 4-deficient mice have reduced bone destruction following mixed anaerobic infection. Infect. Immun. 68:4681-4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang, Y., L. Magli, and M. Russo. 1999. Bacterium-dependent induction of cytokines in mononuclear cells and their pathologic consequences in vivo. Infect. Immun. 67:2125-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kobayashi, K., N. Takahashi, E. Jimi, N. Udagawa, M. Takami, S. Kotake, N. Nakagawa, M. Kinosaki, K. Yamaguchi, N. Shima, H. Yasuda, T. Morinaga, K. Higashio, T. Martin, and T. Suda. 2000. Tumor necrosis factor alpha stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction. J. Exp. Med. 191:275-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kong, Y., U. Feige, I. Sarosi, B. Bolon, A. Tafuri, S. Morony, C. Capparelli, J. Li, R. Elliott, S. McCabe, T. Wong, G. Campagnuolo, E. Moran, E. Bogoch, G. Van, L. Nguyen, P. Ohashi, D. Lacey, E. Fish, W. Boyle, and J. Penninger. 1999. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature 402:304-309. [DOI] [PubMed] [Google Scholar]
- 15.Kong, Y., H. Yoshida, I. Sarosi, H. Tan, E. Timms, C. Capparelli, S. Morony, A. Oliveira-dos-Santos, G. Van, A. Itie, W. Khoo, A. Wakeham, C. Dunstan, D. Lacey, T. Mak, W. Boyle, and J. Penninger. 1999. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 397:315-323. [DOI] [PubMed] [Google Scholar]
- 16.Lee, S., and J. Lorenzo. 1999. Parathyroid hormone stimulates TRANCE and inhibits osteoprotegerin messenger ribonucleic acid expression in murine bone marrow cultures: correlation with osteoclast-like cell formation. Endocrinology 140:3552-3561. [DOI] [PubMed] [Google Scholar]
- 17.McSheehy, P., and T. Chambers. 1986. Osteoblastic cells mediate osteoclastic responsiveness to parathyroid hormone. Endocrinology 118:824-828. [DOI] [PubMed] [Google Scholar]
- 18.Minkin, C. 1982. Tartrate-resistant acid phophatase as a marker of osteoclast function. Calcif. Tissue Int. 34:285-290. [DOI] [PubMed] [Google Scholar]
- 19.Moriyama, H., C. Huang, M. Abramson, and M. Kato. 1984. Bone resorption factors in chronic otitis media. Otolaryngol. Head Neck Surg. 92:322-328. [DOI] [PubMed] [Google Scholar]
- 20.Mundy, G., and G. Roodman. 1987. Bone and mineral research, p. 209-280. Elsevier, Amsterdam, The Netherlands.
- 21.Nair, S., S. Meghji, M. Wilson, K. Reddi, P. White, and B. Henderson. 1996. Bacterially induced bone destruction: mechanisms and misconceptions. Infect. Immun. 64:2371-2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parfitt, A. 1998. Osteoclast precursors as leukocytes: importance of the area code. Bone 23:491-494. [DOI] [PubMed] [Google Scholar]
- 23.Puzas, J., D. Hicks, S. Reynolds, and R. O'Keefe. 1994. Regulation of osteoclastic activity in infection. Methods Enzymol. 236:47-58. [DOI] [PubMed] [Google Scholar]
- 24.Roodman, G. 1999. Cell biology of the osteoclast. Exp. Hematol. 27:1229-1241. [DOI] [PubMed] [Google Scholar]
- 25.Simonet, W., D. Lacey, C. Dunstan, M. Kelley, M. Chang, R. Luthy, H. Nguyen, S. Wooden, L. Bennett, T. Boone, G. Shimamoto, M. DeRose, R. Elliott, A. Colombero, H. Tan, G. Trail, J. Sullivan, E. Davy, N. Bucay, L. Renshaw-Gegg, T. Hughes, D. Hill, W. Pattison, P. Campbell, and W. Boyle. 1997. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 89:309-310. [DOI] [PubMed] [Google Scholar]
- 26.Sundquist, K., M. Cecchini, and S. J. Marks. 1995. Colony-stimulating factor-1 injections improve but do not cure skeletal sclerosis in osteopetrotic (op) mice. Bone 16:39.. [PubMed] [Google Scholar]
- 27.Thomson, B., G. Mundy, and T. Chambers. 1987. Tumor necrosis factors alpha and beta induce osteoblastic cells to stimulate osteoclastic bone resorption. J. Immunol. 138:775-779. [PubMed] [Google Scholar]
- 28.Thomson, B., J. Saklatvala, and T. Chambers. 1986. Osteoblasts mediate interleukin-1 stimulation of bone resorption by rat osteoclasts. J. Exp. Med. 164:104-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yasuda, H., N. Shima, N. Nakagawa, K. Yamaguchi, M. Kinosaki, M. Goto, S. Mochizuki, E. Tsuda, T. Morinaga, N. Udagawa, N. Takahashi, T. Suda, and K. Higashio. 1999. A novel molecular mechanism modulating osteoclast differentiation and function. Bone 25:109-113. [DOI] [PubMed] [Google Scholar]
- 30.Yasuda, H., N. Shima, N. Nakagawa, K. Yamaguchi, M. Kinosaki, S. Mochizuki, A. Tomoyasu, K. Yano, M. Goto, A. Murakami, E. Tsuda, T. Morinaga, K. Higashio, N. Udagawa, N. Takahashi, and T. Suda. 1998. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc. Natl. Acad. Sci. USA 95:3597-3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zubery, Y., C. Dunstan, B. Story, L. Kesavalu, J. Ebersole, S. Holt, and B. Boyce. 1998. Bone resorption caused by three periodontal pathogens in vivo in mice is mediated in part by prostaglandin. Infect. Immun. 66:4158-4162. [DOI] [PMC free article] [PubMed] [Google Scholar]