Abstract
O antigen is part of the lipopolysaccharide present in the outer membrane of gram-negative bacteria and is highly polymorphic. In this study, we obtained sequences of the O-antigen gene clusters for the Yersinia pseudotuberculosis antigens IA, IIA, and IVB. We propose that the IIA gene cluster was derived from the IVB cluster, one of the very few cases in which a parent gene cluster is identified, and that the IA gene cluster could be a hybrid of the IVB and IB gene clusters. All three O antigens contain 6-deoxy-d-mannoheptose, and we identified six genes for the biosynthetic pathway for the precursor of this sugar, GDP-6-deoxy-d-mannoheptose.
Lipopolysaccharide (LPS), an important component of the outer membrane of gram-negative bacteria, usually consists of three distinct regions: lipid A, core oligosaccharide, and O-specific polysaccharide (O antigen). The O antigen consists of repeats of an O unit of generally two to six sugars, and in most species it is highly polymorphic, with many forms that can vary in the types of sugars present, in their relative arrangement within the repeat unit, and in the linkage between repeat units (12). The genes for O-antigen synthesis are normally grouped together on the chromosome in a gene cluster which maps between hemH and gsk in Yersinia pseudotuberculosis.
We, among others, have undertaken an extensive study of the genetic basis of O-antigen variation by sequencing and identifying O-antigen genes, mostly in Salmonella enterica and Escherichia coli (see references 24 and 25 for reviews). Comparison of sequences has provided evidence for the origin of new O-antigen forms by recombination between gene clusters in S. enterica (5, 40) and evidence for recent interspecies transfer of an entire O-antigen gene cluster (28, 33).
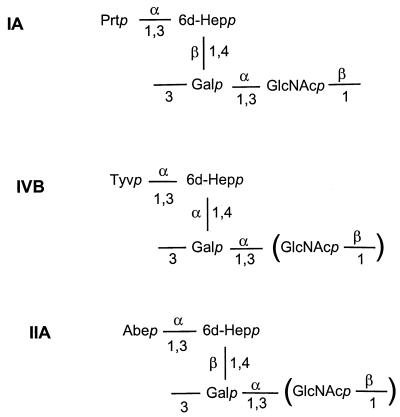
Y. pseudotuberculosis O antigens are of particular interest, since the reported forms include all five naturally occurring 3,6-dideoxyhexose (DDH) sugars (abequose, colitose, paratose, tyvelose, and ascarylose) (6), otherwise rarely found in nature. The IA, IIA, and IVB O antigens have similar structures but differ in the presence of paratose, tyvelose, and abequose, respectively (Fig. 1). The biosynthetic pathways for the activated nucleotide forms of these DDH sugars are related (21), and the genes involved for all three have been identified in S. enterica (4, 14, 20, 35, 40) and Y. pseudotuberculosis (11, 15). Genes ddhABCD for the conversion of glucose-1-phosphate to CDP-4-keto-3,6-dideoxy-d-glucose are common to the three pathways. This compound can then be converted either to CDP-abequose by CDP-abequose synthetase (abe) or to CDP-paratose synthetase (prt). CDP-paratose can then be converted into CDP-tyvelose by CDP-tyvelose epimerase (tyv).
FIG. 1.
Structures of Y. pseudotuberculosis O antigens IA, IVB, and IIA (17, 27, 30).
We previously reported sequences of the DDH genes from the IA and IIA O-antigen gene clusters (11, 15) and found the order to be the same as in S. enterica. In this study we report the complete DNA sequences of the O antigens of Y. pseudotuberculosis IA, IIA, and IVB. The sequences reveal DDH genes, 6-deoxy-d-mannoheptose pathway genes, the chain length determinant gene (wzz), the O-unit flippase gene (wzx), the O-antigen polymerase gene (wzy), and glycosyltransferase genes. Sequence comparisons suggest that the IIA gene cluster was formed by introducing an abe gene into a IVB gene cluster, providing one of the few clear cases of such a relationship. It also appears that the IA gene cluster is a hybrid of IVB and IB gene clusters.
Plasmids and bacterial strains.
Plasmid pPR1670 was as described by Hobbs and Reeves (11). Plasmids constructed for sequencing were maintained in E. coli K-12 strain DH10B. The Y. pseudotuberculosis strains H892/87 (serotype IA, laboratory name M444) and H715/86 (IVB, M454) were obtained from S. Aleksic, Institute of Hygiene, Hamburg, Germany. Y. pseudotuberculosis IIA strain M85 was obtained from D. Hughes, NSW Dairy Corp. Lab (Australia).
DNA isolation, manipulation, and sequencing.
We used the JUMPstart sequence (primer 412 [ATTGGTAGCTGTAAGCCAAGGGCGGTAGCGT]), which is a 39-bp element present upstream of many polysaccharide gene clusters (10), and the gsk sequence (primer 413 [GCGGGCATTGACTGGAAAGTAGTGTTTGGAC]), which is present downstream of O-antigen gene clusters in Y. pseudotuberculosis, to amplify the IIA and IVB O-antigen gene clusters by doing long PCR. PCR fragments of ∼20 kb were obtained from strains M85 and M454, subjected to DNase I digestion, and cloned into pGEM-T to make banks for sequencing as described previously (39). The products of 32 individual PCRs were pooled to make each bank to limit the effect of PCR errors.
The IA sequence was obtained from three overlapping DNA fragments. The sequence from positions 4417 to 10136 was obtained from a partial Sau3A bank made from the insert of pPR1670 (11). The sequence from positions 1 to 4416 and from positions 10137 to 20511 was obtained from DNase I banks made from PCR products amplified from chromosomal DNA by using the JUMPstart or gsk primers and a primer based on the sequence of pPR1670.
The DNA template for sequencing was prepared by using the 96-well format plasmid DNA Miniprep kit from Advanced Genetic Technologies Corp. and the procedure developed by The Institute for Genome Research (34). Sequencing was performed with an Applied Biosystems 377 automated DNA sequencer. Sequence data were assembled by using the Phred/Phrap package of the University of Washington Genome Center, and the sequence annotation was done by using the program Artemis from the Sanger Centre. We used the algorithm described by Eisenberg et al. (8) to identify potential transmembrane segments from the amino acid sequence.
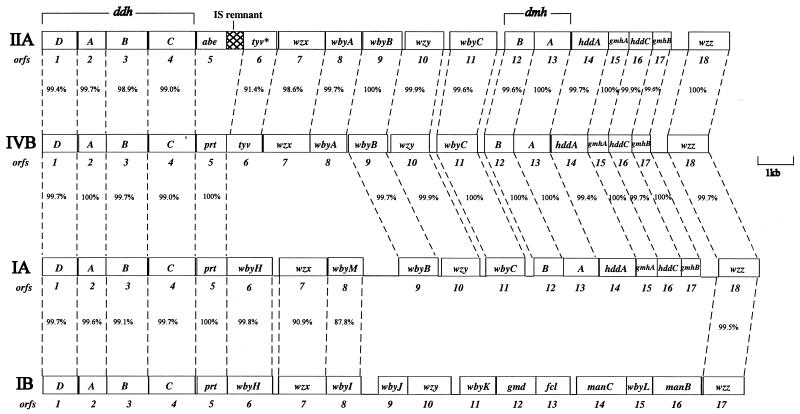
Sequences of 20,511, 19,535, and 19,457 bp, which cover the entire O-antigen gene cluster and the 5′ half of the gsk gene, were obtained from IA, IIA, and IVB, respectively. Analysis showed that there are 18 genes per gene cluster, including one obviously defective gene in IIA (Fig. 2). All were transcribed from JUMPstart to gsk. The nucleotide and amino acid sequences of each gene were used to search available databases for indications of function.
FIG. 2.
O-antigen gene clusters of Y. pseudotuberculosis IVB, IIA, IA, and IB. The 18 open reading frames are numbered 1 to 18 in map order. The IB gene cluster (29) is included for comparison.
DDH biosynthetic pathway genes.
The IIA, IA, and IVB O antigens contain abequose, paratose, and tyvelose, respectively (Fig. 1). We have previously sequenced the region from positions 1 to 6753 of the IIA O-antigen gene cluster and identified the ddhABCD and abe genes which are responsible for the synthesis of CDP-abequose (11). The ddhABCD genes are also present in the IA and IVB O-antigen gene clusters (Fig. 2) and are almost identical (99.4 to 100%) in the three gene clusters. The prt genes of IA and IVB are 100% identical to each other and Y. pseudotuberculosis IB and 99.8% identical to those of Yersinia pestis (29) (note that Y. pestis is a clone of Y. pseudotuberculosis [1]). The tyv gene of IVB is 68% identitical to that of S. enterica serovar Typhimurium (14). Thus, all of the genes necessary for the synthesis of CDP-paratose (ddhABCD and prt) and CDP-tyvelose (ddhABCD, prt, and tyv) are found in IA and IVB, respectively.
6dDHep biosynthetic pathway genes.
6-Deoxy-d-mannoheptose (6dDHep) is present in all three O antigens and is not otherwise found in Y. pseudotuberculosis (29). It is closely related to l-glycero-d-manno-heptose (LDHep), which is present in the LPS inner core of many species of gram-negative bacteria (23). d-Glycero-d-manno-heptose (DDHep) has also been reported in the LPS outer core region of some species and since carbon six, the asymmetric carbon atom that differentiates LDHep and DDHep, is reduced in 6dDHep and no longer asymmetric, one can envisage either LDHep or DDHep as the precursor for 6dDHep.
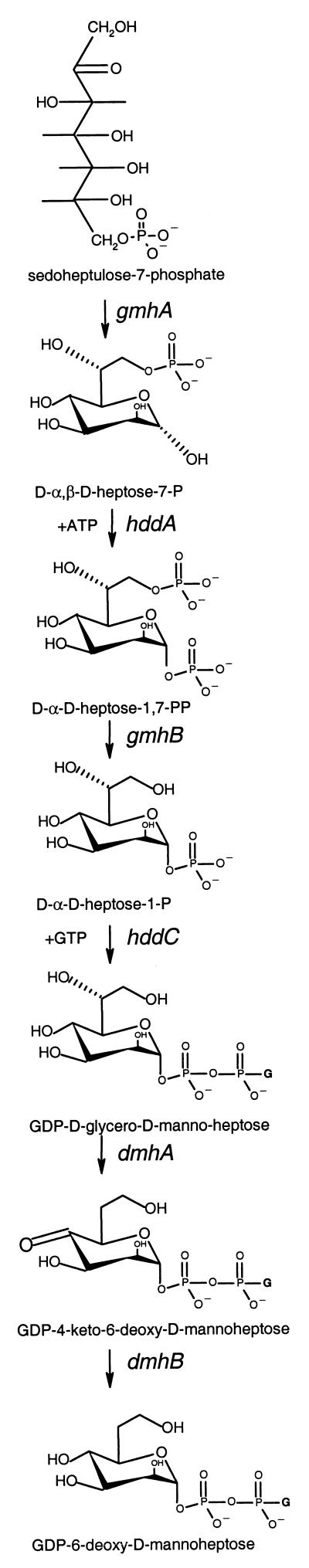
Kneidinger et al. (16) have recently shown that DDHep of Aneurinibacillus thermoaerophilus capsule is synthesized as precursor GDP-DDHep from sedoheptulose 7-phosphate via a four-enzyme pathway (see steps 1 to 4 of Fig. 3). Our three Y. pseudotuberculosis O-antigen gene clusters have in common Orf14 to Orf17, which are homologous to the GDP-DDHep pathway genes of A. thermoaerophilus, suggesting that it is the GDP-DDHep pathway that is extended to give the 6dDHep precursor (Fig. 3). Orf14, Orf15, Orf16, and Orf17 show 50, 61, 42, and 42% identity to HddA, GmhA, HddC, and GmhB of A. thermoaerophilus, respectively. It seems clear that the Y. pseudotuberculosis genes have the same functions as those of A. thermoaerophilus and have been given the same names.
FIG. 3.
Proposed biosynthetic pathway of GDP-6-deoxy-d-mannoheptose.
Biosynthesis of 6-deoxyhexoses starts with nucleotide diphospho sugars and involves firstly oxidation of C-4 and then the reduction of C-6, followed by reduction of C-4 to give a specific 6-deoxyhexose (26). We suggest that GDP-6dDHep is formed in this way from GDP-DDHep (Fig. 3, steps 5 and 6). Orf13 of the three gene clusters is similar to Gmd, which carries out the oxidation of C-4 and the reduction of C-6 on GDP-mannose. GDP-DDHep differs from GDP-mannose only in having a CH2OH substitution on C-6, and we suggest that Orf13 converts GDP-DDHep to GDP-4-keto-6-deoxy-d-mannoheptose. This leaves only the final C-4 reduction step, and we suggest that this is carried out by Orf12, which is similar to many sugar dehydratases, converting GDP-4-keto-6-deoxy-d-mannoheptose to GDP-6dDHep. We have named the Orf13 and Orf12 genes dmhA and dmhB, respectively.
The proposed GDP-6dDHep pathway includes four genes of the GDP-DDHep pathway and two additional genes. The six genes are present in the same order in a capsular gene cluster of Burkholderia mallei (7), with amino acid identity levels between B. mallei and Y. pseudotuberculosis gene products ranging from 35 to 53%. This capsule is a homopolymer of 6dDHep, and the presence of the set of six genes in the same order in B. mallei confirms our designation of genes for the GDP-6dDHep pathway.
Genes wzz, wzx, and wzy.
A putative wzz gene was found in each cluster. The three genes (orf18 in each case) are highly similar (Fig. 2) and show 91% identity to wzz of Y. pestis (29) and 92% identity to wzz of Y. pseudotuberculosis IIA, which was sequenced and studied by Stevenson et al. (32). The protein has two predicted transmembrane segments, as for many other Wzz proteins.
The presumptive wzy genes (orf10) are near identical in the three gene clusters (Fig. 2). The deduced proteins have many predicted transmembrane segments and were most similar to Wzy proteins of E. coli O157 (39), E. coli O111 (37), S. enterica O4 (14), and E. coli K-12 (31). BLOCKMAKER and PSI-BLAST operations were carried out (38) and, after four iterations, the four input proteins and many other distantly related Wzy proteins but no other proteins were retrieved (E value ≤ 4e × 10−35). We propose that orf10 is the wzy gene.
The presumptive wzx genes (orf7) were first identified as encoding an integral inner membrane protein with 12 predicted transmembrane segments. Orf7 of IA shows 99% identity to Wzx of Y. pestis and 90% identity to Wzx of Y. pseudotuberculosis IB (29), and orf7 of IA was identified as wzx. The orf7 genes of IVB and IIA are 98.6% identical and were identified as wzx by carrying out BLOCKMAKER and PSI-BLAST operations as described above.
Transferase genes and comments on structures of IIA and IVB.
Sugar tranferase genes can usually be identified by being most similar to other sugar transferase genes but often cannot be allocated to specific transferase specificities on this basis. We found three putative transferase genes for each of the three O-antigen gene clusters. None of the predicted proteins had the several putative transmembrane segments found in WecA and WbaP, which add a sugar phosphate to UndPP to initiate synthesis of the O unit (14, 22) and, since all structures include GlcNAc (30), we suggest that, as for E. coli GlcNAc-containing O antigens, synthesis is initiated by the transfer of GlcNAc-1-P to UndP by WecA (3), the same reaction that initiates synthesis of enterobacterial common antigen (ECA). Skurnik et al. (29) showed that WecA starts the O-antigen biosynthesis in Y. enterocolitica serotype O8 when the gene cluster was transferred to E. coli (41). It has also been shown that Y. pestis expresses ECA (36), and we found that the wecA gene was present in an ECA gene cluster in the Y. pestisgenome (http://www.sanger.ac.uk/Projects/Y_pestis) (74%identical to E. coli wecA). It seems that in Yersinia sp. as in E. coli, WecA can act as first transferase for O antigens containing GlcNAc, and given that only three transferases were identified within the IA, IIA, and IVB O-antigen gene clusters, we propose that WecA initiates synthesis in all three.
The wbyC genes of the three clusters are virtually identical (Fig. 2) and were most similar to wbbP of E. coli Dysenteriae 1 (34% identity) (9), and WbyC could be the transferase for the same linkage in the three Y. pseudotuberculosis gene clusters.
WbyB, also common to all three gene clusters, is similar to many putative sugar transferases, and we suggest that it is the transferase for putting the 6dDHep residue onto galactose since this linkage is also common to all three structures.
The third putative sugar transferase genes, wbyA in IIA and IVB and wbyM in IA, are both between wzx and wbyB (Fig. 2). wbyA is similar to wbaV of S. enterica groups B and D (62 and 65% identity, respectively), which encode transferases for abequose or tyvelose (19), although both forms are nonspecific for the dideoxyhexose (19, 20). We suggest that WbyA is the tyvelose or abequose transferase of IVB and IIA. WbyM is only 21% identical to WbyA but is similar to several sugar transferases and is presumably the paratose transferase, since paratose is unique to IA and this is the only linkage specific to IA. It is of particular interest that wbyM is 87.8% identical to wbyI, a putative glycosyltransferase, of Y. pseudotuberculosis IB (29). IA and IB both have paratose 1-3 linked to mannose and 6dDHep, respectively, which are closely related sugars, adding strong support for wbyM and wbyI being paratose transferases.
The IIA gene cluster was derived from that of IVB.
The IIA cluster differs from the IVB cluster by the presence of an abe gene in place of the prt gene of IVB and because the tyv gene of IIA is defective and not functional. The similarity between IIA and IVB sequences for all but abe, tyv, and prt indicates that one gene cluster is derived from the other, whereas the levels of identity, ranging from 98 to 100% (Fig. 2), indicate relatively recent divergence. The structures for IIA and IVB differ only in the presence of abequose or tyvelose, respectively, as the DDH sugar, and replacement of prt and tyv by abe is precisely what is required to change the DDH sugar. The tyv gene is useful only in the presence of a prt gene, and the defective tyv gene in IIA indicates that the ancestral form had both tyv and prt. The formation of the IIA cluster with abe must have involved replacement of prt and tyv by abe in a recombination event probably mediated by the insertion sequence (IS), of which a substantial remnant can still be seen. The defective tyv gene of IIA and the IS remnant adjacent to abe had been observed previously (11, 15) and led us to postulate an IS-mediated recombination event. The IVB gene cluster, for which we now present the sequence, has the characteristics expected of the ancestral form for the IIA gene cluster.
Given that in both S. enterica and Y. pseudotuberculosis the tyv, prt, abe, and asc genes that code for the later steps in DDH synthesis always map downstream of the ddh genes, it is reasonable to assume this arrangement in the donor strain, which allows homologous recombination at one end and IS-mediated recombination at the other as proposed for the origin of the S. enterica D2 gene cluster (40).
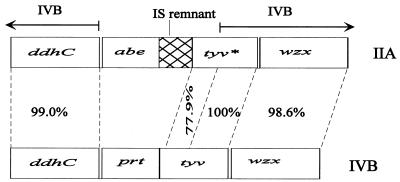
The situation is complicated by the observation that, whereas the 3′ end of the tyv genes (bases 5820 to 6753 of IIA) is identical in the IIA and IVB gene clusters, the 5′ end of the IIA tyv gene is defective (Fig. 4). The first 76 bp are missing, and the remaining part of the 5′ end differs from the functional IVB gene by 18%, including several deletions and an overall frameshift. It appears that the two parts of the tyv gene may have entered at different times, suggesting two steps in the assembly of the IIA gene cluster.
FIG. 4.
The region around the tyv gene of the O-antigen gene clusters of Y. pseudotuberculosis IIA and IVB indicates the probable origin of the IIA gene cluster. Note that the 5′ end of tyv* of IIA has accumulated many mutations, and this part shares 77.9% identity with the corresponding region of IVB. The asterisk in tyv* indicates a defective gene.
The IA gene cluster is potentially a hybrid of IVB and IB gene clusters.
The 5′ end of the IA gene cluster to prt and the 3′ end from wbyB are nearly identical to the corresponding segments of IVB (Fig. 2). However, the intervening segment of IA, including the wbyH and wbyM genes, is absent in IIA and IVB, but is present, with wbyI replacing wbyM, in IB (wbyM and the very similar wbyI are both putative paratose transferases as discussed above). The IB gene cluster also has the DDH genes common to IA, IIA, and IVB. The IA gene cluster is in effect a chimera of those of IVB and IB (see Fig. 2). There is also a 1,012-bp intergenic region between wbyI and wbyB in IA that is not similar to any known sequence.
It seems clear that one or more of IA, IB, and IVB arose by recombination, with IVB perhaps being the recipient of the segment wbyH to wbyM/I from IB to form IA. If so, it is interesting that wzx and wbyI/M have undergone 10% divergence, whereas wbyH has undergone very little (Fig. 2). The Wzx proteins are responsible for the translocation of two different O units across the inner membrane, whereas WbyI and WbyM are proposed to put the same residue (paratose) onto different sugars in IA and IB, so the divergence may reflect functional differences.
wbyH is 96% identical to wbyH of Y. pseudotuberculosis IB and Y. pestis, which was proposed (29) to be a sugar pathway gene. As discussed above, we have identified the genes for the synthesis of CDP-paratose and GDP-6dDHep, and this leaves no obvious function for wbyH. Skurnik et al. (29) also did not assign a function to wbyH in the IB gene cluster, with all required functions accounted for by other genes.
Concluding remarks.
There are 12 O-antigen forms characterized in Y. pseudotuberculosis (2), and we now have sequences for four of them, including the three presented in this study. The ddhABCD genes are located at the 5′ end of the gene cluster (Fig. 2) in all four clusters, and these four genes are highly conserved.
The three new sequences are all for 6-deoxy-d-mannoheptose containing O antigens, and this has enabled us to identify the biosynthetic pathway genes which are at the 3′ end of the gene cluster just upstream of wzz gene (Fig. 2). It is probably no coincidence that the ddh and DDHep genes are conserved and at the 5′ and 3′ ends, respectively, of the gene clusters, since both are present in several gene clusters. This pattern for such pathway genes is seen, for example, in E. coli, S. enterica, and Streptococcus pneumoniae for rml genes, which are quite commonly present in these species, where it facilitates recombination among gene clusters (13, 18).
Nucleotide sequence accession numbers.
The Y. pseudotuberculosis IA, IIA, and IVB O-antigen gene cluster sequences have been deposited in GenBank under accession numbers AF461768, AF461770, and AF461769, respectively.
Acknowledgments
This investigation was supported by the Australian Research Council.
Editor: D. L. Burns
REFERENCES
- 1.Achtman, M., K. Zurth, G. Morelli, G. Torrea, A. Guiyoule, and E. Carniel. 1999. Yersinia pestis, the cause of plague, is a recently emerged clone of Yersinia pseudotuberculosis. Proc. Natl. Acad. Sci. USA 96:14043-14048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aleksic, S., G. Suchan, J. Bockemühl, and V. Aleksic. 1991. An extended antigenic scheme for Yersinia pseudotuberculosis, p. 235-238. In T. Une, T. Maruyama, and M. Tsubokura (ed.), Current investigations of the microbiology of yersiniae. Contributions to microbiology and immunology, vol. 12. Karger, Basel, Switzerland. [PubMed] [Google Scholar]
- 3.Alexander, D. C., and M. A. Valvano. 1994. Role of the rfe gene in the biosynthesis of the Escherichia coli O7-specific lipopolysaccharide and other O-specific polysaccharides containing N-acetylglucosamine. J. Bacteriol. 176:7079-7084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown, P. K., L. K. Romana, and P. R. Reeves. 1992. Molecular analysis of the rfb gene cluster of Salmonella serovar Muenchen (strain M67): genetic basis of the polymorphism between groups C2 and B. Mol. Microbiol. 6:1385-1394. [DOI] [PubMed] [Google Scholar]
- 5.Curd, H., D. Liu, and P. R. Reeves. 1998. Relationships among the O-antigen gene clusters of Salmonella enterica groups B, D1, D2, and D3. J. Bacteriol. 180:1002-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies, D. A. L. 1961. Dideoxysugars of Pasteurella pseudotuberculosis-specific polysaccharides, and the occurrence of ascarylose. Nature 191:91-97. [DOI] [PubMed] [Google Scholar]
- 7.DeShazer, D., D. M. Waag, D. L. Fritz, and D. E. Woods. 2001. Identification of a Burkholderia mallei polysaccharide gene cluster by subtractive hybridization and demonstration that the encoded protein capsule is an essential virulence determinant. Microb. Pathog. 30:253-269. [DOI] [PubMed] [Google Scholar]
- 8.Eisenberg, D., E. Schwarz, M. Komaromy, and R. Wall. 1984. Analysis of membrane and surface protein sequences with the hydrophobic moment plot. J. Mol. Biol. 179:125-142. [DOI] [PubMed] [Google Scholar]
- 9.Gohmann, S., P. A. Manning, C. A. Alpert, M. J. Walker, and K. N. Timmis. 1994. Lipopolysaccharide O-antigen biosynthesis in Shigella dysenteriae serotype 1: analysis of the plasmid-carried rfp determinant. Microb. Pathog. 16:53-64. [DOI] [PubMed] [Google Scholar]
- 10.Hobbs, M., and P. R. Reeves. 1994. The JUMPstart sequence: a 39-bp element common to several polysaccharide gene clusters. Mol. Microbiol. 12:855-856. [DOI] [PubMed] [Google Scholar]
- 11.Hobbs, M., and P. R. Reeves. 1995. Genetic organisation and evolution of Yersinia pseudotuberculosis 3,6-dideoxyhexose biosynthetic genes. Biochim. Biophys. Acta 1245:273-277. [DOI] [PubMed] [Google Scholar]
- 12.Jann, K., and B. Jann. 1984. Structure and biosynthesis of O-antigen, p. 138-186. In E. T. Rietschel (ed.), Handbook of endotoxin, vol. 1. Elsevier Scientific Publishers, Amsterdam, The Netherlands. [Google Scholar]
- 13.Jiang, S.-M., L. Wang, and P. R. Reeves. 2001. Molecular characterization of Streptococcus pneumoniae type 4, 6B, 8, and 18C capsular polysacharide gene clusters. Infect. Immun. 69:1244-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang, X. M., B. Neal, F. Santiago, S. J. Lee, L. K. Romana, and P. R. Reeves. 1991. Structure and sequence of the rfb (O antigen) gene cluster of Salmonella serovar typhimurium (strain LT2). Mol. Microbiol. 5:695-713. [DOI] [PubMed] [Google Scholar]
- 15.Kessler, A., A. Haase, and P. R. Reeves. 1993. Molecular analysis of the 3,6-dideoxyhexose pathway genes of Yersinia pseudotuberculosis serogroup IIa. J. Bacteriol. 175:1412-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kneidinger, B., M. Graninger, M. Puchberger, P. Kosma, and P. Messner. 2001. Biosynthesis of nucleotide-activated d-glycero-d-manno-heptose. J. Biol. Chem. 276:20935-20944. [DOI] [PubMed] [Google Scholar]
- 17.Komandrova, N. A., R. P. Gorshkova, V. V. Isakov, and Y. S. Ovodov. 1984. Structure of O-specific polysaccharide isolated from lipopolysaccharide of Yersinia pseudotuberculosis 1A serovar. Bioorg. Khim. 10:232-237. [PubMed] [Google Scholar]
- 18.Li, Q., and P. R. Reeves. 2000. Genetic variation of dTDP-l-rhamnose pathway genes in Salmonella enterica. Microbiology 146:2291-2307. [DOI] [PubMed] [Google Scholar]
- 19.Liu, D., L. Lindquist, and P. R. Reeves. 1995. Transferases of O-antigen biosynthesis in Salmonella enterica: dideoxhexosyl transferases of groups B and C2 and acetyltransferase of group C2. J. Bacteriol. 177:4084-4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu, D., N. K. Verma, L. K. Romana, and P. R. Reeves. 1991. Relationships among the rfb regions of Salmonella serovars A, B, and D. J. Bacteriol. 173:4814-4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu, H. W., and J. S. Thorson. 1994. Pathways and mechanisms in the biogenesis of novel deoxysugars by bacteria. Annu. Rev. Microbiol. 48:223-256. [DOI] [PubMed] [Google Scholar]
- 22.Meier-Dieter, U., K. Barr, R. Starman, L. Hatch, and P. D. Rick. 1992. Nucleotide sequence of the Escherichia coli rfe gene involved in the synthesis of enterobacterial common antigen. J. Biol. Chem. 267:746-753. [PubMed] [Google Scholar]
- 23.Raetz, C. R. H. 1990. Biochemistry of endotoxins. Annu. Rev. Biochem. 59:129-170. [DOI] [PubMed] [Google Scholar]
- 24.Reeves, P. R. 1994. Biosynthesis and assembly of lipopolysaccharide, p. 281-314. In A. Neuberger and L. L. M. van Deenen (ed.), Bacterial cell wall, vol. 27. Elsevier Science Publishers, Amsterdam, The Netherlands. [Google Scholar]
- 25.Reeves, P. R. 1993. Evolution of Salmonella O antigen variation by interspecific gene transfer on a large scale. Trends Genet. 9:17-22. [DOI] [PubMed] [Google Scholar]
- 26.Robyt, J. F. 1998. Essentials of carbohydrate chemistry. Springer-Verlag, New York, N.Y.
- 27.Samuelsson, K., B. Lindberg, and R. R. Brubaker. 1974. Structure of O-specific side chains of lipopolysaccharides from Yersinia pseudotuberculosis. J. Bacteriol. 117:1010-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shepherd, J. G., L. Wang, and P. R. Reeves. 2000. Comparison of O-antigen gene clusters of Escherichia coli (Shigella) Sonnei and Plesiomonas shigelloides O17: Sonnei gained its current plasmid-borne O-antigen genes from P. shigelloides in a recent event. Infect. Immun. 68:6056-6061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skurnik, M., A. Peippo, and E. Ervela. 2000. Characterization of the O-antigen gene clusters of Yersinia pseudotuberculosis and the cryptic O-antigen gene cluster of Yersinia pestis shows that the plague bacillus is most closely related to and has evolved from Y. pseudotuberculosis serotype O:1b. Mol. Microbiol. 37:316-330. [DOI] [PubMed] [Google Scholar]
- 30.Skurnik, M., and L. Zhang. 1996. Molecular genetics and biochemistry of Yersinia lipopolysaccharide. APMIS 104:849-872. [PubMed] [Google Scholar]
- 31.Stevenson, G., B. Neal, D. Liu, M. Hobbs, N. H. Packer, M. Batley, J. W. Redmond, L. Lindquist, and P. R. Reeves. 1994. Structure of the O-antigen of Escherichia coli K-12 and the sequence of its rfb gene cluster. J. Bacteriol. 176:4144-4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stevenson, G. S., A. Kessler, and P. R. Reeves. 1995. A plasmid-borne O-antigen chain length determinant and its relationship to other chain length determinants. FEMS Microbiol. Lett. 125:23-30. [DOI] [PubMed] [Google Scholar]
- 33.Sugiyama, T., N. Kido, Y. Kato, N. Koide, T. Yoshida, and T. Yokochi. 1998. Generation of Escherichia coli O9a serotype, a subtype of E. coli O9, by transfer of the wb* gene cluster of Klebsiella O3 into E. coli via recombination. J. Bacteriol. 180:2775-2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Utterback, T. R., L. A. McDonald, and R. A. Fuldner. 1995. A reliable, efficient protocol for 96-well plasmid DNA miniprep with rapid DNA quantification for high-throughput automated DNA sequencing. Genome Sci. Technol. 1:1-8. [Google Scholar]
- 35.Verma, V., and P. R. Reeves. 1989. Identification and sequence of rfbS and rfbE, which determine antigenic specificity of group A and group D Salmonella. J. Bacteriol. 171:5694-5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vinogradov, E. V., Y. A. Knirel, J. E. Thomas-Oates, A. S. Shashkov, and V. L. L'vov. 1994. The structure of the cyclic enterobacterial common antigen (ECA) from Yersinia pestis. Carbohydr. Res. 258:223-232. [DOI] [PubMed] [Google Scholar]
- 37.Wang, L., H. Curd, W. Qu, and P. R. Reeves. 1998. Sequencing of Escherichia coli O111 O-antigen gene cluster and identification of O111-specific genes. J. Clin. Microbiol. 36:3182-3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang, L., W. Qu, and P. R. Reeves. 2001. Sequence analysis of four Shigella boydii O-antigen loci: implication for Escherichia coli and Shigella relationships. Infect. Immun. 69:6923-6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang, L., and P. R. Reeves. 1998. Organization of Escherichia coli O157 O antigen gene cluster and identification of its specific genes. Infect. Immun. 66:3545-3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xiang, S. H., M. Hobbs, and P. R. Reeves. 1994. Molecular analysis of the rfb gene cluster of a group D2 Salmonella enterica strain: evidence for its origin from an insertion sequence-mediated recombination event between group E and D1 strains. J. Bacteriol. 176:4357-4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang, L., J. Radziejewska-Lebrecht, D. Krajewska-Pietrasik, P. Toivanen, and M. Skurnik. 1997. Molecular and chemical characterization of the lipopolysaccharide O-antigen and its role in the virulence of Yersinia enterocolitica serotype O8. Mol. Microbiol. 23:63-76. [DOI] [PubMed] [Google Scholar]