Abstract
Several lines of evidence indicate that the monocytes of subjects with localized juvenile periodontitis (LJP) are functionally distinct from cells of age- and race-matched nonperiodontitis (NP) subjects. Among the abnormalities are the propensity to secrete large amounts of prostaglandin E2 and the induction of immunoglobulin G2 (IgG2) antibodies. The experiments described here were performed to further characterize the LJP monocytes and to determine if these cells mature differently than NP monocytes. When adherent monocytes from LJP subjects were cultured in the presence of human serum, both macrophages and cells with the morphology of immature monocyte-derived dendritic cells (MDDC) were observed. Within 4 days the prevalence of the immature MDDC was approximately twofold higher in LJP cultures than in NP cultures. In addition to their dendritic morphology, these cells were CD11c+ and CD14− or CD14low and stimulated potent autologous mixed leukocyte reactions, consistent with differentiation to the MDDC phenotype. Like LJP monocytes, cultures of MDDC generated with interleukin-4 and granulocyte-macrophage colony-stimulating factor selectively induced IgG2 in cultures of pokeweed mitogen-stimulated NP leukocytes. Together, these data suggest that the monocytes of LJP subjects have a propensity to differentiate into MDDC and that this differentiation may be related to the high levels of IgG2 that are observed in the sera of LJP subjects. As high levels of circulating IgG2 are correlated with less severe disease, the propensity of LJP monocytes to differentiate into MDDC may have important implications for both the host response against oral pathogens and the progression of LJP.
Localized juvenile periodontitis (LJP) is a form of early-onset periodontitis that tends to run in families. Several oral pathogens have been linked to the etiology of the disease, including Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis (4, 22, 36, 37). However, mounting evidence suggests that alterations in the host response may contribute to the pathogenesis of LJP. Several studies have highlighted abnormalities in the myeloid compartment of LJP subjects. For example, LJP neutrophils exhibit reduced chemotactic and calcium responses (7, 10) and have altered diacylglycerol metabolism (32) compared to cells from nonperiodontitis (NP) individuals. The peripheral blood of LJP subjects contains abnormally large numbers of immature granulocytes, which express low levels of CD16 (25). It also appears that the monocytes of LJP subjects are somewhat abnormal, as these cells produce abnormally large amounts of prostaglandin E2 (PGE2) in response to stimulation with lipopolysaccharide (26, 30). Our group has been particularly intrigued by the unique relationship that appears to exist between LJP monocytes and antibody production.
LJP patients exhibit elevated levels of circulating immunoglobulin G2 (IgG2) compared to age- and race-matched NP subjects (23). In contrast, the levels of other isotypes of IgG are similar in NP and LJP subjects. Much of the antibody response against the oral pathogens associated with the disease is IgG2 (4, 22, 35). It is likely that this antibody is protective, as IgG2 titers are positively correlated with reduced severity of disease (2). In a recent study, we reported that monocytes control IgG2 production in LJP subjects (18, 38). When LJP monocytes are cultured with pokeweed mitogen (PWM)-stimulated T and B cells from NP individuals, a dose-dependent increase in IgG2 production is observed. In contrast, increasing the number of monocytes from NP subjects does not affect the production of IgG2. These data are consistent with other reports of abnormalities in the myeloid cells of LJP subjects (11, 17, 30, 31) and suggest that the high levels of IgG2 that are observed in LJP patients may be attributed to the monocytes.
The remarkable ability of LJP monocytes to selectively promote IgG2 production prompted the hypothesis that LJP and NP monocytes mature differently. Peripheral blood monocytes are precursors of a variety of mature cells, including distinct populations of splenic, lung, and liver macrophages, as well as a potent population of antigen-presenting cells known as dendritic cells. Our data indicate that during culture the adherent monocytes of LJP and NP subjects mature into both macrophages and monocyte-derived dendritic cells (MDDC). However, by 4 days of culture the percentage of MDDC that emerge from LJP monocytes is more than double the percentage of MDDC that emerge from NP monocytes. Furthermore, like LJP monocytes, small numbers of interleukin-4 (IL-4)- and granulocyte-macrophage colony-stimulating factor (GM-CSF)-generated MDDC from NP subjects selectively promote IgG2 production. Thus, it appears likely that the increased levels of IgG2 in LJP patients may be attributable to increased numbers of MDDC.
MATERIALS AND METHODS
Materials.
Human AB serum was obtained from Biowhittaker (Walkersville, Md.). Recombinant IL-4 and GM-CSF were obtained from R&D Systems (Minneapolis, Minn.). Fluorescently coupled antibodies against CD11c, CD3, CD8, CD19, and CD14 were obtained from BD-Pharmingen (San Diego, Calif.). Magnetic bead-coupled anti-CD19, anti-CD8, and anti-CD14 were obtained from Miltenyi Biotec (Auburn, Calif.). PWM was obtained from Invitrogen (Rockville, Md.).
Human subjects.
Human studies were performed in compliance with all relevant federal guidelines and the institutional policies of Virginia Commonwealth University. Subjects for study were obtained by the Clinical Research Center for Periodontal Disease, School of Dentistry, Virginia Commonwealth University, Richmond, Va. Patients with LJP were 35 years old or less and had localized patterns of severe periodontal destruction limited to the first molar or incisor teeth and up to two additional teeth. The NP control subjects were age and race matched and had no evidence of attachment loss, except for recession on the buccal surface of anterior teeth at no more than one site and no pocket greater than 3 mm. Different LJP and NP subjects were used to replicate data within an experimental group. In some cases, subjects were rescreened to assess the stability of the phenotypes that were observed.
Lymphocyte separation.
Peripheral blood mononuclear cells (peripheral blood leukocytes [PBL]) were obtained from heparinized blood of LJP or NP subjects by density centrifugation using lymphocyte separation medium (Organon Teknika Corporation, Durham, N.C.). The blood cells were centrifuged at 400 × g for 20 min, and the PBL were collected from the interface and washed three times in Hanks' balanced salt solution. After washing, the PBL were suspended in RPMI 1640 supplemented with serum and antibiotics.
Adherent monocyte cultures.
Adherent monocytes were prepared by culturing PBL in RPMI 1640 containing 10% human AB serum (human serum medium) in six-well culture plates (diameter, 35 mm; Costar) for 1 h. The cell monolayers were washed to remove nonadherent cells and then cultured in fresh human serum medium as indicated below.
MDDC cultures.
Immature MDDC were generated as described previously (27). Briefly, adherent monocytes were cultured in 10% fetal calf serum containing 500 U of recombinant human IL-4 per ml and 800 U of recombinant human GM-CSF per ml for 5 days.
Antibody production.
PBL were depleted of monocytes by adherence to tissue culture wells. The nonadherent cells (T and B cells) were suspended in medium containing 10% dimethyl sulfoxide and 50% fetal calf serum and frozen until they were used. The cells were thawed by using a special medium and methods that have been described previously (13). Under these conditions, the cell viability is at least 90% for PWM-stimulated cells, and the number of PFU is the same as the number of PFU for fresh lymphocytes. The adherent monocytes were cultured in the presence of IL-4 and GM-CSF to generate MDDC. After 7 days of culture, the adherent cells were harvested and added to thawed T and B cells from the same subject for antibody production assays. A total of 106 T and B cells were resuspended in RPMI 1640 (Mediatech, Herndon, Va.) supplemented with 10% heat-inactivated fetal bovine serum (HyClone, Logan, Utah), 2 mM glutamine, 100 U of penicillin per ml, and 100 mg of streptomycin sulfate (Sigma, St. Louis, Mo.) per ml and cultured in 75-mm tubes (1 ml/tube; Falcon, Lincoln Park, N.J.) at 37°C in a humidified atmosphere containing 5% CO2. Nine microliters of PWM was added to each cell culture to induce antibody production. Cultures contained 200 MDDC, 2,000 MDDC, or no MDDC from the same subject. The cultures were incubated for 7 days, and then supernatant fluids were collected and assayed for IgG2 or IgG1 as outlined below.
MLR.
Adherent monocytes from NP and LJP subjects were used as antigen-presenting cells in autologous and allogeneic mixed leukocyte reactions (MLRs). The adherent cells were cultured in human serum medium for 6 days and then subjected to 3,000 rads of irradiation. The responder cells were CD4+ T cells isolated from the peripheral blood of the same subject (autologous MLR) or an NP subject (allogeneic MLR). All allogeneic MLRs were performed with T cells from the same NP subject. CD4+ T cells were isolated by negative selection with magnetic beads as previously described (38). In these experiments, CD14+ cells, CD19+ cells, and CD8+ cells were depleted from the nonadherent fraction of PBL. The purity of CD4+ T-cell preparations was verified by fluorescence-activated cell sorter analyses. The T cells were cultured with antigen-presenting cells in human serum medium for 3 days, and then the cultures were pulsed with 1 μCi of [3H]thymidine per ml for 16 h. Cells were harvested onto Unifilter GF/C microtiter plates (Packard), and the incorporated radiolabel was quantified with a TopCount scintillation counter. A considerable degree of variability was observed among the NP and LJP subjects studied in both the autologous and allogeneic MLRs. Hence, the autologous MLR data are presented as percentages of the allogeneic MLR data. As the value approaches 100%, the autologous MLR becomes more robust, indicating the presence of more potent antigen-presenting cells (dendritic cells) in the cultures.
Phenotypic analyses of LJP and NP monocytes.
Adherent monocytes were cultured in human serum medium for 4 days. For fluorescence-activated cell sorter analysis, adherent monocytes were incubated for 10 min with 10 μg of mouse IgG (Jackson Laboratories) per ml in 0.5% bovine serum albumin (BSA)-phosphate-buffered saline (PBS) to block Fc receptors and then incubated for 30 min with murine anti-human phycoerythrin-labeled anti-CD11c (5 μg/ml), fluorescein isothiocyanate-labeled anti-CD14 (5 μg/ml), or phycoerythrin-labeled or fluorescein isothiocyanate-labeled murine isotype-matched controls (5 μg/ml). Samples were analyzed by flow cytometry by using a FACScan (Becton Dickinson) and the Cell Quest Pro software package. The data are presented below as the percentage of cells with the MDDC phenotype (CD14− CD11c+ cells). For immunocytochemistry experiments, adherent monocytes were cultured in eight-well chamber slides (Costar). Nonspecific binding was blocked with 0.5% BSA-PBS, and then the cells were incubated with 5 μg of murine anti-CD11c per ml, with 5 μg of anti-CD14 (BD-Pharmingen) per ml, or with 5 μg of murine isotype-matched controls per ml for 30 min at 41°C (there was no blocking antibody in these experiments). After washing, the cells were incubated with 2 μg of Fab2′ goat anti-mouse IgG per ml coupled to Alexa-488 (Molecular Probes) for 30 min at 41°C. Samples were washed and then fixed in 1% paraformaldehyde in PBS and stored in 5% N-propyl-gallate (Sigma) to prevent quenching. The fluorescent signals were minimal for samples incubated with isotype-matched control antibodies (data not shown). The fluorescent signals were visualized with an Olympus Fastscan 2000 12-bit digital camera mounted on an Olympus IX70 fluorescent microscope (Olympus America, Melville, N.Y.). Images were compiled with Photoshop 5.5 (Adobe Systems).
IgG assays.
IgG levels in supernatant fluids were determined by a sandwich enzyme-linked immunosorbent assay as described previously (38). Briefly, Fc-specific goat anti-mouse antibody (10 μl/ml; ICN, Aurora, Ohio) was placed in a carbonate buffer (pH 9.6) and used to coat microtiter plates (Dynex, Chantilly, Va.). Mouse anti-human IgG1 and IgG2 monoclonal antibodies (1 μg/ml) (IgG1, clone HP6001; IgG2, clone HP6002; both obtained from Sigma) were then added to the plates to serve as capture antibodies. The plates were incubated overnight at 4°C, and this was followed by a 1-h application of BSA-PBS to minimize nonspecific binding at room temperature. A known standard for human IgG1 and IgG2 antibodies and a control (pooled human sera) (The Binding Site, San Diego, Calif.) and the culture supernatant fluids were applied to different wells in plates and incubated for 1 h at room temperature. Horseradish peroxidase-conjugated goat anti-human immunoglobulin (Sigma) was added, and the plates were incubated for 1 h at room temperature. The plates were washed three times with PBS containing 0.05% Tween 20 between steps. The color was developed by using O-phenylenediamine dihydrochloride (Sigma) as the substrate. Optical density at 450 nm was measured with a micro plate reader (Dynex). The IgG concentration in each test sample was calculated by using the standard curve generated in each assay.
Statistical analyses.
All experiments were replicated with at least four different NP or LJP subjects. Each experimental analysis was performed in triplicate, and the mean and standard deviation for each set of replicates were determined. In most cases, statistical significance was determined by unpaired t tests, with P < 0.05 as the cutoff for significance. Data from the MLR experiments were analyzed by using repeated-measures analysis of variance of the log response. The results were then back-transformed to the original units for display.
RESULTS
NP and LJP monocytes have distinct morphologies.
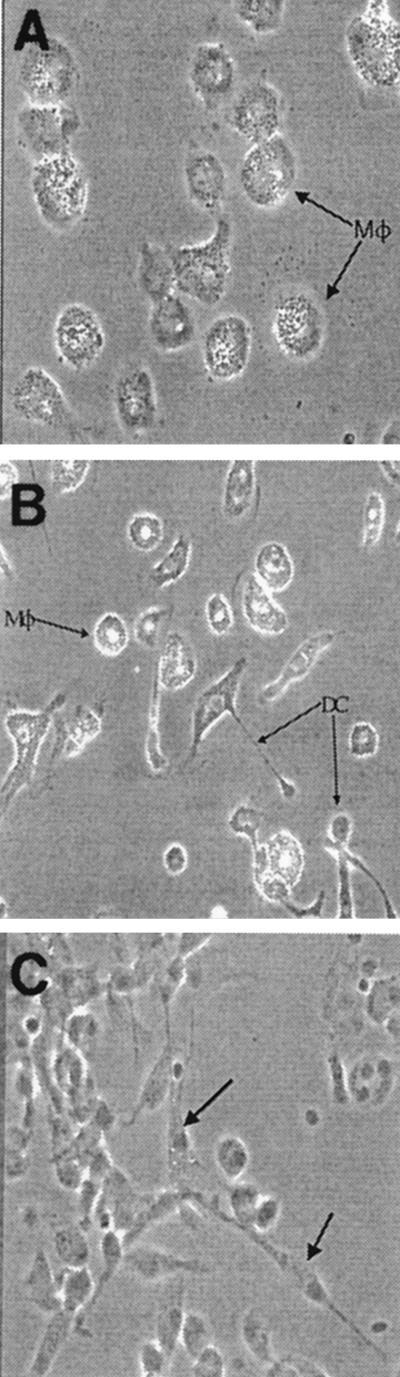
Leukocytes were prepared from the heparinized peripheral blood of LJP and NP subjects, and the adherent cells were cultured in medium containing human serum for up to 7 days. When the cultures were initiated, both NP and LJP cells exhibited monocyte morphology (data not shown). Within 4 days, most of the cells in the NP cultures had acquired the morphology of mature macrophages (Fig. 1A). The LJP cultures also contained such cells, but a cell type with a distinct morphology was observed in addition to the macrophages (Fig. 1B). The frequency of these cells in LJP cultures was approximately twice that in NP cultures (Fig. 2). Upon close inspection, the morphology of the adherent monocytes in LJP cultures was reminiscent of the morphology of very immature MDDC, which exhibit an almost fibroblastoid appearance (28). Indeed, when adherent monocytes from an NP subject were treated with IL-4 and GM-CSF to induce differentiation to the MDDC phenotype, the same cell morphology was observed early in the cultures (Fig. 1C). Together, these data suggested that LJP monocytes might be predisposed to differentiate into MDDC. The experiments described below were designed to test this hypothesis.
FIG. 1.
Morphologies of adherent monocytes from NP and LJP subjects. Adherent monocytes from an NP subject (A) and an LJP subject (B) were cultured in human serum-containing medium for 4 days. Photomicrographs of the cultures are shown. NP cultures contained primarily macrophages (Mφ), while LJP cultures contained both macrophages and immature MDDC (DC). For comparison, a photomicrograph of MDDC (generated with IL-4 and GM-CSF) is shown in panel C (MDDC are indicated by arrows). The results of a representative experiment are shown (n = 5).
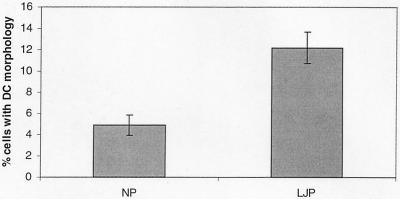
FIG. 2.
Quantification of morphologically distinct MDDC-like cells in LJP and NP cultures. Adherent monocytes from NP and LJP peripheral blood were cultured in human serum-containing medium for 4 days. The cultures were scored for the percentage of cells with the distinct morphology that arose in cultures of LJP monocytes. The data include data from four NP and four LJP subjects and are means ± standard errors. The percentage of MDDC was significantly higher in LJP cultures (P < 0.01, unpaired t test). DC, dendritic cell.
LJP adherent monocytes express MDDC markers.
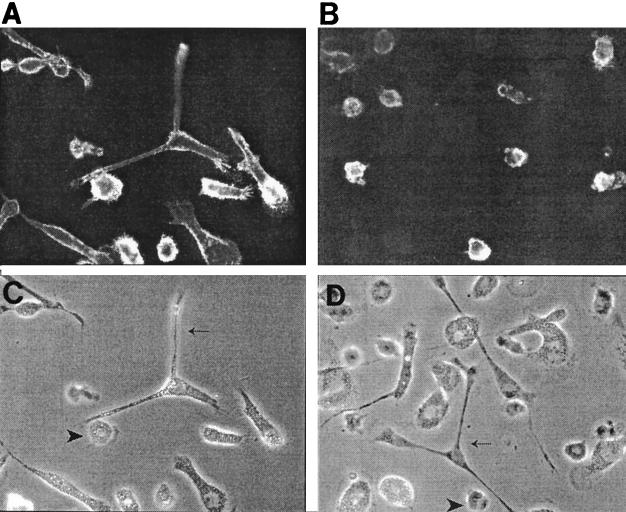
Immature MDDC are known to express high levels of CD11c and low levels of the macrophage marker CD14. Therefore, flow cytometric and immunohistochemical analyses were performed to characterize the cell surface phenotypes of LJP monocytes (Fig. 3). The cells with macrophage morphology expressed both CD14 and CD11c, a phenotype that is consistent with macrophages. In contrast, CD14 expression was very low or absent on the putative immature MDDC that arose in the LJP cultures. The percentages of CD11c+ C14low cells in 4-day cultures of NP and LJP adherent monocytes were determined by flow cytometry. Cultures of adherent monocytes from five NP and six LJP subjects were examined, and the percentages of immature MDDC (CD11c+ CD14− cells) for the two groups were 5.0% ± 1.9% and 13.0% ± 2.9% (mean ± standard error), respectively; the LJP value is significantly different from the NP value (P < 0.05, as determined by an unpaired t test). These data are strikingly similar to the morphological analysis data and indicate that the frequency of immature MDDC is approximately twofold higher in cultures of LJP monocytes than in cultures of NP monocytes.
FIG. 3.
Phenotypic analysis of immature MDDC. Adherent monocytes from a representative LJP subject were cultured in human serum-containing medium for 4 days. The cells were screened for expression of CD11c (A) or CD14 (B) as described in Materials and Methods. For comparison, light micrographs of the cell cultures used for CD11c and CD14 analyses are shown in panels C and D, respectively. Macrophages (arrowheads) expressed both markers, while immature MDDC (arrows) expressed only CD11c.
LJP adherent monocytes elicit potent autologous MLR.
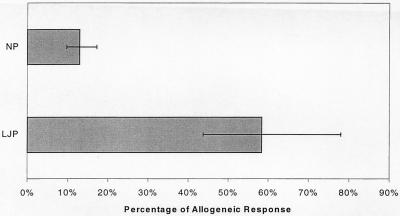
Other distinct characteristics of MDDC are their ability to activate allogeneic T cells and their remarkable ability to elicit autologous MLRs (5). Therefore, the autologous MLR assay was used as a functional assay for the presence of MDDC in cultures of LJP adherent monocytes. Adherent monocytes were prepared from the peripheral blood of NP and LJP subjects, cultured in human serum for 6 days, and then irradiated. These antigen-presenting cells were then cultured with allogeneic or autologous T cells for 5 additional days, and the incorporation of [3H]thymidine was measured to obtain an indication of T-cell activation. T cells for the allogeneic MLR were obtained from a single NP subject. The absolute magnitudes of the autologous and allogeneic MLRs differed considerably from study subject to study subject. For the NP group, the allogeneic responses ranged from 6,442 to 113,892 cpm (median, 41,844 cpm) and the autologous responses ranged from 552 to 38,669 cpm (median, 6,141 cpm). For the LJP group, the allogeneic responses ranged from 18,909 to 39,752 cpm (median, 32,535 cpm) and the autologous responses ranged from 9,261 to 29,133 cpm (median, 19,106 cpm). However, a striking difference was apparent when the magnitude of the autologous MLR was compared to the magnitude of the allogeneic MLR. In Fig. 4, the autologous MLR data are presented as percentages of the allogeneic MLR data. In LJP subjects, the autologous MLR was nearly as high as the allogeneic MLR, whereas the autologous MLR in NP subjects was much lower. The autologous MLR in LJP subjects was about 60% of the allogeneic MLR, whereas the NP autologous response was only about 13% of the allogeneic response. The increased ability of LJP monocytes to stimulate the autologous MLR is consistent with the approximate doubling of the number of MDDC in the LJP monocyte population (Fig. 2) (see above).
FIG. 4.
LJP monocytes stimulate potent MLRs. Adherent monocytes from LJP and NP subjects were used as antigen-presenting cells for autologous or allogeneic T cells. Three LJP and four NP subjects were studied. All allogeneic T cells were derived from a single NP donor. The uptake of [3H]thymidine was measured to obtain an indication of T-cell activation. The absolute values of the autologous and allogeneic MLRs varied considerably among the individual subjects. Therefore, the data were expressed to compare the autologous response to the allogeneic response for each individual and are presented as percentages of the allogeneic responses. The error bars indicate the 95% confidence intervals.
MDDC selectively induce IgG2.
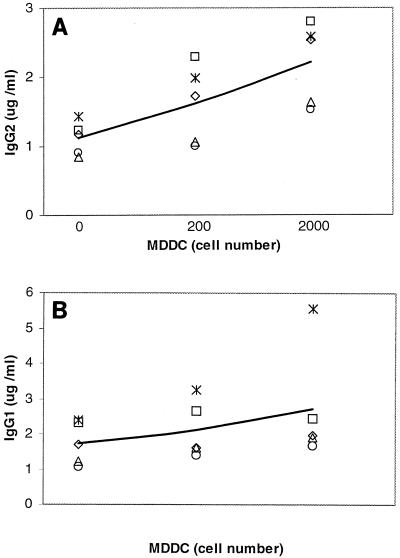
Given the well-characterized role of MDDC in regulation of the immune response, we considered the possibility that these cells could induce the high level of IgG2 production that is observed in cultures of LJP PBL. To address this possibility, IL-4 and GM-CSF (27) were used to generate MDDC from the adherent monocytes of NP subjects. The MDDC were added to NP PBL, the cultures were stimulated with PWM (a polyclonal activator), and the levels of IgG1 and IgG2 in the cultures were quantified. An increase in IgG2 was observed in PBL that were cultured with MDDC (Fig. 5A). The trend towards increased IgG2 was apparent with T and B cells cultured with 200 MDDC, and a clear approximately twofold increase in IgG2 production (1.1 ± 0.2 μg/ml for T and B cells alone versus 2.2 ± 0.3 μg/ml with MDDC; P < 0.02) was induced by 2,000 MDDC. In contrast, addition of MDDC to LJP PBL did not increase the IgG2 response, which is consistent with the higher percentage of MDDC in LJP monocyte cultures (data not shown). A trend toward more IgG1 production (Fig. 5B) was also apparent, but this was attributable to a single individual and the effect did not reach statistical significance (the means ± standard errors for IgG1 production were 1.7 ± 0.3, 2.1 ± 0.4, and 2.8 ± 0.7 μg/ml for T and B cells alone and T and B cells cultured with 200 and 2,000 MDDC, respectively), suggesting that MDDC have a selective effect on induction of IgG2. Interestingly, a similar effect was observed when NP PBL were treated with GM-CSF, a cytokine that promotes monocyte differentiation into MDDC (data not shown). These results were consistent with our previous observation that LJP monocytes selectively enhanced the production of IgG2 (18, 38) and suggested that this effect could be attributed to the MDDC in these cultures.
FIG. 5.
Selective induction of IgG2 by MDDC. NP T and B cells were cultured with PWM in the presence or absence of 200 or 2,000 MDDC for 7 days. Culture supernatants were harvested and screened for the presence of IgG1 and IgG2 as outlined in Materials and Methods. Each symbol represents data obtained with cells from an individual subject. Five subjects were studied. The line connects the means for the five determinations at each dose. (A) IgG2; (B) IgG1. Addition of 2,000 MDDC induced significantly higher levels of IgG2 (1.1 ± 0.1 μg/ml for T and B cells alone versus 2.2 ± 0.3 μg/ml for T and B cells cultured with 2,000 MDDC; P < 0.02).
DISCUSSION
Several lines of evidence indicate that the adherent monocytes of LJP subjects exhibit both phenotypic and functional abnormalities (10, 18, 30, 31, 38). Among these is the production of large amounts PGE2 in response to lipopolysaccharides (30). We have recently shown that LJP monocytes also selectively promote the production of the IgG2 subclass of immunoglobulins (18, 38) and that this is likely mediated by soluble factors that are produced by the monocytes. Based on these observations, we predicted that LJP monocytes would differentiate into a population of cells that selectively promoted the production of IgG2. The results of this study supported this hypothesis and indicated that LJP monocytes differentiate not only into macrophages but also into increased numbers of cells with the morphological, phenotypic, and functional characteristics of immature MDDC. Like LJP monocytes, cultures of IL-4- and GM-CSF-generated MDDC were shown to selectively induce the production of IgG2. Together, these data indicate that LJP monocytes have the propensity to differentiate into MDDC and that this is related to the high levels of IgG2 that are produced by LJP subjects.
Immediately ex vivo, the adherent monocytes of LJP and NP subjects exhibited similar morphologies. However, after 4 to 5 days of culture in 10% human serum, the frequency of cells with MDDC morphology was approximately twofold higher in LJP cultures than in NP cultures. At present, it is not certain when the LJP monocytes commit to differentiating into MDDC. We anticipate that this commitment occurs in vivo and may be related to the genetic predisposition to develop LJP (15, 24, 29). To begin to address this possibility, we quantified the levels of MDDC precursors in NP and LJP peripheral blood. Our preliminary data indicate that the frequencies of myeloid dendritic cell precursors (defined as CD3−, CD19−, CD56−, CD14−, CD16−, CD11c+, and HLA-DR+) are similar in NP and LJP peripheral blood. Alternatively, the differentiation of monocytes into MDDC in LJP subjects may be a consequence of the microbial insults associated with the disease. However, the fact that a propensity to differentiate into MDDC is observed in monocytes derived from LJP subjects whose disease is apparently inactive argues against this possibility. Perhaps the most attractive hypothesis for the skewing of monocyte differentiation in LJP subjects is that this process is controlled by one or more of the genes that are associated with the disease. For example, there is precedence for association of early-onset periodontitis with polymorphisms in cytokine genes (12, 16). Cytokine levels and/or the response of monocytes to cytokines, in turn, may be under control of the genes that are associated with LJP. We are currently conducting experiments to determine the mechanism of induction of MDDC in LJP subjects.
In previous studies, we have demonstrated that IgG2 is selectively induced by LJP monocytes and their secretions (18, 38). Interestingly, MDDC generated with IL-4 and GM-CSF had similar effects on the production of IgG2, suggesting that the differentiation of LJP monocytes into this phenotype has important implications for the production of IgG2. There are several potential roles for MDDC in the production of IgG2. Immature MDDC express high levels of class II major histocompatibility complex (MHC) proteins and costimulatory molecules and hence are very potent antigen-presenting cells (1) (9). In addition, MDDC express a variety of pattern recognition receptors and hence are well adapted for uptake and processing of oral pathogens, such as P. gingivalis and A. actinomycetemcomitans (1, 9). In this study, we demonstrated that the MDDC induce IgG2 in a PWM-driven system, and this suggests that MDDC also have antigen-independent effects on the production of IgG2. Among these is the production of cytokines that might skew the antibody response towards IgG2. Several lines of evidence suggest that IgG2 production is dependent on gamma interferon, a Th1 cytokine (19, 20), and MDDC are known to secrete at least two cytokines that induce the Th1 response, IL-12 and IL-18 (6) (14, 21). Our preliminary experiments indicated that neutralizing antibodies against IL-12 and IL-18 reduce PWM-stimulated IgG2 production, suggesting that the MDDC-derived cytokines are indeed involved in IgG2 production. We have also demonstrated that PWM-stimulated IgG2 production is absolutely dependent on the lipid cytokines PGE2 and platelet-activating factor (PAF) and that PAF acts upstream of PGE2 by binding to receptors on LJP monocytes (18). Recent experiments have indicated that the phospholipase that catabolizes PAF, PAF acetylhydrolase, is expressed at much lower levels in MDDC than in macrophages (S. Al-Darmaki et al., unpublished data). Thus, higher levels of PAF should be available in cultures containing LJP monocytes (which differentiate into both macrophages and MDDC) than in cultures containing NP monocytes (which primarily differentiate into macrophages), and this should result in higher levels of IgG2 production. Together, these studies suggest that LJP monocytes differentiate into a cell type that is uniquely adapted for inducing IgG2.
Induction of IgG2 is just one of the unique adaptations of MDDC that allow them to drive the immune response against the oral pathogens that are associated with periodontal disease. These organisms (P. gingivalis and A. actinomycetemcomitans, for example) express a variety of cell surface carbohydrates, and much of the antibody response is directed against these structures (3, 4, 36). Cell surface oligosaccharides also facilitate the phagocytosis of oral bacteria by antigen-presenting cells, such as macrophages and immature MDDC. MDDC are especially well suited for the uptake of oral bacteria as they express a family of pattern recognition receptors that recognize microbial carbohydrates (1, 9). Indeed, Cutler et al. have recently demonstrated that P. gingivalis is readily phagocytosed by immature dendritic cells both in vitro and in vivo and that uptake of P. gingivalis induces dendritic cell maturation (8). Thus, immature MDDC readily engulf the oral bacteria, process antigenic peptides, and load these molecules onto MHC proteins. As MDDC express high levels of MHC class II proteins and costimulatory molecules, they are highly efficient in the presentation of oral pathogen antigens to T cells (1, 9). As noted above, MDDC also produce or facilitate the accumulation of the Th1-associated cytokines that are essential for the production of IgG2 (6, 14, 21). Much of the IgG2 response is directed against carbohydrates (3, 4, 36), the very structures that are highly expressed on the surfaces of oral pathogens and initiate the uptake of these organisms by antigen-presenting cells. The IgG2 subclass of antibodies can activate the alternative pathway of complement, and IgG2 is an effective opsonin when it is paired with the H131 allotype of FcγRII (33, 34). Thus, the MDDC that arise from LJP monocytes promote the production of a subclass of antibody that is uniquely suited to facilitate the immune response against oral pathogens. The utility of this response is underscored by the observation that the severity of LJP is inversely correlated with the levels of IgG2 antibody (2).
Together, these data suggest that the differentiation of LJP monocytes into MDDC is an adaptation that promotes a protective immune response that facilitates the destruction and clearance of oral pathogens. However, as MDDC-derived cytokines are also associated with the induction of Th1 cytokines, such as gamma interferon, and the inflammatory response, these cells also contribute to the pathology that is associated with LJP and other periodontal diseases. Hence, it is likely that the clinical impact of the MDDC is a balance between the destructive effects of inflammation and the protective effects of anti-carbohydrate IgG2. A clearer understanding of the maintenance of this balance and the factors that affect it may lead to more effective treatments for severe periodontal diseases like LJP.
Acknowledgments
This work was supported by grant DE-13102 from the National Institute of Dental and Craniofacial Research.
We thank Robert Tombes (Department of Biology, Virginia Commonwealth University) for use of his fluorescent microscope and digital camera.
Editor: R. N. Moore
REFERENCES
- 1.Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y. J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767-811. [DOI] [PubMed] [Google Scholar]
- 2.Califano, J. V., J. C. Gunsolley, K. Nakashima, H. A. Schenkein, M. E. Wilson, and J. G. Tew. 1996. Influence of anti-Actinobacillus actinomycetemcomitans Y4 (serotype b) lipopolysaccharide on severity of generalized early-onset periodontitis. Infect. Immun. 64:3908-3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Califano, J. V., H. A. Schenkein, and J. G. Tew. 1992. Immunodominant antigens of Actinobacillus actinomycetemcomitans serotype b in early-onset periodontitis patients. Oral Microbiol. Immunol. 7:65-70. [DOI] [PubMed] [Google Scholar]
- 4.Califano, J. V., R. E. Schifferle, J. C. Gunsolley, A. M. Best, H. A. Schenkein, and J. G. Tew. 1999. Antibody reactive with Porphyromonas gingivalis serotypes K1-6 in adult and generalized early-onset periodontitis. J. Periodontol. 70:730-735. [DOI] [PubMed] [Google Scholar]
- 5.Caux, C., S. Lebecque, Y. J. Liu, and J. Banchereau. 1999. Developmental pathways of human myeloid dendritic cells, p. 63-92. In M. T. Lotze and A. W. Thomson (ed.), Dendritic cells: biology and clinical applications. Academic Press, New York, N.Y.
- 6.Cella, M., D. Scheidegger, K. Palmer-Lehmann, P. Lane, A. Lanzavecchia, and G. Alber. 1996. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J. Exp. Med. 184:747-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Champagne, C. M., J. Vaikuntam, M. L. Warbington, L. Rose, M. A. Daniel, and T. E. Van Dyke. 1998. Cytoskeletal actin reorganization in neutrophils from patients with localized juvenile periodontitis. J. Periodontol. 69:209-218. [DOI] [PubMed] [Google Scholar]
- 8.Cutler, C. W., R. Jotwani, K. A. Palucka, J. Davoust, D. Bell, and J. Banchereau. 1999. Evidence and a novel hypothesis for the role of dendritic cells and Porphyromonas gingivalis in adult periodontitis. J. Periodontal Res. 34:406-412. [DOI] [PubMed] [Google Scholar]
- 9.Cutler, C. W., R. Jotwani, and B. Pulendran. 2001. Dendritic cells: immune saviors or Achilles' heel? Infect. Immun. 69:4703-4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daniel, M. A., G. McDonald, S. Offenbacher, and T. E. Van Dyke. 1993. Defective chemotaxis and calcium response in localized juvenile periodontitis neutrophils. J. Periodontol. 64:617-621. [DOI] [PubMed] [Google Scholar]
- 11.Daniel, M. A., and T. E. Van Dyke. 1996. Alterations in phagocyte function and periodontal infection. J. Periodontol. 67:1070-1075. [DOI] [PubMed] [Google Scholar]
- 12.Diehl, S. R., Y. F. Wang, C. N. Brooks, J. A. Burmeister, J. V. Califano, S. B. Wang, and H. A. Schenkein. 1999. Linkage disequilibrium of interleukin-1 genetic polymorphisms with early-onset periodontitis. J. Periodontol. 70:418-430. [DOI] [PubMed] [Google Scholar]
- 13.Donaldson, S. L., G. A. Miller, P. L. Rice, R. R. Ranney, and J. G. Tew. 1981. The maintenance of B-cell and T-cell function in frozen and stored human lymphocytes. J. Clin. Immunol. 1:106-112. [DOI] [PubMed] [Google Scholar]
- 14.Fukao, T., S. Matsuda, and S. Koyasu. 2000. Synergistic effects of IL-4 and IL-18 on IL-12-dependent IFN-gamma production by dendritic cells. J. Immunol. 164:64-71. [DOI] [PubMed] [Google Scholar]
- 15.Hart, T. C. 1996. Genetic risk factors for early-onset periodontitis. J. Periodontol. 67:355-366. [DOI] [PubMed] [Google Scholar]
- 16.Hart, T. C., and K. S. Kornman. 1997. Genetic factors in the pathogenesis of periodontitis. Periodontol. 2000 14:202-215. [DOI] [PubMed] [Google Scholar]
- 17.Hart, T. C., L. Shapira, and T. E. Van Dyke. 1994. Neutrophil defects as risk factors for periodontal diseases. J. Periodontol. 65:521-529. [DOI] [PubMed] [Google Scholar]
- 18.Ishihara, Y., J. B. Zhang, S. M. Quinn, H. A. Schenkein, A. M. Best, S. E. Barbour, and J. G. Tew. 2000. Regulation of immunoglobulin G2 production by prostaglandin E-2 and platelet-activating factor. Infect. Immun. 68:1563-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawano, Y., T. Noma, and J. Yata. 1994. Regulation of human IgG subclass production by cytokines. IFN-gamma and IL-6 act antagonistically in the induction of human IgG1 but additively in the induction of IgG2. J. Immunol. 153:4948-4958. [PubMed] [Google Scholar]
- 20.Kitani, A., and W. Strober. 1993. Regulation of C gamma subclass germ-line transcripts in human peripheral blood B cells. J. Immunol. 151:3478-3488. [PubMed] [Google Scholar]
- 21.Koch, F., U. Stanzl, P. Jennewein, K. Janke, C. Heufler, E. Kampgen, N. Romani, and G. Schuler. 1996. High level IL-12 production by murine dendritic cells: upregulation via MHC class II and CD40 molecules and downregulation by IL-4 and IL-10. J. Exp. Med. 184:741-746. (Erratum, 184:1590.) [DOI] [PMC free article] [PubMed]
- 22.Lu, H., J. V. Califano, H. A. Schenkein, and J. G. Tew. 1993. Immunoglobulin class and subclass distribution of antibodies reactive with the immunodominant antigen of Actinobacillus actinomycetemcomitans serotype b. Infect. Immun. 61:2400-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu, H., M. Wang, J. C. Gunsolley, H. A. Schenkein, and J. G. Tew. 1994. Serum immunoglobulin G subclass concentrations in peridontally healthy and diseased individuals. Infect. Immun. 62:1677-1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marazita, M. L., J. A. Burmeister, J. C. Gunsolley, T. E. Koertge, K. Lake, and H. A. Schenkein. 1994. Evidence for autosomal dominant inheritance and race-specific heterogeneity in early-onset periodontitis. J. Periodontol. 65:623-630. [DOI] [PubMed] [Google Scholar]
- 25.Nemoto, E. M., S. Nakamura, S. Shoji, and H. Horiuchi. 1997. Circulating promyelocytes and low levels of CD16 expression on polymorphonuclear leukocytes accompany early-onset periodontitis. Infect. Immun. 65:3906-3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Offenbacher, S., and G. E. Salvi. 1999. Induction of prostaglandin release from macrophages by bacterial endotoxin. Clin. Infect. Dis. 28:505-513. [DOI] [PubMed] [Google Scholar]
- 27.Romani, N., D. Reider, M. Heuer, S. Ebner, E. Kampgen, B. Eibl, D. Niederwieser, and G. Schuler. 1996. Generation of mature dendritic cells from human blood. An improved method with special regard to clinical applicability. J. Immunol. Methods 196:137-151. [DOI] [PubMed] [Google Scholar]
- 28.Sato, M., K. Iwakabe, A. Ohta, M. Sekimoto, M. Nakui, T. Koda, S. Kimura, and T. Nishimura. 2000. Functional heterogeneity among bone marrow-derived dendritic cells conditioned by T(h)1-and T(h)2-biasing cytokines for the generation of allogeneic cytotoxic T lymphocytes. Int. Immunol. 12:335-342. [DOI] [PubMed] [Google Scholar]
- 29.Schenkein, H. A. 1998. Inheritance as a determinant of susceptibility for periodontitis. J. Dent. Educ. 62:840-851. [PubMed] [Google Scholar]
- 30.Shapira, L., W. A. Soskolne, M. N. Sela, S. Offenbacher, and V. Barak. 1994. The secretion of PGE2, IL-1 beta, IL-6, and TNF alpha by adherent mononuclear cells from early onset periodontitis patients. J. Periodontol. 65:139-146. [DOI] [PubMed] [Google Scholar]
- 31.Shapira, L., W. A. Soskolne, and T. E. Van Dyke. 1996. Prostaglandin E2 secretion, cell maturation, and CD14 expression by monocyte-derived macrophages from localized juvenile periodontitis patients. J. Periodontol. 67:224-228. [DOI] [PubMed] [Google Scholar]
- 32.Tyagi, S. R., D. J. Uhlinger, J. D. Lambeth, C. Champagne, and T. E. Van Dyke. 1992. Altered diacylglycerol level and metabolism in neutrophils from patients with localized juvenile periodontitis. Infect. Immun. 60:2481-2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Schie, R. C. A. A., and M. E. Wilson. 2000. Evaluation of human Fc gamma RIIA (CD32) and Fc gamma RIIIB (CD16) polymorphisms in Caucasians and African-Americans using salivary DNA. Clin. Diagn. Lab. Immunol. 7:676-681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson, M. E., P. M. Bronson, and R. G. Hamilton. 1995. Immunoglobulin G2 antibodies promote neutrophil killing of Actinobacillus actinomycetemcomitans. Infect. Immun. 63:1070-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson, M. E., and R. G. Hamilton. 1992. Immunoglobulin G subclass response of localized juvenile periodontitis patients to Actinobacillus actinomycetemcomitans Y4 lipopolysaccharide. Infect. Immun. 60:1806-1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilson, M. E., and R. E. Schifferle. 1991. Evidence that the serotype b antigenic determinant of Actinobacillus actinomycetemcomitans Y4 resides in the polysaccharide moiety of lipopolysaccharide. Infect. Immun. 59:1544-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zambon, J. J., V. I. Haraszthy, G. Hariharan, E. T. Lally, and D. R. Demuth. 1996. The microbiology of early-onset periodontitis: association of highly toxic Actinobacillus actinomycetemcomitans strains with localized juvenile periodontitis. J. Periodontol. 67(Suppl.):282-290. [DOI] [PubMed] [Google Scholar]
- 38.Zhang, J. B., S. M. Quinn, M. Rausch, J. C. Gunsolley, H. A. Schenkein, and J. G. Tew. 1996. Hyper-immunoglobulin G2 production by B cells from patients with localized juvenile periodontitis and its regulation by monocytes. Infect. Immun. 64:2004-2009. [DOI] [PMC free article] [PubMed] [Google Scholar]