Abstract
In mice injected intravenously with Candida albicans, administration of anti-interleukin-18 (IL-18) antibodies increased the yeast load in the kidneys. There was no effect on the organ load with Candida when gamma interferon (IFN-γ)-deficient mice were treated with anti-IL-18 antibodies, suggesting that the protective effect of IL-18 is mediated through endogenous IFN-γ.
Candida albicans or mannoproteins derived from the yeast cell wall induce gamma interferon (IFN-γ) production by human mononuclear cells, and IFN-γ is a key cytokine for defense against candidiasis (8). The important role of endogenous IFN-γ in resistance to systemic candidiasis has been demonstrated in knockout mice deficient in IFN-γ, which are highly susceptible to C. albicans infection (1). Moreover, administration of recombinant IFN-γ to wild-type mice infected with C. albicans improves the outcome of the infection (8). Interleukin-18 (IL-18) serves as a costimulus for IFN-γ production in the context of costimulation with microbial products (14). When endogenous IL-18 is neutralized by administration of antibodies to IL-18 (3), there is little, if any, IFN-γ production after challenge with endotoxin. These data have led to the hypothesis that IL-18 is important for the host defense against disseminated candidiasis.
The aim of the present study was to investigate whether endogenous IL-18 is involved in host defense against Candida infection. IL-18 bioactivity was blocked by neutralizing anti-mouse IL-18 antibodies. In addition, we assessed whether the effects of IL-18 during disseminated candidiasis are mediated through production of IFN-γ by studying IFN-γ-deficient mice with disseminated candidiasis treated with anti-IL-18 antibodies. The role of tumor necrosis factor (TNF) in the subsequent IL-18 synthesis was investigated with mice deficient in TNF and lymphotoxin (LT).
CBA mice (females, 20 to 25 g, 6 to 8 weeks old) were purchased from Jackson Laboratories (Bar Harbor, Maine). IFN-γ−/− mice and their wild-type littermates (BALB/c genetic background) were generously provided by Organon (Oss, The Netherlands). Homozygous TNF−/− LT−/− and wild-type TNF+/+ LT+/+ mice (genetic background, C57BL/6J × 129sv) were obtained as mating pairs (kindly provided by F. Amiot, CEA, Fontenay-aux-Roses). Anti-mouse IL-18 polyclonal antibodies were produced in rabbits using recombinant mature murine IL-18 (Peprotech, Rocky Hill, N.J.) (3). Normal rabbit serum (NRS) was used in the control groups.
The mice were injected intravenously (i.v.) with C. albicans (strain UC 820; 105 CFU/mouse). EDTA-blood was collected from the retroorbital plexus for plasma IL-18 concentration measurements at various time points: 1, 2, 4, 8, 24, 48, and 72 h after infection. Animals received either 200 μl of anti-IL-18 antiserum intravenously 10 min before infection and on days 2 and 4 after infection or a similar volume of NRS. Subgroups of 10 animals were killed on day 1, 3, or 7 of infection. The number of viable Candida cells in the kidneys was determined as previously described (7) and expressed as log CFU per gram of tissue.
IL-18 concentrations were determined by electrochemiluminescence, using a biotinylated rat anti-mouse IL-18 antibody (Igen, Gaithersburg, Md.) and a ruthenilated goat antimouse antibody (Peprotech, Princeton, N.J.). The reaction was quantitated using the Origen 1.5 Analyzer (Igen) (15). IL-1β and TNF-α levels were determined by specific radioimmunoassays (11). Murine IL-6 and IFN-γ concentrations were measured using commercial enzyme-linked immunosorbent assay kits (Pelikine; CLB, Amsterdam, The Netherlands). Detection limits were 20 pg/ml (TNF, IL-1α, IL-1β, and IL-6) and 40 pg/ml (IL-18 and IFN-γ). The differences between groups were analyzed by the Mann-Whitney U test.
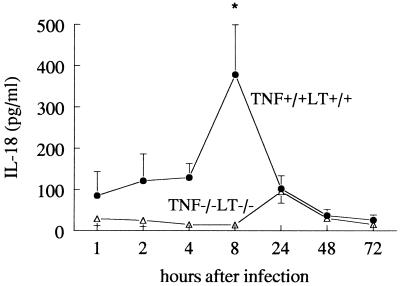
IL-18 concentrations were below the detection limit (40 pg/ml) in both uninfected TNF+/+ LT+/+ and TNF−/− LT−/− mice. Intravenous administration of C. albicans to TNF+/+ LT+/+ mice induced circulating IL-18 concentrations, which reached peak elevations at 8 h postinfection (279 ± 144 pg/ml) (Fig. 1). In Candida-infected TNF+/+ LT+/+ mice, the circulating IL-18 concentrations were significantly higher at 2, 4, 8, and 24 h postinfection than those in uninfected mice. Similar IL-18 concentrations were measured in CBA mice and IFN-γ+/+ BALB/c mice (not shown). TNF and/or LT was required for the induction of IL-18, since the peak of circulating IL-18 levels was absent in TNF−/− LT−/− mice (Fig. 1). For TNF−/− LT−/− mice, the IL-18 concentrations were slightly increased above background levels for uninfected mice at 24 h after infection (98 ± 11 pg/ml), similar to the increase in TNF+/+ LT+/+ mice. These findings are in line with data showing that neutralization of endogenous TNF and LT using soluble TNF p55 receptors reduced circulating IL-18 following i.v. treatment with concanavalin A (2).
FIG. 1.
Circulating IL-18 during disseminated candidiasis. Groups of 10 TNF+/+ LT+/+ and TNF−/− LT−/− mice were injected i.v. with 105 C. albicans CFU. IL-18 concentrations in the serum of TNF+/+ LT+/+ (closed circles) and TNF−/− LT−/− (open triangles) mice were measured by enhanced chemiluminescence at various time points after infection (the experiment was performed twice, with a total of 10 animals per time point). Asterisk, P < 0.05
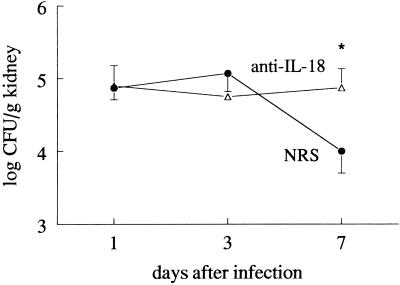
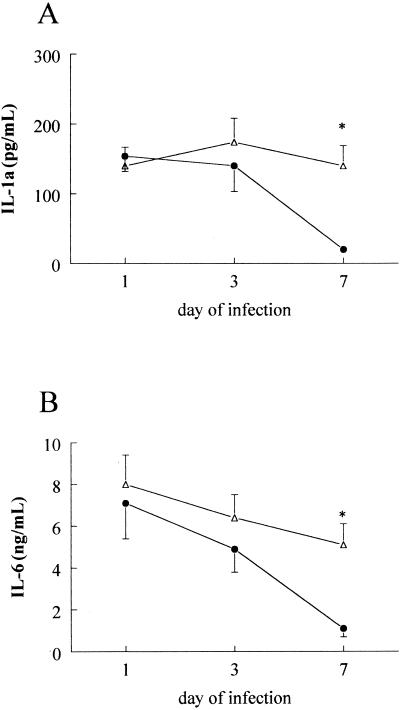
The role of endogenous IL-18 for the defense against C. albicans infection was investigated by administering neutralizing anti-IL-18 antibodies to the mice prior to infection. C. albicans CFU in the kidneys decreased 10-fold within 7 days of infection in mice injected with NRS, whereas neutralization of IL-18 by anti-IL-18 antibodies prevented the elimination of the microorganisms (Fig. 2). Circulating concentrations of IL-1α and IL-6 on days 1 and 3 of infection were not influenced by administration of anti-IL-18 antibodies. However, the increased outgrowth of Candida in the organs of the anti-IL-18-treated mice on day 7 was accompanied by higher circulating concentrations of IL-1α and IL-6 than for NRS-treated mice (Fig. 3). TNF, IL-1β, and IFN-γ concentrations were below the detection limit in all samples on days 1, 3, and 7 after infection.
FIG. 2.
The role of endogenous IL-18 in the defense against disseminated candidiasis. CBA mice received 200 μl of either NRS (closed circles) or anti-IL-18 antiserum (open triangles) and were thereafter injected i.v. with 105 C. albicans CFU. Outgrowth of the microorganism in the kidneys was assessed on days 1, 3, and 7 after infection in groups of 10 animals. Asterisk, P < 0.05
FIG. 3.
Cytokine concentrations during disseminated candidiasis. CBA mice received 200 μl of either NRS (closed circles) or anti-IL-18 antiserum (open triangles) and were thereafter injected i.v. with 105 C. albicans CFU. Circulating concentrations of IL-1α (A) and IL-6 (B) were measured on days 1, 3, and 7 after infection in subgroups of 10 animals. Asterisk, P < 0.05.
These data imply an important role of endogenous IL-18 in the defense against disseminated candidiasis, and such findings are supported by other studies showing that IL-18 is essential for host defense against mycobacterial infections (16) and Cryptococcus neoformans (5), Leishmania major (13), and Salmonella infections (9). In a recent study, it has been shown that recombinant IL-18 restores the Th1 response to C. albicans in caspase-1-deficient mice, which are unable to process the inactive precursors in bioactive IL-18 and IL-1β (10). Promising therapeutic properties of IL-18 in experimental infections with C. neoformans (6) or Leishmania spp. (13) have also been suggested.
To investigate whether the effect of IL-18 is mediated through endogenous IFN-γ, neutralizing anti-IL-18 antibodies were given to mice deficient in IFN-γ before infection with C. albicans. In contrast to the effects in wild-type mice (Fig. 2), there was no effect of anti-IL-18 antibodies on Candida outgrowth in the kidneys of IFN-γ−/− mice (94% compared to the outgrowth in IFN-γ-deficient mice treated with NRS; P > 0.05), demonstrating that the effects of endogenous IL-18 during disseminated candidiasis are mediated by IFN-γ. These findings are consistent with previous data demonstrating the importance of IFN-γ for the protective effects of IL-18 during infection with C. neoformans (6) or Salmonella enterica serovar Typhimurium (9). The finding that IL-18 has a relatively late effect, on day 7 of infection, is consistent with IFN-γ-mediated stimulation of macrophages, which is known to occur at least 7 days after infection, as has been shown previously with IFN-γ−/− mice (1), whereas no effect of the anti-IL-18 antibodies was found during the first phase of infection, when neutrophil-mediated mechanisms are more important (1). However, since IFN-γ−/− mice are highly susceptible to disseminated candidiasis, it is possible that an additive effect of IL-18 on the anti-Candida defense through IFN-γ-independent mechanisms is difficult to substantiate with these mice. IFN-γ-independent effects of IL-18 have been found in lethal endotoxemia and experimental models of streptococcal cell wall arthritis (4, 12).
In conclusion, endogenous IL-18 plays a protective role in the defense against disseminated infection with C. albicans. The production of IL-18 during disseminated candidiasis requires endogenous TNF and/or LT, and its protective effects are likely mediated through intermediary stimulation of endogenous IFN-γ synthesis.
Acknowledgments
This study was partially supported by NIH grant AI-15614 (to C.A.D.).
Editor: T. R. Kozel
REFERENCES
- 1.Balish, E., R. D. Wagner, A. Vasquez-Torres, C. Pierson, and T. Warner. 1998. Candidiasis in interferon-gamma knock-out (IFN-gamma-/-) mice. J. Infect. Dis. 178:478-487. [DOI] [PubMed] [Google Scholar]
- 2.Faggioni, R., J. Jones-Carson, D. A. Reed, C. A. Dinarello, K. R. Feingold, C. Grunfeld, and G. Fantuzzi. 2000. Leptin-deficient (ob/ob) mice are protected from T-cell mediated hepatotoxicity: role of tumor necrosis factor alpha and IL-18. Proc. Natl. Acad. Sci. USA 97:2367-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fantuzzi, G., D. A. Reed, and C. A. Dinarello. 1999. IL-12-induced IFNgamma is dependent on caspase-1 processing of the IL-18 precursor. J. Clin. Investig. 104:761-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joosten, L. A., F. A. van de Loo, E. Lubberts, M. M. Helsen, M. G. Netea, J. W. M. van der Meer, and W. B. van den Berg. 2000. An IFN-gamma-independent proinflammatory role of IL-18 in murine streptococcal cell wall arthritis. J. Immunol. 165:6553-6558. [DOI] [PubMed] [Google Scholar]
- 5.Kawakami, K., Y. Koguchi, M. H. Qureshi, Y. Kinjo, S. Yara, M. Miyazato, M. Kurimoto, K. Takeda, S. Akira, and A. Saito. 2000. Reduced host resistance and Th1 response to Cryptococcus neoformans in interleukin-18 deficient mice. FEMS Microbiol. Lett. 186:121-126. [DOI] [PubMed] [Google Scholar]
- 6.Kawakami, K., M. H. Qureshi, T. Zhang, H. Okamura, H. Kurimoto, and A. Saito. 1997. IL-18 protects mice against pulmonary and disseminated infection with Cryptococcus neoformans by inducing IFN-gamma production. J. Immunol. 159:5528-5534. [PubMed] [Google Scholar]
- 7.Kullberg, B. J., M. G. Netea, J. H. A. J. Curfs, M. Keuter, J. F. G. M. Meis, and J. W. M. Van der Meer. 1998. Recombinant murine granulocyte colony-stimulating factor protects against acute disseminated Candida albicans infection in non-neutropenic mice. J. Infect. Dis. 177:175-181. [DOI] [PubMed] [Google Scholar]
- 8.Kullberg, B. J., J. W. Van 't Wout, C. Hoogstraten, and R. Van Furth. 1993. Recombinant interferon-γ enhances resistance to acute disseminated Candida albicans infection in mice. J. Infect. Dis. 168:436-443. [DOI] [PubMed] [Google Scholar]
- 9.Mastroeni, P., S. Clare, S. Khan, J. A. Harrison, H. E. Hormaeche, H. Okamura, M. Kurimoto, and H. Dougan. 1999. Interleukin 18 contributes to host resistance and gamma interferon production in mice infected with virulent Salmonella typhimurium. Infect. Immun. 67:478-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mencacci, A., A. Bacci, E. Cenci, C. Montagnoli, S. Fiorucci, A. Casagrande, R. A. Flavell, F. Bistoni, and L. Romani. 2000. Interleukin 18 restores defective Th1 immunity to Candida albicans in caspase 1-deficient mice. Infect. Immun. 68:5126-5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Netea, M. G., P. N. M. Demacker, B. J. Kullberg, O. C. Boerman, I. Verschueren, A. F. H. Stalenhoef, and J. W. M. Van der Meer. 1996. Low-density-lipoprotein receptor deficient mice are protected against lethal endotoxinemia and severe Gram-negative infections. J. Clin. Investig. 97:1366-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Netea, M. G., G. Fantuzzi, B. J. Kullberg, R. Stuyt, E. J. Pulido, R. C. McIntyre, J. W. M. Van der Meer, and C. A. Dinarello. 2000. Neutralization of interleukin-18 reduces neutrophil tissue accumulation and protects mice against lethal Escherichia coli and Salmonella typhimurium endotoxemia. J. Immunol. 164:2644-2649. [DOI] [PubMed] [Google Scholar]
- 13.Ohkusu, K., T. Yoshimoto, K. Takeda, T. Ogura, S.-I. Kashiwamura, Y. Iwakura, S. Akira, H. Okamura, and K. Nakanishi. 2000. Potentiality of interleukin-18 as a useful reagent for treatment and prevention of Leishmania major infection. Infect. Immun. 68:2449-2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okamura, H., K. Nagata, T. Komatsu, T. Tanimoto, Y. Nukata, F. Tanabe, K. Akita, K. Torigoe, T. Okura, S. Fukuda, and M. Kurimoto. 1995. A novel costimulatory factor for gamma interferon induction found in the livers of mice causes endotoxic shock. Infect. Immun. 63:3966-3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puren, A. J., G. Fantuzzi, Y. Gu, M. S.-S. Su, and C. A. Dinarello. 1998. Interleukin-18 (IFNγ inducing factor) induces IL-8 and IL-1β via TNFα production from non-CD14+ human mononuclear cells. J. Clin. Investig. 101:711-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sugawara, I., H. Yamada, H. Kaneko, S. Mizuno, K. Takeda, and S. Akira. 1999. Role of interleukin-18 (IL-18) in mycobacterial infection in IL-18-gene-disrupted mice. Infect. Immun. 67:2585-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]