Abstract
The 42-kDa carboxyl-terminal processing fragment of Plasmodium falciparum merozoite surface protein 1 (MSP-142) is an anti-erythrocytic stage malaria vaccine candidate. In this study, MSP-142 was expressed by using the Bombyx mori nuclear polyhedrosis virus-silkworm expression system, and the antigenicity and immmunogenicity of the recombinant protein, Bmp42, were evaluated. The average yield of Bmp42, as determined by a sandwich enzyme-linked immunosorbent assay (ELISA), was 379 μg/ml of infected silkworm hemolymph, which was >100-fold higher than the level attainable in cell culture medium. N-terminal amino acid sequencing revealed that Bmp42 was correctly processed in silkworm cells. Data from immunoblotting, as well as from the inhibition ELISA, suggested that the conformational B-cell epitopes of MSP-142 were recreated in Bmp42. Immunization of rabbits with Bmp42 in complete Freund's adjuvant generated high-titer antibody responses against the immunogen. Specificity analyses of the anti-Bmp42 antibodies using several recombinant MSP-119 proteins expressing variant and conserved B-cell epitopes suggested that the anti-Bmp42 antibodies recognized primarily conserved epitopes on MSP-119. Furthermore, the anti-Bmp42 antibodies were highly effective in inhibiting the in vitro growth of parasites carrying homologous or heterologous MSP-142. Our results demonstrated that the baculovirus-silkworm expression system could be employed to express biologically and immunologically active recombinant MSP-142 at elevated levels; thus, it is an attractive alternative for producing a protective MSP-142 vaccine for human use.
Malaria is the most prevalent vector-borne disease worldwide and is the third leading cause of death from infectious disease (57). Over 40% of the world's population lives in areas at risk of malaria. Some 1.5 to 2.7 million people die of malaria each year, and 300 to 500 million clinical cases are reported each year. It is now widely recognized that effective vaccines against malaria would significantly aid in the global control of the disease. Among a number of promising malaria vaccines, Plasmodium falciparum merozoite surface protein 1 (MSP-1) is a leading candidate for a human erythrocytic malaria vaccine. MSP-1 is synthesized during schizogony as a 195-kDa glycoprotein (19) and is proteolytically processed into fragments of 83, 30, 38, and 42 kDa, designated MSP-183, MSP-130, MSP-138, and MSP-142, respectively (20, 39). During erythrocytic invasion, MSP-142 is cleaved to yield 33- and 19-kDa fragments (MSP-133 and MSP-119, respectively) (4, 5). MSP-119, which contains two epidermal growth factor-like domains, remains anchored to the merozoite membrane and is carried into the invaded erythrocyte (3). The sequence of MSP-119 is highly conserved (29, 31, 32) and contains a series of cysteine residues that are evolutionarily conserved among different Plasmodium species (12).
Early studies have shown that immunization of Aotus monkeys with MSP-1 protects against malaria (50). Moreover, the carboxyl-terminal fragment of MSP-1 alone can induce immunity. Accordingly, vaccination of monkeys with recombinant MSP-142 or MSP-119 from P. falciparum or vaccination of mice with the recombinant 15-kDa carboxyl-terminal fragment of Plasmodium yoelii MSP-1 protects the animals from lethal malarial infections (9, 11, 34). Protective immunity induced by MSP-1-based polypeptides is thought to be primarily antibody dependent, as monoclonal antibodies against MSP-1, MSP-142, or MSP-119 and antibodies from Aotus monkeys protected by vaccination with MSP-1 or MSP-142 can inhibit parasite invasion and growth in vitro (3, 9, 24, 45). Human antibody responses to MSP-119 also correlate well with clinical immunity to P. falciparum (2, 15, 48). Although MSP-119 is the target of protective antibodies, studies with mice and with blood lymphocytes isolated from people living in areas where malaria is endemic have shown that MSP-119 lacks sufficient T-cell epitopes to elicit a universal response in genetically diverse populations (1, 56). Additional T-cell epitopes from the N-terminal region of MSP-142 seem to be more efficient or efficacious in inducing protection (9, 14, 27).
A variety of expression systems have been explored for production of MSP-1-based recombinant vaccines, and these systems include bacterial, yeast, and baculovirus hosts (8, 22, 33, 37, 42, 51). A common challenge is to produce a correctly folded polypeptide with a reasonably high yield. Previous studies have suggested that correct folding of MSP-1 is critical to its immunogenicity (22, 37) and to the production of parasite-inhibiting antibodies (8, 38). MSP-1-based recombinant proteins prepared in bacterial expression systems (16, 17, 22) and MSP-1-derived synthetic peptides (10, 44) are less effective in inducing immunity. The reduced effectiveness may result from the inability of proper folding to produce a suitable conformation necessary to induce protective immunity. The yeast expression system has been used to produce a correctly folded MSP-119 (33), which when fused with a TT universal T-cell epitope, P30P2, induced protective immunity in monkeys (34, 35) and parasite-inhibiting antibodies in rabbits (51). However, expression of MSP-142 in the same yeast system yielded antigenically and immunologically poor recombinant proteins (8). An antigenically and immunologically active MSP-142 was successfully produced by using the baculovirus-insect cell culture system (8, 42, 51). The MSP-142 produced in this fashion can induce parasite-inhibiting antibodies in rabbits (8) and/or protective immunity in monkeys (9, 51). Nevertheless, the use of baculovirus to produce MSP-142 is not without shortcomings. Expression of recombinant proteins using the prototypic baculovirus Autographa californica nuclear polyhedrosis virus relies on infecting cultured insect cells, which is costly in large-scale production. Meanwhile, the expression level is less than 10 mg/liter of cell culture medium. To deal with these shortcomings, we chose to express MSP-142 in silkworm larvae by using the silkworm-specific baculovirus Bombyx mori nuclear polyhedrosis virus (BmNPV). By using this in vivo expression system, a number of recombinant proteins of pharmaceutical and agricultural importance, including human interferons (13, 40), human growth hormone (30, 52), human macrophage colony-stimulating factor (46), human granulocyte-macrophage colony-stimulating factor (47), viral proteins (54, 58), and grass carp growth hormone (18), have been successfully expressed with biological activities comparable to those of the native counterparts. The expression levels of these recombinant proteins vary, but up to 13 mg/larva has been reported (43). Thus, gram quantities of recombinant proteins can potentially be obtained with small-scale rearing of silkworm larvae. Because of the availability of silkworm larva production in southern People's Republic of China and the enhanced expression level in vivo, use of the BmNPV-silkworm expression system to produce MSP-142-based vaccines is an attractive option.
MATERIALS AND METHODS
Cloning of P. falciparum MSP-142.
The DNA fragment encoding MSP-142 of the P. falciparum 3D7 isolate (from Ala1333 to Ser1705) was amplified by PCR from the transfer vector pMbac-MSP-142 (F. Alonso-Caplen, personal communication). Forward primer 42k-F-SmaIN (5′ TAG GCC CCC GGG ATG AAA TTC TTA GTC AAC GTT GCC 3′) was designed to include the honeybee melittin signal sequence on pMbac in the final MSP-142 construct; an end clamp sequence (TAG GCC) was introduced into the 5′ end of the primer to facilitate restriction enzyme digestion of the PCR product, and an SmaI site (underlined) was included following the end clamp. By the same strategy, reverse primer 42k-R-XbaIN (5′ TAG GCC CCT CTA GAT TAG GAA CTG CAG AAA ATA 3′) was designed to prime the 3′ end of MSP-142; an XbaI site (underlined) was included following the end clamp. PCR products were purified by using GeneClean (Bio 101, Inc., Vista, Calif.).
The PCR-amplified MSP-142 DNA was ligated into the BmNPV-based transfer vector pBM030 (23, 41) following SmaI/XbaI digestion (Pharmacia Biotech, Uppsala, Sweden). The ligation product was electroporated into electrocompetent Escherichia coli DH10B (Gibco BRL, Gaithersburg, Md.). The orientation of the MSP-142 reading frame in the resulting plasmid, pBM030-MSP-142, was confirmed by restriction mapping analyses and DNA sequencing.
Insect cell transfection and construction of recombinant BmNPV.
Genomic BmNPV DNA was prepared from the hemolymph of silkworm larvae previously inoculated with the wild-type virus (41). Two micrograms of viral DNA and 4 μg of pBM030-MSP-142 were cotransfected into BmN cells by using LipofectAMINE reagent (Gibco BRL), and the transfected cells were incubated at 27°C until occlusion bodies were observable. The culture medium was used as the primary stock for screening recombinant viruses. Recombinant BmNPV carrying the MSP-142 DNA was isolated by plaque assays (53). Viral plaques lifted onto ZetaProbe membranes (Bio-Rad, Hercules, Calif.) were screened with the 32P-labeled MSP-142 DNA probe for the presence of recombinant viruses. Trapped viruses from MSP-142-positive plaques were used for subsequent rounds of the screening procedure. The recombinant virus preparation was considered to be pure when no occlusion bodies could be observed after a prolonged period of infection of a BmN cell culture.
Expression and purification of recombinant MSP-142 (Bmp42) from silkworm hemolymph.
Early-fifth-instar silkworm larvae (body weight, 2.5 to 4.0 g) were used for infection. After each larva was anesthetized on ice for 20 min, approximately 3.6 × 105 PFU of recombinant virus was injected longitudinally underneath the dorsal cuticle with a short-needle (29-gauge) syringe. After 6 to 7 days of infection, hemolymph samples were harvested in the presence of 5 to 10 mM dithiothreitol. The Bmp42 was purified by immunoaffinity chromatography using the MSP-1-specific monoclonal antibody MAb5.2. This antibody was produced against purified parasite MSP1 (49), and it is specific for conserved epitopes within the C-terminal 19-kDa fragment of MSP-1 or MSP-119 (33). Furthermore, it recognizes the disulfide-dependent conformational epitopes on MSP-119 (7). The protein concentrations of the eluted fractions were determined by a bicinchoninic acid assay (Pierce, Rockford, Ill.), and the purity of the isolated Bmp42 was examined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and silver staining.
Immunoblotting.
Purified Bmp42 fractions were separated by SDS-PAGE (36) in the presence or absence of β-mercaptoethanol and were electrophoretically transferred to a polyvinylidene difluoride membrane. The blotted membrane was blocked with 5% nonfat powdered milk in 0.05% Tween 20-phosphate-buffered saline (PBSTM). The membrane was then incubated with 20 μg of MAb5.2 in 10 ml of PBSTM at room temperature for 1 h. After it was washed with 0.05% Tween 20-phosphate-buffered saline (PBST), the membrane was incubated for 1 h in PBSTM containing alkaline phosphatase-conjugated goat anti-mouse immunoglobulin G (IgG) (H and L chain specific; Bio-Rad) at a 1:5,000 dilution in PBSTM. The membrane was washed with PBST, and reactive protein bands were visualized by incubation with the enzyme substrate nitroblue tetrazolium-5-bromo-4-chloro-3-indolylphosphate (Sigma, St. Louis, Mo.).
N-terminal amino acid sequencing.
Purified Bmp42 was first electrophoretically transferred to a polyvinylidene difluoride membrane. The bound polypeptide was sequenced by automatic Edman sequencing chemistry with a Hewlett-Packard HP G1005A protein sequencing system. The amino acid sequence was determined by calibrating with phenylthiohydantoin-amino acid standards.
Rabbit immunization.
Four New Zealand White rabbits (rabbits 7857, 7858, 7859, and 7860) were used. A total of four immunizations were given intramuscularly at 3-week intervals. Each injection consisted of 100 μg of Bmp42 in complete Freund's adjuvant (CFA) (Sigma). The amount of mycobacterium in CFA was successively halved in subsequent immunizations. Serum samples were collected 1 week before immunization (preimmune controls) and 18 to 21 days after each immunization.
ELISA.
Rabbit serum antibodies were assayed for binding to Bmp42 or yeast-expressed recombinant MSP-119 proteins (rMSP-119s) (33) by using an enzyme-linked immunosorbent assay (ELISA) as described previously (7). Briefly, vinyl plates were coated with 0.08 μg of antigen per ml, washed with BBS (167 mM borate, 134 mM NaCl; pH 8.0), and blocked with 1% bovine serum albumin (BSA) in BBS. Rabbit sera were serially diluted in 1% BSA-BBS and added to antigen-coated wells for incubation at room temperature for 1 h. The plates were washed with 0.5 M NaCl in BBS, an appropriate dilution of peroxidase-conjugated goat anti-rabbit IgG (H and L chain specific; Cappel, Durham, N.C.) was added, and then the preparations were similarly incubated for 1 h. The plates were then washed with 0.5 M NaCl-BBS and finally with BBS. A peroxidase substrate solution [H2O2 and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonate)] was added, and the optical density at 410 nm (OD410) was determined with a Dynatech 605 ELISA reader. The end point ELISA titer was the serum dilution that produced an OD410 of 0.2, which was >4 standard deviations above the background absorbance values.
Inhibition ELISA.
Monoclonal antibody MAb5.2 and polyclonal anti-MSP-1 antibodies (obtained previously from rabbits K103 and K104 immunized with purified parasite MSP-1) (8) were diluted to a point on the descending portion of the ELISA titration curve against coated rMSP-119. The diluted antibodies were separately mixed with various concentrations of inhibitor (soluble rMSP-119 or Bmp42), incubated for 1 h, and added to rMSP-119-coated plates for ELISA as described above.
Sandwich ELISA.
Plates were coated with MAb5.2 (50 ng/well), washed with PBSTM, and blocked with 1% BSA-PBS. Serially diluted purified Bmp42 (with the protein content quantified by a Bradford assay) or hemolymph samples were added to the wells and incubated at room temperature for 1 h. The plates were washed with PBST and incubated with appropriately diluted rabbit anti-Bmp42 antiserum at room temperature for 1 h. After the plates were washed with PBSTM, 1/2,000-diluted goat anti-rabbit IgG (heavy and light chain-specific)-horseradish peroxidase conjugate (enzyme immunoassay grade affinity purified; Bio-Rad) was added and similarly incubated. The plates were washed in PBSTM and finally in PBST. A peroxidase substrate solution was added, and the OD405 values were determined as described above. The amounts of Bmp42 in hemolymph samples were calculated from the OD405 values that were in the descending portion of the ELISA standard curve for the purified Bmp42.
In vitro parasite growth inhibition assay.
Preimmune and immune (tertiary and quaternary bleed) rabbit sera were evaluated for their ability to inhibit parasitic growth in vitro as described previously (25). Briefly, rabbit sera were heat inactivated at 58°C for 40 min and absorbed with fresh normal human erythrocytes. Parasite cultures (isolate 3D7 or FVO) were synchronized by sorbitol lysis to select for late trophozoite-schizont stages. Infected erythrocyte preparations were adjusted to give 0.1% parasitemia and 0.8% hematocrit by the addition of fresh erythrocytes. Rabbit preimmune or immune sera were added to infected erythrocyte cultures at a final concentration of 20%. Based on our past experience, decreasing the concentration of the antisera to less than 10% should have significantly lowered the inhibitory activity. The cultures were incubated in duplicate at 37°C in 2% O2-8% CO2-90% N2 for 72 h. Samples were pooled, and thin blood smears were prepared. The percentage of parasitemia was determined microscopically. The degree of growth inhibition was calculated as follows: percent inhibition = {[(P − O) − (I − O)]/(P − O)} × 100, where P is the parasitemia at 72 h for cultures incubated in preimmune sera, I is the parasitemia at 72 h for cultures incubated in immune sera, and O is the initial parasitemia at zero time.
RESULTS
Expression of Bmp42 in silkworm larvae.
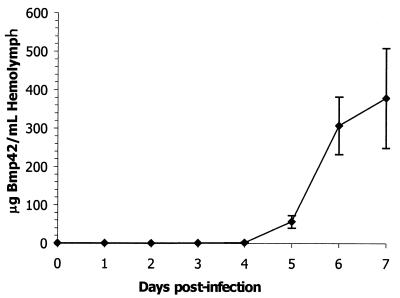
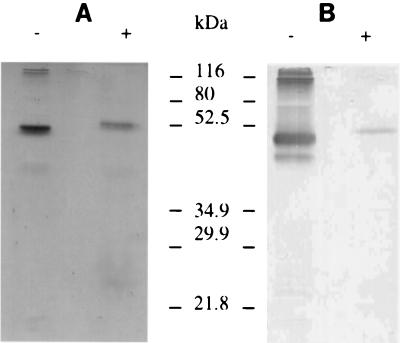
Bmp42 was expressed in vivo by infecting silkworm larvae with recombinant BmNPV harboring the MSP-142 DNA. To monitor expression of Bmp42, infected larvae were sacrificed daily over a period of 7 days, and the level of Bmp42 in the hemolymph was determined by a sandwich ELISA. Figure 1 shows the Bmp42 expression level over a 7-day period. Bmp42 was first detected on day 3, and the expression level dramatically increased from day 5 and reached a maximum on day 7. Based on these results, silkworm larvae were subsequently infected for 7 days and hemolymph samples were harvested and pooled for purification. Bmp42 was readily purified by immunoaffinity chromatography using MAb5.2 as the ligand (Fig. 2A). The recombinant protein migrated at a molecular mass of 48 kDa in SDS-PAGE gels under nonreducing conditions. Immunoblotting of Bmp42 using MAb5.2 under nonreducing and reducing conditions is shown in Fig. 2B. The immunoreactivity of the reduced Bmp42 was dramatically diminished, and there was a concomitant decrease in mobility, suggesting that disulfide bonds were present in the nonreduced protein.
FIG. 1.
In vivo expression of Bmp42. The expression of Bmp42 in silkworm hemolymph was monitored by a sandwich ELISA for a total of 7 days. The value for each time point is the average for the hemolymph samples from three separately infected larvae.
FIG. 2.
Analysis of Bmp42 purified by immunoaffinity chromatography: silver staining (A) and immunoblotting (B) of purified Bmp42 electrophoresed in an SDS-15% polyacrylamide gel in the absence (−) or presence (+) of β-mercaptoethanol. MAb5.2 was used for immunoblotting.
N-terminal amino acid sequence analysis.
The N-terminal amino acid sequence of the purified Bmp42 was determined to be D*-P*-S*-P*-M-A-I-S-V-T-M…, indicating that the melittin signal sequence was cleaved at the expected site; due to introduction of the signal sequence, the N terminus of the recombinant Bmp42 has four additional amino acid residues (marked by asterisks) derived from this peptide.
Antigenic analysis.
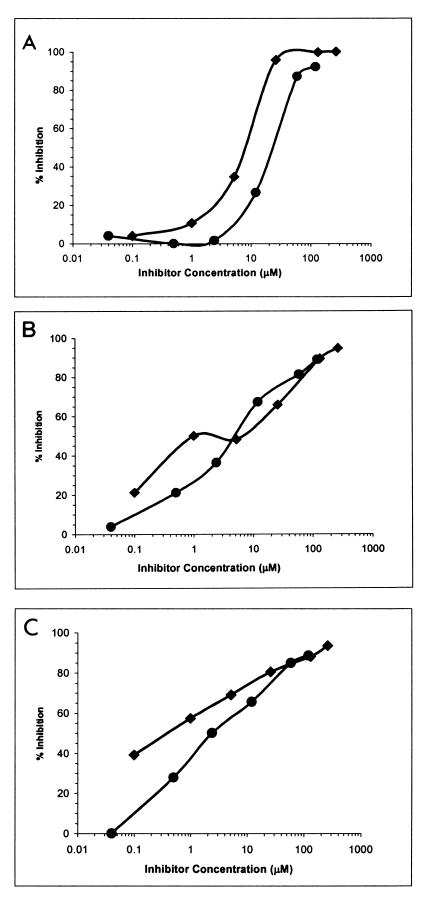
The antigenicity of Bmp42 was evaluated in an inhibition ELISA by using monoclonal (MAb5.2) and polyclonal (rabbit anti-MSP-1) antibodies that are specific for the carboxyl terminus of native MSP-1. A correctly folded rMSP-119 (33) was used as a standard for comparison to Bmp42. Binding of the antibodies to immobilized rMSP-119 was performed in the presence of various concentrations of the competitors, soluble rMSP-119 and Bmp42 (Fig. 3). In the case of MAb5.2 (Fig. 3A), the antigen concentrations required to achieve 50% inhibition in the ELISA were in similar ranges for rMSP-119 (∼8 μM) and Bmp42 (∼24 μM), differing by less than 1 order of magnitude. The maximum levels of inhibition were also similar for the two inhibitors. For the two rabbit anti-MSP-1 serum samples, K103 and K104 (8), the extents of competition were also similar when either rMSP-119 or Bmp42 was used (Fig. 3B and C). The results indicate that the three antibody preparations recognized rMSP-119 and Bmp42 equally well. Since the conformation of rMSP-119 has been shown to approximate the native conformation of MSP-1 (33), the similarity in the reactivities of Bmp42 and rMSP-119 suggests that Bmp42 may be antigenically similar to MSP-1.
FIG. 3.
Inhibition ELISA. Binding of MAb5.2 (A), anti-MSP-1 antibody from rabbit K103 (B), and anti-MSP-1 antibody from rabbit K104 (C) to coated rMSP-119 was studied in the presence of soluble rMSP-119 (⧫) or Bmp42 (•) as the inhibitor. The following concentrations of antibody and antisera were used: MAb5.2, 0.15 μg/ml; K103, 1/12,500; and K104, 1/25,000.
Immunogenicity of Bmp42.
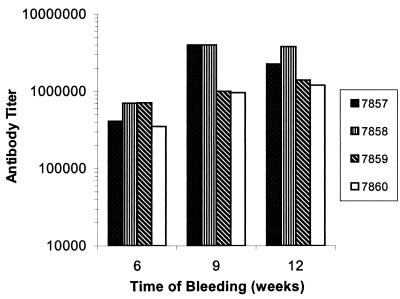
New Zealand White rabbits were hyperimmunized with Bmp42 in CFA, and secondary, tertiary, and quaternary sera (collected 6, 9, and 12 weeks after the first immunization, respectively) were assayed for anti-Bmp42 antibody titers. As depicted in Fig. 4, high anti-Bmp42 antibody titers (average, >1/300,000) were detected in all secondary sera, indicating that the Bmp42 was highly immunogenic. Results obtained with tertiary and quaternary sera generally showed an increase in the titer response. In all rabbits, a titer of 1/1,000,000 or more could be achieved and maintained in both the tertiary and quaternary bleeds. These results clearly show that the Bmp42 expressed in silkworms was highly effective in inducing a specific antibody response.
FIG. 4.
End point titers of rabbit anti-Bmp42 antibodies. Rabbits 7857, 7858, 7859, and 7860 were immunized with 100 μg of Bmp42 in CFA per dose, and the antibody titers were assayed at different time points. The end point titer was set at an OD410 value of 0.2, which was more than 4 standard deviations above the background OD410 value. The titers of the tertiary bleeds (9 weeks) for rabbits 7857 and 7858 were actually >1/4,000,000.
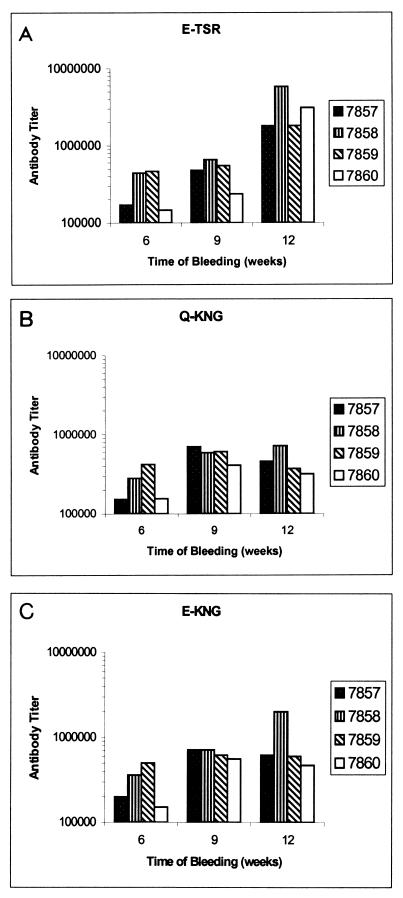
Specificity of the anti-Bmp42 antibody response.
The reactivities of the anti-Bmp42 sera towards conserved and variant epitopes of MSP-119 were studied by performing ELISAs (33). The end point ELISA titers of the rabbit antisera towards three rMSP-119s expressing the variant epitopes, E-TSR, E-KNG, and Q-KNG (6, 21, 32, 55), were determined. As shown in Fig. 5, the overall responses of the antisera to each rMSP-119 variant were similar, indicating that the majority of the anti-Bmp42 antibodies were targeted towards conserved B-cell epitopes of MSP-119 and that these epitopes were immunogenic. Interestingly, a higher antibody titer to the E-TSR variant, which is found in the Bmp42 sequence, was obtained only after repeated immunizations (Fig. 5A), suggesting that the variant epitopes were less immunogenic than a conserved determinant(s).
FIG. 5.
Specificity test for the rabbit anti-Bmp42 antibodies to different rMSP-119 variants. The anti-Bmp42 antibody responses against the E-TSR variant (A), the Q-KNG variant (B), and the E-KNG variant (C) are shown.
Inhibitory activity of anti-Bmp42 antibodies against homologous and heterologous malaria parasites.
The tertiary and quaternary sera were evaluated for inhibitory effects on the in vitro growth of homologous parasites. As shown in Table 1, significant levels of inhibition of growth were observed with the tertiary-bleed sera, with three of four sera having >70% growth inhibition. Further enhancement of parasite-inhibiting activities was observed after the quaternary immunization. An increase in inhibitory activity ranging from 89 to 96% was observed in all serum samples. These sera were also able to inhibit the growth of parasites carrying a heterologous MSP-142 (FVO isolate, Q-KNG variant) to the same extent as the sera carrying the homologous MSP-142 (Table 1).
TABLE 1.
In vitro parasitic growth inhibition assay performed with rabbit anti-Bmp42 seraa
| Rabbit | Titer | % Growth inhibition
|
|
|---|---|---|---|
| 3D7 strain | FVO strain | ||
| Tertiary bleed | |||
| 7857 | >1/4,000,000 | 81 | NT |
| 7858 | >1/4,000,000 | 92 | NT |
| 7859 | 1/1,000,000 | 73 | NT |
| 7860 | 1/960,000 | 53 | NT |
| Quaternary bleed | |||
| 7857 | 1/2,250,000 | 89 | 80 |
| 7858 | 1/3,800,000 | 96 | 91 |
| 7859 | 1/1,400,000 | 94 | 95 |
| 7860 | 1/1,200,000 | 90 | 84 |
Rabbit immune sera (heat inactivated) from the tertiary and quaternary bleeds were tested. Preimmune antibodies were also tested as a control. See Materials and Methods for details concerning the assay.
DISCUSSION
The baculovirus A. californica nuclear polyhedrosis virus has been employed to express recombinant MSP-142 proteins in cultured insect cells (8, 51). These proteins elicited parasite-inhibiting antibodies in vitro and protective immunity in vivo (8, 9, 26, 51). To further enhance the yield of the recombinant vaccine in a more cost-effective way, the BmNPV-silkworm expression system was evaluated in this study. The average maximal expression level of Bmp42 in vivo was 379 μg/ml of hemolymph, while in vitro expression in infected BmN cell cultures was only 2.4 to 3.5 μg/ml of culture medium. Thus, a >100-fold increase in expression level was achieved with silkworm larvae.
Our results clearly demonstrated that Bmp42 was antigenically similar to the native form. The presence of a reduction-sensitive disulfide-dependent conformational epitope(s) in Bmp42 was confirmed by immunoblot analyses with MAb5.2, which recognizes a disulfide-dependent conformational epitope in the carboxyl terminus of native MSP-1. This observation agrees with previous findings (8, 38) that the disulfide-dependent conformation is essential to the antigenicity of MSP-142. The antigenicity of Bmp42 was further examined with an inhibition ELISA. This assay has been employed to show that most, if not all, of the conformational B-cell epitopes of MSP-142 reside within the MSP-119 region (27, 33). Thus, a panel of correctly folded yeast-expressed rMSP-119s, all of which have been shown to efficiently compete with native MSP-1 for binding to polyclonal anti-MSP-1 antibodies in an ELISA (33), was used to evaluate the antigenicity of Bmp42. From our data, the similar extents of ELISA inhibition obtained with rMSP-119 and Bmp42 for the polyclonal anti-MSP-1 antibodies, as well as monoclonal antibody MAb5.2, indicate that these antibodies recognize common epitopes on both antigens. rMSP-119 and Bmp42 were therefore highly cross-reactive. As rMSP-119 closely mimics MSP-142 (33), our results suggest that the conformation of Bmp42 closely mimics the native form.
The effectiveness of Bmp42 as an anti-erythrocytic malaria vaccine was evaluated in immunogenicity studies, as well as in analyses of the specificity of anti-Bmp42 antibody responses. The rapid induction of extremely high anti-Bmp42 titers in immunized rabbits indicates that Bmp42 is highly immunogenic. Furthermore, the specificity of the anti-Bmp42 antibodies with respect to recognition of conserved versus variant epitopes on MSP-119 was investigated by using rMSP-119s expressing the E-TSR, E-KNG, and Q-KNG variants (6, 21, 29, 31, 32, 55). Rabbit anti-Bmp42 antibodies cross-reacted equally well with the variant forms of rMSP-119, suggesting that conserved B-cell epitopes on MSP-119 are immunodominant. While these results support the results of previous studies in which rabbits and Aotus monkeys were used (25-28), our findings shed new light on the immunogenicity of variant and conserved epitopes on MSP-119. As shown in Fig. 5, anti-variant epitope antibodies were prominent only after repeated hyperimmunizations. The data strongly suggest that the variant epitopes are much less immunogenic than the conserved determinants on MSP-119. Development of variant-specific antibodies was not observed in similar immunization studies of rabbits with a baculovirus-expressed MSP-142 or BVp42 (26, 33). It is possible that subtle antigenicity differences between BVp42 and Bmp42 may account for further diminishment of the immunogenicity of the variant epitopes on BVp42.
In contrast to immunizations with the rMSP-142s, immunizations of rabbits with a yeast-expressed MSP-119, P30P2MSP-119, in CFA has been shown to induce variant-specific antibodies that can dominate the anti-MSP-119 antibody response (51; G. Hui, A. Stower, and D. Kaslow, unpublished data). Thus, variant-specific epitopes are not inherently less immunogenic than conserved determinants. Rather, the vaccine constructs themselves may influence the relative immunogenicity of these epitopes. Conserved B epitopes within MSP-119 may be rendered more immunogenic when T helper cells specific for T epitopes within the N-terminal region of MSP-142 provide specific help for the corresponding B cells. On the other hand, inclusion of the tetanus toxoid T epitopes (P30P2) in the P30P2MSP-119 construct was not able to focus the development of antibody responses on the conserved determinants. Thus, besides providing additional T-cell help for broader induction of immunity in genetically diverse populations, the ability to consistently induce strong antibody responses to conserved regions of MSP-119 by rMSP-142 antigens may be another key advantage of MSP-142-based vaccines over the minimal MSP-119 constructs.
Previous studies with Aotus monkeys showed that the presence of parasite-inhibiting activity in antisera against MSP-1 and BVp42 correlates with protection against infection with P. falciparum (9, 50). Induction of parasite-inhibiting antibodies may therefore be a crucial factor in protection against erythrocytic malaria and may provide an indirect measurement of the protective efficacy of an anti-erythrocytic malaria vaccine. In this study, the ability of the anti-Bmp42 antibodies to inhibit parasite proliferation was demonstrated in an in vitro parasitic growth inhibition assay. Moreover, these antibodies can inhibit parasites (FVO strain) carrying heterologous MSP-142. The MSP-142 (FVO) allele is the opposite allele of MSP-142 (3D7) and carries the variant sequence Q-KNG, compared to the E-TSR variant (3D7) (25, 33). An important observation is that the anti-Bmp42 antibodies (quaternary bleed) strongly inhibited heterologous parasites despite the presence of increased levels of anti-variant antibodies to MSP-119. This not only supports the previous finding (26) that antibodies against conserved regions of MSP-142 inhibit parasites but also strongly suggests that variant-specific antibodies do not interfere with the biological activities of the inhibitory anti-Bmp42 antibodies. This has important implications for the deployment of MSP-142- or MSP-119-based vaccines in populations exposed to malaria since preexisting antibodies may contain significant levels of anti-variant antibodies.
In conclusion, an antigenically and immunologically active recombinant MSP-142 was produced by using the BmNPV-silkworm expression system. The rapid induction of highly cross-reactive parasite-inhibiting antibodies after immunization and the subsequent increase in the parasite-inhibiting activity after further immunizations clearly demonstrated the effectiveness of Bmp42 as an anti-erythrocytic malaria vaccine. By expressing MSP-142 in silkworm larvae, the yield of the protein was dramatically improved. Taken altogether, our data strongly support further development of the BmNPV-silkworm system to produce MSP-142 for use in vaccination studies with monkeys and humans.
Acknowledgments
We thank Susumu Maeda for providing BmNPV (T3 isolate), transfer vector pBM030, and the BmN cell line and David Kaslow for providing the rMSP-119s. Also, the kind gift of transfer vector pMbac-MSP-142 from F. Alonso-Caplen of The Salk Institute is highly appreciated.
This work was supported by grant AF/256/96 from the Industry and Technology Development Council, Hong Kong Government; by a direct grant from the Chinese University of Hong Kong, Hong Kong; and by a grant from the Queen Emma Foundation, Honolulu, Hawaii.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Ahlborg, N., I. T. Ling, A. A. Holder, and E. M. Riley. 2000. Linkage of exogenous T-cell epitopes to the 19-kilodalton region of Plasmodium yoelii merozoite surface protein 1 (MSP119) can enhance protective immunity against malaria and modulate the immunoglobulin subclass response to MSP119. Infect. Immun. 68:2102-2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Yaman, F., B. Genton, K. J. Kramer, S. P. Chang, G. S. N. Hui, M. Baisor, and M. P. Alpers. 1996. Assessment of the role of naturally acquired antibody levels to Plasmodium falciparum merozoite surface protein-1 in protecting Papua New Guinean children from malaria morbidity. Am. J. Trop. Med. Hyg. 54:443-448. [DOI] [PubMed] [Google Scholar]
- 3.Blackman, M. J., H.-G. Heidrich, S. Donachie, J. S. McBride, and A. A. Holder. 1990. A single fragment of a malaria merozoite surface protein remains on the parasite during red cell invasion and is the target of invasion-inhibiting antibodies. J. Exp. Med. 172:379-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blackman, M. J., I. T. Ling, S. C. Nicholls, and A. A. Holder. 1991. Proteolytic processing of the Plasmodium falciparum merozoite surface protein-1 produces a membrane-bound fragment containing two epidermal growth factor-like domains. Mol. Biochem. Parasitol. 49:29-34. [DOI] [PubMed] [Google Scholar]
- 5.Blackman, M. J., H. Whittle, and A. A. Holder. 1991. Processing of the Plasmodium falciparum merozoite surface protein-1: identification of a 33-kilodalton secondary processing product which is shed prior to erythrocyte invasion. Mol. Biochem. Parasitol. 49:35-44. [DOI] [PubMed] [Google Scholar]
- 6.Chang, S. P., K. J. Kramer, K. M. Yamaga, A. Kato, S. E. Case, and W. A. Siddiqui. 1988. Plasmodium falciparum: gene structure and hydropathy profile of the major merozoite surface antigen (gp195) of the Uganda-Palo Alto isolate. Exp. Parasitol. 67:1-11. [DOI] [PubMed] [Google Scholar]
- 7.Chang, S. P., G. S. N. Hui, A. Kato, and W. A. Siddiqui. 1989. Generalized immunological recognition of the major merozoite surface antigen (gp195) of Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 86:6343-6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang, S. P., H. L. Gibson, C. T. Lee-Ng, P. J. Barr, and G. S. N. Hui. 1992. A carboxyl-terminal fragment of Plasmodium falciparum gp195 expressed by a recombinant baculovirus induces antibodies that completely inhibit parasite growth. J. Immunol. 149:548-555. [PubMed] [Google Scholar]
- 9.Chang, S. P., S. E. Case, W. L. Gosnell, A. Hashimoto, K. J. Kramer, L. Q. Tam, C. Q. Hashiro, C. M. Nikaido, H. L. Gibson, C. T. Lee-Ng, P. J. Barr, B. T. Yokota, and G. S. N. Hui. 1996. A recombinant baculovirus 42-kilodalton C-terminal fragment of Plasmodium falciparum merozoite surface protein 1 protects Aotus monkeys against malaria. Infect. Immun. 64:253-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheung, A., J. Leban, A. R. Shaw, B. Merkli, J. Stocker, C. Chizzolini, C. Sander, and L. H. Perrin. 1986. Immunization with synthetic peptides of a Plasmodium falciparum surface antigen induces antimerozoite antibodies. Proc. Natl. Acad. Sci. USA 83:8328-8332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daly, T. M., and C. A. Long. 1993. A recombinant 15-kilodalton carboxyl-terminal fragment of Plasmodium yoelii yoelii 17XL merozoite surface protein 1 induces a protective immune response in mice. Infect. Immun. 61:2462-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Del Portillo, H. A., S. Longacre, E. Khouri, and P. H. David. 1991. Primary structure of the merozoite surface antigen 1 of Plasmodium vivax reveals sequences conserved between different Plasmodium species. Proc. Natl. Acad. Sci. USA 88:4030-4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deng, J., S. Wang, Q. Yang, X. Cheng, and L. Li. 1995. High-level expression of human beta-interferon gene in the silkworm with new constructed BmNPV vector. Chin. J. Biotechnol. 11:109-117. [PubMed] [Google Scholar]
- 14.Egan, A., M. Waterfall, M. Pinder, A. A. Holder, and E. Riley. 1997. Characterization of human T- and B-cell epitopes in the C terminus of Plasmodium falciparum merozoite surface protein 1: evidence for poor T-cell recognition of polypeptides with numerous disulfide bonds. Infect. Immun. 65:3024-3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Egan, A. F., J. A. Chappel, P. A. Burghaus, J. S. Morris, J. S. McBride, A. A. Holder, D. C. Kaslow, and E. M. Riley. 1995. Serum antibodies from malaria-exposed people recognize conserved epitopes formed by the two epidermal growth factor motifs of MSP119, the carboxyl-terminal fragment of the major merozoite surface protein of Plasmodium falciparum. Infect. Immun. 63:456-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Etlinger, H. M., P. Caspers, H. Matile, H.-J. Schoenfeld, D. Stueber, and B. Takacs. 1991. Ability of recombinant or native proteins to protect monkeys against heterologous challenge with Plasmodium falciparum. Infect. Immun. 59:3498-3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herrera, S., M. A. Herrera, B. L. Perlaza, Y. Burki, P. Caspers, H. Döbeli, D. Rotmann, and U. Certa. 1990. Immunization of Aotus monkeys with Plasmodium falciparum blood-stage recombinant proteins. Proc. Natl. Acad. Sci. USA 87:4017-4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ho, W. K. K., Z. Q. Meng, H. R. Lin, C. T. Poon, Y. K. Leung, K. T. Yan, N. Dias, A. P. K. Che, J. Liu, W. M. Zheng, Y. Sun, and A. O. L. Wong. 1998. Expression of grass carp growth hormone by baculovirus in silkworm larvae. Biochim. Biophys. Acta 1381:331-339. [DOI] [PubMed] [Google Scholar]
- 19.Holder, A. A., and R. R. Freeman. 1982. Biosynthesis and processing of a Plasmodium falciparum schizont antigen recognized by immune serum and a monoclonal antibody. J. Exp. Med. 156:1528-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holder, A. A., and R. R. Freeman. 1984. The three major antigens on the surface of Plasmodium falciparum merozoites are derived from a single high molecular weight precursor. J. Exp. Med. 160:624-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holder, A. A., M. J. Lockyer, K. G. Odink, J. S. Sandhu, V. Riveros-Moreno, S. C. Nicholls, Y. Hillman, L. S. Davey, M. L. V. Tizard, R. T. Schwarz, and R. R. Freeman. 1985. Primary structure of the precursor to the three major surface antigens of Plasmodium falciparum merozoites. Nature 317:270-273. [DOI] [PubMed] [Google Scholar]
- 22.Holder, A. A., R. R. Freeman, and S. C. Nicholls. 1988. Immunization against Plasmodium falciparum with recombinant polypeptides produced in Escherichia coli. Parasite Immunol. (Oxford) 10:607-617. [DOI] [PubMed] [Google Scholar]
- 23.Horiuchi, T., Y. Marumoto, Y. Saeki, Y. Sato, M. Furusawa, A. Kondo, and S. Maeda. 1987. High-level expression of the human-α-interferon gene through the use of an improved baculovirus vector in the silkworm, Bombyx mori. Agric. Biol. Chem. 51:1573-1580. [Google Scholar]
- 24.Hui, G. S. N., and W. A. Siddiqui. 1987. Serum from Pf195 protected Aotus monkeys inhibits Plasmodium falciparum growth in vitro. Exp. Parasitol. 64:519-522. [DOI] [PubMed] [Google Scholar]
- 25.Hui, G. S. N., A. Hashimoto, and S. P. Chang. 1992. Roles of conserved and allelic regions of the major merozoite surface protein (gp195) in immunity against Plasmodium falciparum. Infect. Immun. 60:1422-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hui, G. S. N., C. Hashiro, C. Nikaido, S. E. Case, A. Hashimoto, H. Gibson, P. J. Barr, and S. P. Chang. 1993. Immunological cross-reactivity of the C-terminal 42-kilodalton fragment of Plasmodium falciparum merozoite surface protein 1 expressed in baculovirus. Infect. Immun. 61:3403-3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hui, G. S. N., W. L. Gosnell, S. E. Case, C. Hashiro, C. Nikaido, A. Hashimoto, and D. C. Kaslow. 1994. Immunogenicity of the C-terminal 19-kDa fragment of the Plasmodium falciparum merozoite surface protein 1 (MSP1), YMSP119 expressed in S. cerevisiae. J. Immunol. 153:2544-2553. [PubMed] [Google Scholar]
- 28.Hui, G. S. N., C. Nikaido, C. Hashiro, D. C. Kaslow, and W. E. Collins. 1996. Dominance of conserved B-cell epitopes of the Plasmodium falciparum merozoite surface protein, MSP1, in blood-stage infections of naïve Aotus monkeys. Infect. Immun. 64:1502-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jongwutiwes, S., K. Tanabe, and H. Kanbara. 1993. Sequence conservation in the C-terminal part of the precursor to the major merozoite surface proteins (MSP1) of Plasmodium falciparum from field isolates. Mol. Biochem. Parasitol. 59:95-100. [DOI] [PubMed] [Google Scholar]
- 30.Kadono-Okuda, K., M. Yamamoto, Y. Higashino, K. Taniai, Y. Kato, S. Chowdhury, J. Xu, S. K. Choi, M. Sugiyama, K. Nakashima, S. Maeda, and M. Yamakawa. 1995. Baculovirus-mediated production of the human growth hormone in larvae of the silkworm, Bombyx mori. Biochem. Biophys. Res. Commun. 213:389-396. [DOI] [PubMed] [Google Scholar]
- 31.Kaneko, O., M. Kimura, F. Kawamoto, M. U. Ferreira, and K. Tanabe. 1997. Plasmodium falciparum: allelic variation in the merozoite surface protein 1 gene in wild isolates from southern Vietnam. Exp. Parasitol. 86:45-57. [DOI] [PubMed] [Google Scholar]
- 32.Kang, Y., and C. A. Long. 1995. Sequence heterogeneity of the C-terminal, cys-rich region of the merozoite surface protein-1 (MSP-1) in field samples of Plasmodium falciparum. Mol. Biochem. Parasitol. 73:103-110. [DOI] [PubMed] [Google Scholar]
- 33.Kaslow, D. C., G. Hui, and S. Kumar. 1994. Expression and antigenicity of Plasmodium falciparum major merozoite surface protein (MSP119) variants secreted from Saccharomyces cerevisiae. Mol. Biochem. Parasitol. 63:283-289. [DOI] [PubMed] [Google Scholar]
- 34.Kumar, S., A. Yadava, D. B. Keister, J. H. Tian, M. Ohl, K. A. Perdue-Greenfield, L. H. Miller, and D. C. Kaslow. 1995. Immunogenicity and in vivo efficacy of recombinant Plasmodium falciparum merozoite surface protein-1 in Aotus monkeys. Mol. Med. 1:1-8. [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar, S., W. Collins, A. Egan, A. Yadava, O. Garraud, M. J. Blackman, J. A. Patino, C. Diggs, and D. C. Kaslow. 2000. Immunogenicity and efficacy in Aotus monkeys of four recombinant Plasmodium falciparum vaccines in multiple adjuvant formulations based on the 19-kilodalton C terminus of merozoite surface protein 1. Infect. Immun. 68:2215-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 37.Ling, I. T., S. A. Ogun, and A. A. Holder. 1994. Immunization against malaria with a recombinant protein. Parasite Immunol. (Oxford) 16:63-67. [DOI] [PubMed] [Google Scholar]
- 38.Locher, C. P., and L. Q. Tam. 1993. Reduction of disulfide bonds in Plasmodium falciparum gp195 abolishes the production of growth-inhibitory antibodies. Vaccine 11:1119-1123. [DOI] [PubMed] [Google Scholar]
- 39.Lyon, J. A., R. H. Geller, J. D. Haynes, J. D. Chulay, and J. L. Weber. 1986. Epitope map and processing scheme for the 195,000-dalton surface glycoprotein of Plasmodium falciparum merozoites deduced from cloned overlapping segments of the gene. Proc. Natl. Acad. Sci. USA 83:2989-2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maeda, S., T. Kawai, M. Obinata, H. Fujiwara, T. Horiuchi, Y. Saeki, Y. Sato, and M. Furusawa. 1985. Production of human α-interferon in silkworm using a baculovirus vector. Nature 315:592-594. [DOI] [PubMed] [Google Scholar]
- 41.Maeda, S. 1989. Gene transfer vectors of a baculovirus, Bombyx mori nuclear polyhedrosis virus, and their use for expression of foreign genes in insect cells, p. 167-181. In J. Mitsuhashi (ed.), Invertebrate cell system applications, vol. I. CRC Press, Boca Raton, Fla. [Google Scholar]
- 42.Murphy, V. F., W. C. Rowan, M. J. Page, and A. A. Holder. 1990. Expression of hybrid malaria antigens in insect cells and their engineering for correct folding and secretion. Parasitology 100:177-183. [DOI] [PubMed] [Google Scholar]
- 43.Palhan, V. B., S. Sumathy, and K. P. Gopinathan. 1995. Baculovirus mediated high-level expression of luciferase in silkworm cells and larvae. BioTechniques 19:97-104. [PubMed] [Google Scholar]
- 44.Patarroyo, M. E., P. Romero, M. L. Torres, P. Clavijo, A. Moreno, A. Martínez, R. Rodríguez, F. Guzman, and E. Cabezas. 1987. Induction of protective immunity against experimental infection with malaria using synthetic peptides. Nature 328:629-632. [DOI] [PubMed] [Google Scholar]
- 45.Pirson, P. J., and M. E. Perkins. 1985. Characterization with monoclonal antibodies of a surface antigen of Plasmodium falciparum merozoites. J. Immunol. 134:1946-1951. [PubMed] [Google Scholar]
- 46.Qiu, P., Y. Ding, J. Qin, K. K. Han, and D. Zhu. 1994. Expression of biologically active monomeric form of human M-CSF in baculovirus infected silkworm, Bombyx mori. Biol. Chem. Hoppe-Seyler 375:413-418. [DOI] [PubMed] [Google Scholar]
- 47.Shi, X., J. Qin, J. Zhu, and D. Zhu. 1996. Expression of biologically active human granulocyte-macrophage colony-stimulating factor in the silkworm (Bombyx mori). Biotechnol. Appl. Biochem. 24:245-249. [PubMed] [Google Scholar]
- 48.Shi, Y. P., U. Sayed, S. H. Qari, J. M. Roberts, V. Udhayakumar, A. J. Oloo, W. A. Hawley, D. C. Kaslow, B. L. Nahlen, and A. A. Lal. 1996. Natural immune response to the C-terminal 19-kilodalton domain of Plasmodium falciparum merozoite surface protein 1. Infect. Immun. 64:2716-2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Siddiqui, W. A., L. Q. Tam, S. C. Kan, K. J. Kramer, S. E. Case, K. L. Palmer, K. M. Yamaga, and G. S. N. Hui. 1986. Induction of protective immunity to monoclonal-antibody-defined Plasmodium falciparum antigens requires strong adjuvant in Aotus monkeys. Infect. Immun. 52:314-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Siddiqui, W. A., L. Q. Tam, K. J. Kramer, G. S. N. Hui, S. E. Case, K. M. Yamaga, S. P. Chang, E. B. T. Chan, and S. C. Kan. 1987. Merozoite surface coat precursor protein completely protects Aotus monkeys against Plasmodium falciparum malaria. Proc. Natl. Acad. Sci. USA 84:3014-3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stowers, A. W., V. Cioce, R. L. Shimp, M. Lawson, G. Hui, O. Muratova, D. Kaslow, R. Robinson, C. A. Long, and L. H. Miller. 2001. Efficacy of two alternate vaccines based on Plasmodium falciparum merozoite surface protein 1 in an Aotus challenge trial. Infect. Immun. 69:1536-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sumathy, S., V. B. Palhan, and K. P. Gopinathan. 1996. Expression of human growth hormone in silkworm larvae through recombinant Bombyx mori nuclear polyhedrosis virus. Protein Expr. Purif. 7:262-268. [DOI] [PubMed] [Google Scholar]
- 53.Summers, M. D., and G. E. Smith. 1987. A manual of methods for baculovirus vectors and insect cell culture procedures. Tex. Agric. Exp. Stn. Bull. 1555.
- 54.Tada, A., A. Fuse, H. Sekine, B. Simizu, A. Kondo, and S. Maeda. 1988. Expression of the E2 open reading frame of papillomaviruses BPV1 and HPV6b in silkworm by a baculovirus vector. Virus Res. 9:357-367. [DOI] [PubMed] [Google Scholar]
- 55.Tanabe, K., M. Mackay, M. Goman, and J. Scaife. 1987. Allelic dimorphism in a surface antigen gene of the malaria parasite Plasmodium falciparum. J. Mol. Biol. 195:273-287. [DOI] [PubMed] [Google Scholar]
- 56.Udhayakumar, V., D. Anyona, S. Kariuki, Y. P. Shi, P. B. Bloland, O. H. Branch, W. Weiss, B. L. Nahlen, D. C. Kaslow, and A. A. Lal. 1995. Identification of T and B cell epitopes recognized by humans in the C-terminal 42-kDa domain of the Plasmodium falciparum merozoite surface protein (MSP)-1. J. Immunol. 154:6022-6030. [PubMed] [Google Scholar]
- 57.World Health Organization. 1998. Fifty facts from the World Health Report—global health situation and trends 1955-2025, p. 2. In The world health report 1998: life in the 21st century—a vision for all. World Health Organization, Geneva, Switzerland.
- 58.Zhou, N., Y. Zhang, W. Jing, Z. Li, and X. Wu. 1995. High expression of HBV S gene in Bombyx mori cell culture and in silkworms. Chin. J. Biotechnol. 11:149-156. [PubMed] [Google Scholar]