Abstract
Several pathogenic fungal organisms enter eukaryotic cells and manipulate the host cell environment to favor their own growth and survival. Aspergillus fumigatus is a saprophytic fungus that causes invasive lung disease in the immunocompromised host. To determine whether A. fumigatus could enter eukaryotic cells, we studied the uptake of two different GFP-expressing A. fumigatus strains into A549 lung epithelial cells, human umbilical vein endothelial (HUVE) cells, and J774 murine macrophages in vitro. A549 cells internalized 30% of the bound conidia whereas HUVE and J774 cells internalized 50 and 90%, respectively. Conidia within A549 cells remained viable for 6 h; however, 60 to 80% of conidia within J774 cells were killed after only 4 h. Live and heat-killed conidia were internalized to the same extent by A549 cells. After 6 h, almost none of the conidia inside A549 cells had germinated, whereas extracellular conidia had developed germ tubes. Internalization of conidia by A549 cells was a temperature-dependent process and required rearrangement of the underlying host cell cytoskeleton; uptake was inhibited by 75% with 0.5 μM cytochalasin D and by 65% with 5 μM colchicine. Fluorescent labeling of infected A549 cells with rhodamine phalloidin provided visible evidence of cytoskeletal alteration as many of the intracellular conidia were contained in actin-coated phagosomes. These data provide evidence that significant numbers of A. fumigatus conidia can be internalized by nonprofessional phagocytes in vitro and these cells may serve as reservoirs for immune cell evasion and dissemination throughout the host.
Fungal infections cause significant morbidity and mortality in both animals and humans. Over the past 2 decades there has been a substantial increase in human fungal infections among immunocompromised individuals (15, 26). The growth of this high-risk group has been attributed to more aggressive cytotoxic chemotherapy, an increase in the number of bone marrow and organ transplant recipients and the emergence of AIDS (15). Aspergillus fumigatus is a saprophytic fungus which causes the most common mold infection worldwide (43). Inhalation of infectious conidia and their deposition in the alveoli may lead to germination and growth of the fungus in the lung, and in severely immunocompromised individuals, this may develop into a potentially fatal infection known as invasive aspergillosis (13). Amphotericin B is still the drug of choice for the treatment of invasive aspergillosis, despite its severe side effects (33). The newer generation triazole drugs (voriconazole, posaconazole) and antifungal peptides such as the echinocandins are showing promising results in clinical trials; however, their long-term efficacy remains to be determined (14, 23, 55). Early diagnosis of invasive aspergillosis is crucial (12), and even with treatment, the mortality rate exceeds 65% (11). Basic research into the pathogenesis of A. fumigatus remains a priority and may help identify new drug targets which are desperately needed.
Many pathogenic microorganisms can enter eukaryotic cells and use this often hostile environment as a niche within which to replicate and/or evade the host immune response. These organisms can enter both professional phagocytes and cells that are not normally phagocytic, such as epithelial or endothelial cells (19). Among the fungi, there are several species that invade mammalian host cells in vitro and in vivo . Candida albicans can survive in macrophages (31) and endothelial cells (18). Following uptake by macrophages, C. albicans forms germ tubes within phagolysosomes, escapes from this acidic compartment, and destroys the macrophage (31). Similarly, phagocytosis of Cryptococcus neoformans by macrophages results in disordered lysosomal trafficking, host cell disruption, and release of the organisms (17).
A. fumigatus conidia have been shown to bind to lung cells (10) and proteins present in the lung (42, 54, 56). In addition, two groups have independently demonstrated internalization of A. fumigatus conidia by A549 cells (10) and primary lung cells (41) using electron microscopy. Preliminary results in our laboratory have confirmed previous reports (10, 41) that A. fumigatus conidia bind to and become internalized by A549 lung epithelial cells. However, neither of these published studies compared conidial uptake to a negative control, nor was the mechanism of uptake elucidated. To facilitate the study of conidia uptake, we developed two strains of A. fumigatus that expressed green fluorescent protein (GFP) for use in cell culture infection models. Initial immunolabeling experiments demonstrated that differentiation of intracellular and extracellular conidia could be achieved in conidium-host cell invasion assays. Therefore, the objectives of this study were threefold: (i) to determine the percent internalization of conidia by three different cultured cell lines relative to a negative control, (ii) to determine whether conidia germinate inside of A549 cells, and (iii) to establish whether the internalization of conidia is accompanied by cytoskeletal arrangements in A549 cells.
MATERIALS AND METHODS
A. fumigatus strains and growth conditions.
Two strains of A. fumigatus were used in this study: A. fumigatus ATCC 13703 (American Type Culture Collection, Manassas, Va.) and CHUV (gift from M. Monod, Laboratoire de Mycologie, Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland). Fungi were grown on YM agar (0.3% yeast extract, 0.3% malt extract, 0.5% peptone, and 0.5% dextrose) for 3 days at 37°C until conidia were fully mature. Conidia were harvested as described previously (56).
Tissue culture.
The type II pneumocyte cell line A549 and murine macrophage line J774 were obtained from the American Type Culture Collection and maintained in RPMI 1640 medium containing 10% fetal bovine serum (Canadian Life Technologies, Burlington, Ontario, Canada), streptomycin (100 mg/liter) and penicillin (16 mg/liter) (both from Sigma-Aldrich Canada, Oakville, Ontario, Canada). Human umbilical vein endothelial (HUVE) cells were isolated from human umbilical veins by the method of Jaffe et al. with modifications (30). Fresh umbilical cords were stored at 4°C and processed within 12 h. The umbilical vein was cannulated with a 14-gauge catheter and secured with a cable tie. The vein was flushed with phosphate-buttered saline (PBS) (until clear), and the other end of the vein was then tied. Each cord was infused with collagenase II (162.5 U/ml; Sigma) in M-199 medium (Canadian Life Technologies) and incubated at 37°C for 8 min. The enzyme solution was removed, and the cells were centrifuged at 800 × g for 5 min. The pellet was gently resuspended in 4 ml of M-199 medium containing 10% (vol/vol) fetal bovine serum (FBS), 10% (vol/vol) fetal calf serum, 2 mM glutamine, penicillin, and streptomycin and then added to gelatin-coated petri dishes. All cells were grown at 37°C in humidified 5% CO2 incubators.
Creation of GFP-expressing A. fumigatus strains.
To generate GFP-expressing strains of A. fumigatus, conidia were transformed by electroporation with 0 or 1.5 μg of linearized gGFP plasmid based on the method adapted from Sanchez and Aguirre (48) by Wiedner et al. (57). Reaction mixtures of 200 to 1,000 μl were plated onto YM agar containing hygromycin (250 μg/ml) and incubated for 3 days at 37°C. Conidia from hygromycin-resistant clones were restreaked twice onto YM agar containing hygromycin to check for stable integration.
Detection of GFP expression in hygromycin-resistant transformants. (i) Immunoblotting.
To prepare conidial proteins, wild-type and GFP strains were harvested and resuspended in 10 ml of lysis buffer (100 mM Tris-Cl [pH 7.4], 2.5 mM EDTA, 5 mM dithiothreitol) and a protease inhibitor cocktail containing 40 μM 4-(2-aminoethyl) benzenesulfonyl fluoride, 20 μM sodium EDTA, 2.6 mM bestatin, 0.28 mM trans-epoxysuccinyl-l-leucylamido (4-guanidino) butane (E-64), 20 μM leupeptin, and 6 μM aprotinin (Sigma). An equal volume of 0.5-mm-diameter acid-washed glass beads was added, and the suspension was vortexed at 30-s intervals (followed by 1 min of cooling) until cell breakage was observed by microscopy. The glass beads were removed, and the suspension was centrifuged at 1,500 × g for 10 min. The supernatant fraction consisted of the whole conidial homogenate. To test for expression of the GFP protein, conidial proteins from the homogenate were separated by sodium dodecyl sulfate-12% polyacrylamide gel (SDS-12% PAGE) (6 μg/lane) and transferred to polyvinylidene difluoride membrane using a submerged tank transfer apparatus according to the manufacturer's directions (Bio-Rad Laboratories, Mississauga, Ontario, Canada). The membrane was blocked overnight in BLOTTO (PBS-5% [wt/vol] skim milk powder). The next day the blot was incubated for 2 h with a polyclonal rabbit anti-GFP antibody (diluted to 1:100; Clontech Laboratories Inc., Palo Alto, Calif.) in BLOTTO and then rinsed four times for 5 min each with PBS-0.05% Tween 20 (PBST). The blot was incubated for 1 h in a solution containing goat anti-rabbit horseradish peroxidase-conjugated secondary antibody (Amersham Pharmacia Biotech Inc., Baie d'Urfé, Québec, Canada) (diluted 1:1,000 in BLOTTO), rinsed five times with PBST as described above, and developed with DAB substrate (6 mg diaminobenzidine tetrahydrochloride (Sigma) in 10 mM Tris-Cl [pH 7.6] containing 0.3% NiCl2 and H2O2 [1 μl/ml]).
(ii) Fluorescence microscopy.
To detect whether transformants expressing GFP protein emitted green fluorescence when excited with blue (488 nm) light, conidia were harvested and 2 × 107 spores were added to 12-mm-diameter poly-l-lysine coated coverslips, fixed with paraformaldehyde, mounted onto slides, and sealed with nail polish. Samples were viewed on a Zeiss Axioplan 2 microscope equipped for epifluorescence with fluorescein filters.
Preparation of an anti-Aspergillus antibody.
A. fumigatus mycelial cell wall proteins were used as antigens for the production of a polyclonal antibody that recognizes A. fumigatus conidia. Fernbach flasks containing 800 ml of YM medium were inoculated with 2.5 × 106 A. fumigatus ATCC 13073 conidia/ml and incubated at 150 rpm for 16 h. Mycelial proteins were prepared using the method of Puente et al. (44) and quantitated by a Bradford assay (5). Female New Zealand White rabbits were injected with 1 mg of A. fumigatus mycelial cell wall proteins in 1.5 ml PBS plus 250 μl of TiterMax adjuvant (Sigma). To test serum for the presence of antibodies recognizing A. fumigatus, conidia from strain 13073 were added to an eight-well chamber slide (Becton Dickinson) and reacted with various dilutions of serum prepared in PBS-10% (vol/vol) goat serum. Bound primary antibody was detected by a goat anti-rabbit Texas Red secondary antibody (Molecular Probes, Eugene, Oreg.) diluted 1:100 in PBS-goat serum. Conidia were fixed with 4% (wt/vol) paraformaldehyde in PBS and viewed with an Olympus AHBS3 Vanox microscope equipped for epifluorescence microscopy.
Immunofluorescence-confocal microscopy. (i) Phagocytosis assays.
A549 and HUVE cells were seeded at 2.5 × 105 cells/well on 12-mm-diameter number 1 coverslips in 24-well plates (Falcon, Becton-Dickinson Canada Inc., Mississauga, Ontario, Canada) and grown for 16 h. J774 cells were seeded at 2.5 × 105 cells/well for 2 h at 37°C. Following cell growth, wells were blocked for 1 h in minimum essential medium (MEM) (Canadian Life Technologies) containing 0.1% (wt/vol) bovine serum albumin (ICN Pharmaceuticals, Montreal, Canada) at 37°C. Cells were infected with 1 ml of 106 spores/ml in MEM-10% (vol/vol) FBS for the indicated times at 37°C. To prepare heat-killed spores, conidia from strain 13073 gGFP4 were first autoclaved for 20 min at 121°C. As a control for nonspecific uptake, A549 and HUVE cells were also incubated with 1-μm-diameter fluorescein-labeled biotinylated polystyrene beads (5 × 106/ml; Molecular Probes) diluted in MEM-10% FBS for 3 h at 37°C. A fivefold higher multiplicity of infection (compared to infection with conidia) was required in order to get a reasonable number of beads bound to a field of cells. After incubation, unbound spores or beads were removed by washing wells three times with PBST. Extracellular spores were labeled with the rabbit antibody raised against conidial proteins. Primary antibody was diluted 1:50 in PBS-10% (vol/vol) goat serum (Canadian Life Technologies) and added to samples on ice for 1 h. Wells were washed three times with PBS and then incubated for 45 min with a goat anti-rabbit Alexa Fluor 594-conjugated secondary antibody (Molecular Probes) diluted 1:400 in PBS-10% (vol/vol) goat serum. Wells were washed with PBS again and fixed for 1 h with PBS-4% (wt/vol) paraformaldehyde (pH 7.4), and the coverslips were mounted onto slides with ProLong antifade from Molecular Probes. To label extracellular beads, samples were incubated for 1 h with a 1:100 dilution of Streptavidin-Alexa Fluor 594 secondary reagent and then fixed and mounted as described above. Samples were viewed with a Zeiss Axioplan 2 microscope equipped with epifluorescence filters using a 63× lens. To analyze uptake of conidia, 10 fields per coverslip were captured (5 for J774 samples) with a Sony DXC-950P 3CCD camera using Eclipse image capturing software (Empix Imaging Inc., Mississauga, Ontario, Canada). The extracellular and intracellular conidia were enumerated after merging the red and green channels in Eclipse.
(ii) Actin-rearrangement assays.
Cells were incubated with strain 13073 gGFP for 2 h and 40 min (rather than 3 h) in order to capture the rearrangement process as conidia were being internalized. Following infection, wells were washed with PBST and fixed with paraformaldehyde, and the cells were permeabilized by incubating for 1 h in PBS-10% (vol/vol) goat serum-0.5% (wt/vol) saponin. Coverslips were incubated for 20 min at room temperature with rhodamine phalloidin (Molecular Probes) diluted 1:50 in PBS to stain cellular actin filaments. Samples were washed with PBS, mounted in ProLong, and viewed with a Zeiss LSM-410 confocal microscope equipped with a krypton-argon laser (Omnichrome), using a 63 × 1.4 numerical aperture lens. Green fluorescence was captured with a 515- to 540-nm band pass filter, and red fluorescence was capture with a 590- to 610-nm band pass filter. Images were processed in Adobe PhotoShop 6.0 (Adobe Systems Incorporated, San Jose, Calif.).
Nystatin protection assay.
Cells were seeded into 24-well plates and infected with conidia as described above for the immunofluorescence. Following infection with GFP strains, wells were washed three times with PBST and then incubated with nystatin at 0, 50 (for J774 cells), or 100 μg/ml (for A549 cells) (250 and 500 U/ml, respectively; Sigma) in MEM for 3 h at 37°C. This amount of nystatin was the minimum fungicidal concentration required to kill 106 conidia (data not shown). A lower amount of nystatin was used with J774 cells (250 U/ml), as higher concentrations were cytotoxic to this cell line. Nystatin at this concentration was sufficient to kill 5 × 105 conidia (the number of conidia bound to J774 cells was always less than 5 × 105 conidia/well) but did not affect the viability of either J774 or A549 cells as determined by crystal violet staining (data not shown). After 3 h, monolayers were lysed by incubating in 0.5% Triton X-100 for 10 min with shaking. Released conidia were diluted, plated onto YM agar (two to three replicate plates/well) and incubated at 37°C. After 24 h colonies were counted to determine total bound and intracellular conidia. The binding of the GFP-transformant and the parental 13073 or CHUV strains to A549 cells was similar when examined by differential interference contrast (DIC) microscopy (data not shown). In addition, the GFP transformants behaved similarly to the parental strains in the nystatin protection assay. For example, the invasion index of the 13073 parental strain was 2.7% ± 1.2% of the initial inoculum, compared to the GFP-transformed strain at 4.0% ± 0.8% (P > 0.05).
Invasion assay in the presence of cytoskeletal inhibitors.
Actin polymerization inhibitors such as cytochalasin D and the microtubule inhibitors colchicine and nocodazole can be used to determine whether actin filaments or microtubules are required for pathogen entry into host cells. Inhibitors were made as stock solutions in dimethyl sulfoxide (cytochalasin D) or deionized distilled H2O (colchicine) and added to the invasion medium at the indicated concentrations. The concentrations of inhibitors (and dimethyl sulfoxide) used did not compromise A549 cell viability as determined by trypan blue exclusion or crystal violet staining (data not shown). Morphology of the cells was also normal as determined by bright-field microscopy (data not shown). Assays were performed with strain 13073 gGFP4. Addition of colchicine or cytochalasin D to conidia alone at the concentrations used in the assay did not cause any defects in conidia germination or growth. The results are representative of two independent experiments and are expressed as the mean ± standard deviation of three replicates.
Statistics.
The Student t test was used for statistical analysis of data.
RESULTS
Expression of gGFP in A. fumigatus conidia.
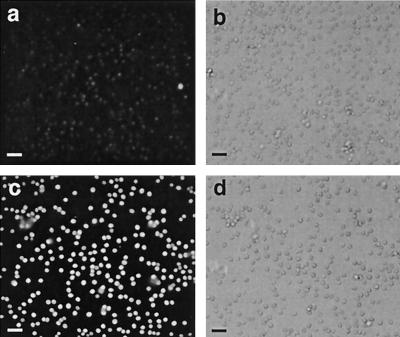
To better understand the interactions between A. fumigatus conidia and host cells we constructed two GFP-expressing strains of A. fumigatus using the gGFP plasmid. In some fungi, the unmodified wild-type GFP protein is not fluorescent (8). gGFP contains the sGFP (S65T) plant codon-optimized gene (7, 27) transcriptionally fused to the Aspergillus nidulans promoter P gpd, known to provide a high level of constitutive expression in ascomycete fungi (35, 49). Conidia from two different A. fumigatus strains, ATCC 13073 and CHUV, were transformed with the gGFP plasmid by electroporation, and five hygromycin-resistant transformants were obtained with both strains. To demonstrate that GFP was expressed in these hygromycin-resistant clones, conidia from wild-type or GFP strains were lysed and the homogenates were run on an SDS-PAGE gel. Immunoblotting of the membrane with an anti-GFP antibody detected a band of the anticipated molecular weight in the transformed strains which was absent in the wild-type strain (Fig. 1). To determine whether this GFP protein was functional, the conidia were observed for green fluorescence under blue light. Under these conditions, the conidia emitted green fluorescence (Fig. 2). In addition, germinating conidia and hyphae were also fluorescent (data not shown). Of the total hygromycin transformants, two from each of the strains were GFP positive; however, only a single clone from each strain was used in the cell uptake studies.
FIG. 1.
Detection of GFP protein in conidia of transformants by immunoblotting. Wild-type (a) or gGFP-transformed (b) conidia from strain 13073 were lysed, and proteins were separated on an SDS-12% PAGE gel and transferred to polyvinylidene difluoride membrane. The membrane was probed with an anti-GFP antibody diluted 1:100 followed by a goat anti-rabbit peroxidase-labeled secondary antibody diluted 1:1,000. Development of the membrane with the DAB substrate revealed a band of 32 kDa in the transformed strain. Molecular mass indicators (in kilodaltons) are labeled on the left.
FIG. 2.
Detection of GFP fluorescence in conidia by epifluorescence. Wild-type (a) or gGFP-transformed (c) conidia from strain 13073 were fixed onto poly-l-lysine-coated coverslips and viewed by epifluorescence using fluorescein filters. (b and d) The corresponding DIC images are shown. Bars = 10 μm.
Phagocytosis of conidia by A549, HUVE, and J774 cells. (i) Measurement by immunostaining.
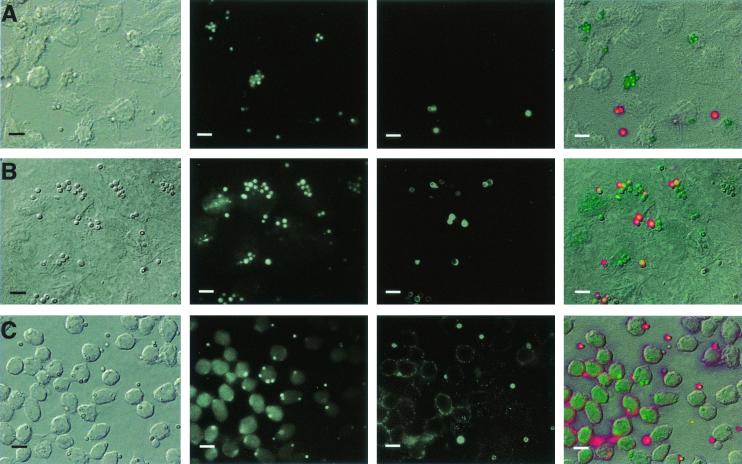
Inhalation of A. fumigatus conidia leads to deposition of spores on the bronchial and alveolar surface (33), and invasion of the pneumocytes lining the alveoli may allow the conidia to breach the epithelial barrier. Previous studies have demonstrated that A. fumigatus can become internalized by several cell types: the type II alveolar cell line A549 (10), primary alveolar type II cells, and by HUVE and tracheal epithelial cells (41) in vitro. However, these studies used electron microscopy to observe internalization and did not measure percent uptake, which is commonly reported when investigating phagocytic uptake of pathogens by host cells (3, 9, 36). Since A549 cells and HUVE cells are capable of internalizing nonspecific particles such as latex beads (21, 25, 58), we investigated whether uptake of A. fumigatus conidia by these cells occurred at a significantly greater rate than nonspecific phagocytosis. We measured the percent internalization of A. fumigatus conidia by three different cultured cells in vitro: the transformed type II pneumocyte cell line A549 (as a model for alveolar epithelial cells); human umbilical vein endothelial cells (as a model for endothelial cell uptake) and the murine macrophage cell line J774 (as a positive control for phagocytosis) (46). Phagocytic uptake was first determined using immunostaining (Fig. 3). As shown in Table 1, the highest invasion index was seen with J774 cells, followed by HUVE and A549 cells. Both GFP strains behaved similarly in the invasion assays.
FIG. 3.
Immunofluorescence microscopy demonstrates phagocytosis of A. fumigatus conidia by A549, HUVE, and J774 cells. A549 (A), HUVE (B), or J774 (C) cells were seeded overnight (A549 and HUVE cells) or for 2 h (J774 cells only) onto 12-mm-diameter coverslips in 24-well plates. Cells were incubated with 106 A. fumigatus 13073 conidia/ml in MEM-10% FBS for 3 h (A549 and HUVE cells) or 1 h (J774 cells) at 37°C. Following infection, samples were washed with PBST and processed for immunofluorescence. From left to right, panels show DIC image, fluorescence image showing the green channel (total bound conidia), fluorescence image of the red channel (extracellular conidia), and a merged overlay of all images. The results are representative of two independent experiments. Bars = 10 μm.
TABLE 1.
Comparison of the extent of invasion of A549, J774, and HUVE cells by two strains of A. fumigatus (ATCC 13073 and CHUV) using fluorescence microscopy and a nystatin protection assaya
| A. fumigatus strain | Extent of invasion of cells by indicated assay
|
||||||
|---|---|---|---|---|---|---|---|
| A549
|
J774
|
HUVE
|
|||||
| Microscopy (invasion indexb) | Nystatin protection
|
Microscopy (invasion index)b | Nystatin protection
|
Microscopy (invasion index)d | |||
| Invasion indexc | % of initial inoculumd | Invasion indexc | % of initial inoculumc | ||||
| 13073 | 39 ± 11 | 35 ± 7 | 2.9 ± 0.5 | 91 ± 8 | 19 ± 4 | 6.0 ± 1.4 | 48 ± 5 |
| CHUV | 30 ± 3 | 29 ± 7 | 1.3 ± 0.3 | 85 ± 10 | 35 ± 4 | 6.6 ± 1.5 | 50 ± 7 |
| Beads | 0 | NDe | 0 | ||||
A549, J774, or HUVE cells were infected with 106 conidia/ml and then analyzed by immunofluorescence microscopy and the nystatin protection assay (A549 and J774 cells only). Polystyrene beads were added to A549 and HUVE cells as a control for nonspecific phagocytosis, and uptake was determined by microscopy. The results are representative of two independent experiments and are expressed as means ± standard deviations of three replicates.
The invasion index determined by microscopy is the number of internalized conidia (green channel − red channel) divided by the number of bound conidia (green channel) per field × 100.
The invasion index determined by the nystatin protection assay is the number of internalized conidia (number of conidia grown in the presence of nystatin) divided by the number of bound conidia (number of conidia grown in the absence of nystatin) × 100.
The percentage of initial inoculum (nystatin protection assay only) is the number of conidia enumerated in the presence of nystatin divided by the initial inoculum × 100.
ND, not determined.
(ii) Measurement by nystatin protection assay.
To confirm the results we obtained by immunostaining, we developed a nystatin protection assay, which is modeled on the gentamicin resistance assay used in bacterial pathogenesis studies (16). We used the antifungal agent nystatin, which is fungicidal to germinating conidia (34, 40) but has a low toxicity to mammalian cells and does not penetrate cell membranes (2). In A549 cells, the nystatin protection assay yielded a similar invasion index for the two A. fumigatus strains (Table 1). Moreover, the nystatin protection assay generated statistically similar invasion indices to the values obtained with immunolabeling for both Aspergillus strains. In contrast, the invasion index of conidia by J774 cells was 19% for strain 13073 and 35% for strain CHUV according to the nystatin protection assay (Table 1)—much lower than that obtained by immunostaining. This discrepancy was likely the result of killing of the internalized conidia by the J774 cells during the 3-h nystatin incubation step. Therefore, the uptake of conidia by J774 cells was threefold greater than A549 cells (as measured by immunostaining), but after 3 h, 60 to 80% of the conidia within J774 cells had been killed, whereas all of the A549-internalized conidia remained viable. In addition to the invasion index, we also calculated the invasion frequency (percent conidia internalized relative to the initial inoculum). A. fumigatus strain 13073 entered A549 and J774 cells with invasion frequencies of 2.9 and 6.0%, respectively, while the CHUV strain entered A549 and J774 cells at a frequency of 1.3 and 6.6% (Table 1).
Phagocytosis of polystyrene beads by A549 and HUVE cells.
To measure the extent of nonspecific phagocytosis, we incubated A549 cells with 1 μm fluorescein-labeled biotinylated polystyrene beads (A. fumigatus conidia are 2 to 3 μm in diameter) and measured the invasion index by immunostaining. Previous studies using polystyrene beads have shown that they are internalized by A549 (21) and HUVE (58) cells under certain conditions. In these studies, internalization was determined by subtracting the number of particles bound to the cell surface at 4°C from the number of microspheres associated with the cells at 37°C (21). In contrast, our assay used immunostaining to differentiate between external and internalized beads. Polystyrene beads adhered to A549 and HUVE cells at a frequency of 10 to 20 beads/50 cells; however, the cells did not internalize the bound beads (Table 1). Therefore, under the conditions used in our assay, uptake of A. fumigatus conidia by A549 and HUVE cells was specific, as these cells did not appear to be randomly internalizing particles.
Inhalation of Aspergillus conidia leads to the deposition of spores on the alveolar surface, and the cells most likely to initially interact with conidia are type I and type II pneumocytes. Therefore, for the rest of our experiments we chose A549 cells as model alveolar epithelial cells and further investigated the interaction of A. fumigatus conidia with this cell line.
Phagocytosis of dead conidia by A549 cells.
To determine whether internalization of conidia by A549 cells required viable conidia, we compared the rates of uptake of live and heat-killed conidia into A549 cells by immunostaining. The invasion index of viable and heat-killed conidia was 28% ± 7% and 37% ± 9 (P > 0.05), respectively. These data suggest that A549 cells internalize viable and heat-killed conidia to an equal extent, which implies that internalization may occur via recognition of a non-heat-labile surface antigen, such as a polysaccharide.
Phagocytosis of conidia by A549 cells: Effect of serum.
To determine whether serum components were required for uptake of conidia by A549 cells, invasion assays were performed in the presence of MEM or MEM plus serum (FBS), and uptake was determined by the nystatin protection assay. There were no significant differences in invasion frequencies between these samples (data not shown) suggesting that the recognition of conidia by A549 surface receptors does not require serum components.
Delay of germination in phagocytosed conidia.
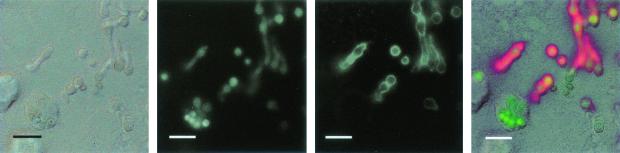
To determine whether conidia germinate inside A549 cells, conidia were incubated with cells for 6 h. The A549 cell monolayer was not damaged by this extended incubation time. Visualization by immunostaining revealed that all of the extracellular conidia had swollen and most had germ tubes ranging from 5 to 10 μm in length, whereas a vast majority of the intracellular conidia had not germinated (Fig. 4). We could not determine whether any of the extracellular germinated conidia had penetrated the cell layer. These data suggest, along with the data from the nystatin protection assay, that internalized conidia remain viable inside A549 cells and that germination is severely restricted for at least 6 h. Whether conidia can be retained in a similar state within alveolar epithelial cells in vivo is unknown.
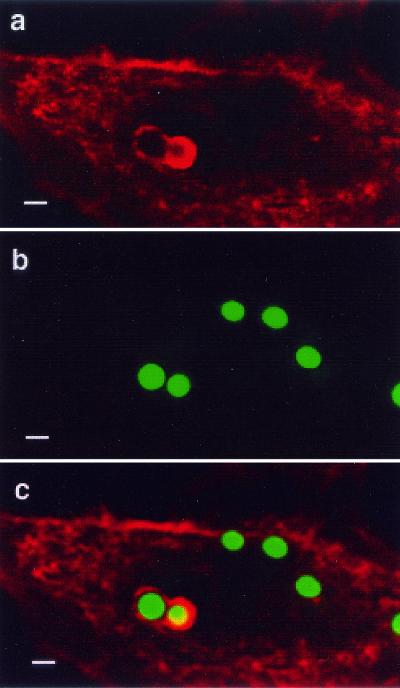
FIG. 4.
Internalized conidia do not germinate in A549 cells. A549 cells were infected with 106 A. fumigatus 13073 conidia/ml in MEM-10% FBS for 6 h at 37°C and then fixed with 4% paraformaldehyde. The following day, samples were immunolabeled and then viewed by DIC and fluorescence microscopy. From left to right, panels show DIC image, fluorescence image showing the green channel (total bound conidia), fluorescence image of the red channel (extracellular conidia/germlings), and a merged overlay of all images. Bars = 10 μm.
Internalization of A. fumigatus conidia by A549 cells is an active process that requires microfilaments and microtubules.
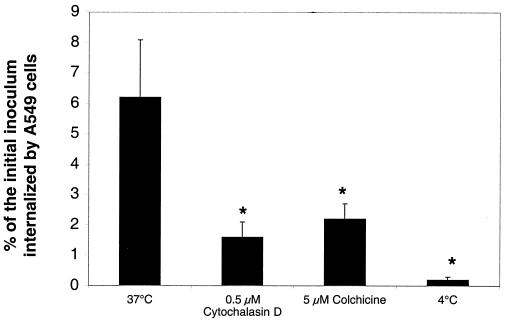
Phagocytosis is the temperature-dependent uptake by cells of particles (usually greater than 0.5 μm in diameter) and leads to the polymerization of actin at the site of entry (45). To determine whether internalization of A. fumigatus conidia into A549 cells required polymerization of actin filaments or microtubules, invasion assays were performed using the nystatin protection assay in the presence and absence of cytochalasin D or colchicine. Cytochalasin D prevents addition of actin monomers to the fast-growing plus ends of filaments (50), and colchicine binds to and prevents polymerization of tubulin (47, 51). The internalization of conidia into A549 cells was inhibited by 75% in the presence of 0.5 μM cytochalasin D and by 65% in the presence of 5 μM colchicine (Fig. 5). These concentrations of cytochalasin D and colchicine had no effect on the germination and growth of conidia alone (data not shown). Thus, both microfilaments and microtubules are utilized during internalization of conidia by A549 cells.
FIG. 5.
Internalization of A. fumigatus conidia in the presence of phagocytosis inhibitors. A549 cells were infected with 106 A. fumigatus 13073 conidia/ml in MEM-10% FBS containing no inhibitors (37°C) or 0.5 μM cytochalasin D or 5 μM colchicine. Invasion assays were also carried out with no inhibitors at 4°C to determine whether uptake was temperature dependent. The number of conidia internalized by the A549 cells was determined using the nystatin protection assay (see Materials and Methods). The results are representative of two independent experiments and are expressed as the means + standard deviations (error bars) of three replicates. ∗, P < 0.05.
Phagocytosis is a temperature-dependent process and particle uptake has been shown to be severely restricted at 4°C (1, 52). To determine whether phagocytosis of conidia by A549 cells was also temperature dependent, invasion assays were carried out at 4°C and uptake was measured by the nystatin protection assay. Internalization of conidia was inhibited by 95% when infections were performed at 4°C versus the control at 37°C (Fig. 5). Taken together, these data suggest that internalization of A. fumigatus conidia by A549 cells occurs by a temperature-dependent phagocytic process, which is dependent on the host cell microfilaments and microtubules.
Internalization of A. fumigatus conidia induces localized actin rearrangement.
Many microbial pathogens such as Salmonella enterica serovar Typhimurium (20) and C. albicans (18) that invade nonprofessional phagocytic cells with rigid cytoskeletons induce visible alterations in the underlying host cytoskeletal structure. Since cytochalasin D inhibited uptake of A. fumigatus conidia by A549 cells, we investigated whether conidia internalization would lead to actin rearrangement which could be visualized in A549 cells. Uninfected A549 cells showed intense staining of cortical and cellular actin filaments (data not shown). In infected cells, approximately 10% of the conidia were found inside vacuoles coated with polymerized actin (Fig. 6). However, as phagocytosis assays had demonstrated that the invasion index of conidia by A549 cells was approximately 30%, the remaining 20% must have already shed their phagosome-actin coat, or the microfilaments were only polymerizing around those organisms that were actively becoming phagocytosed the moment that the fixative was applied. Alternatively, some conidia may have been phagocytosed by a non-actin-dependent mechanism.
FIG. 6.
Rearrangement of actin during the internalization of A. fumigatus conidia by A549 cells. A549 cells were infected with 106 conidia/ml for 160 min. Following infection, actin microfilaments were stained with rhodamine phalloidin. Fluorescence from the red (a) and green (b) channels was collected separately on a Zeiss LSM confocal microscope and then overlaid in Adobe PhotoShop (c). Bars = 2 μm.
DISCUSSION
Life-threatening diseases due to opportunistic fungi such as A. fumigatus have increased over the past 2 decades (15). Although A. fumigatus makes up less than 1% of all airborne conidia, it remains the most-common invasive mold infection among immunocompromised patients worldwide (33). Other species in the genus, namely, Aspergillus terreus and Aspergillus auricomus, possess similar physical attributes (small spore size, ability to grow at 37°C); however, these species cause infection much more rarely (43). Therefore, it has been postulated that A. fumigatus must possess unique virulence factors that allow it to colonize the host (28).
In this report, we transformed two strains of A. fumigatus with gGFP and used them to investigate internalization of conidia by three cultured cell lines. We used differential immunolabeling and a nystatin protection assay to quantify the percent internalization of A. fumigatus conidia by host cells. Previous studies have reported that A. fumigatus conidia are internalized by A549 lung epithelial cells (10) and primary airway type II cells (41). In our study, we confirmed that A549 cells internalize A. fumigatus conidia, and in addition, we determined that the invasion index was approximately 30% and the invasion frequency (relative to the initial inoculum) was approximately 2%. No uptake of 1-μm-diameter polystyrene beads was observed over the assay period. The invasion frequency of conidia into A549 parallels uptake of other pathogenic organisms by these cells: A549 cells internalized 5.7% of the initial inoculum of Mycobacterium tuberculosis (3), 0.2% of the protozoan parasite Encephalitozoon cuniculi (9), and 0.15% of Burkholderia cepacia (36). In contrast, Martin and Mohr (36) observed a 0.008% invasion frequency of the nonpathogenic E. coli HB101 into A549 cells. As a positive control for internalization, we measured uptake of conidia by J774 cells and observed that these professional phagocytes internalized approximately 90% of the bound conidia. Similarly, Káposzta et al. observed that 98% of macrophage-associated C. albicans were phagocytosed by primary macrophages (31). The invasion index for J774 cells calculated by the nystatin protection assay was much lower than that obtained by immunolabeling (27 versus 87% average for the two strains). This was most likely due to killing of the conidia within J774 cells during the nystatin assay incubation period. In contrast, all of the conidia internalized by A549 cells were viable after 6 h. Finally, we measured internalization of conidia by HUVE cells and found that 50% of the bound spores were phagocytosed. This was similar to the uptake of C. neoformans by these cells; Ibrahim et al. (29) showed that 77% of the bound yeasts were internalized by HUVE cells.
The development of the nystatin protection assay allowed us to confirm the internalization results obtained by differential immunolabeling. Since both techniques produced similar internalization rates with A549 cells, we were confident that the nystatin protection assay gave an accurate and objective measurement of invasion. This technique is simple and sensitive and should be applicable to other pathogenic fungi as nystatin is fungicidal toward both C. albicans and C. neoformans (6). Furthermore, comparing the results of immunostaining and nystatin protection may provide quantitative information on the extent of killing by host cells.
To determine whether A. fumigatus conidia germinated following entry into A549 cells, we extended the invasion incubation period from 3 to 6 h. Germination was severely delayed for phagocytosed conidia and this differs from uptake of Candida albicans by HUVE cells. In this case, the yeast cells germinated during the first hour, and by 2 h most of the germlings were internalized by the endothelial cells (18). In their studies of A. fumigatus, DeHart et al. (10) observed that direct hyphal penetration of A549 cells occurred after a twelve h incubation period. Our data suggest that the hyphal penetration of A549 cells observed by Dettart et al. probably occurred via extracellular germlings.
Microbial pathogens use several tactics to gain access to host cells including phagocytosis (9, 37), macropinocytosis (20, 22), receptor-mediated endocytosis (38) and microtubule-dependent internalization (24, 39). Uptake of A. fumigatus conidia by A549 cells occurred via actin-dependent phagocytosis but also required host cell microtubules. Internalization of A. fumigatus conidia did not induce the massive cytoskeletal rearrangements seen in Salmonella uptake into epithelial cells, which enters via membrane ruffling and macropinocytosis (22). Instead, the process of A. fumigatus internalization most likely occurs via engagement of ligands with host cell receptors and resembles the zipper uptake model (53). The identity of these ligands is currently unknown. The microfilament dependence of A. fumigatus uptake resembles epithelial cell invasion by other bacterial pathogens such as M. tuberculosis (3) and S. enterica serovar Typhimurium (20). Although most invasive microbes use host actin to enter nonprofessional phagocytes, there are some organisms that also use microtubules. For example, some strains of Campylobacter jejuni require microtubules but not microfilaments for entry into intestinal cells (4, 39). Following adherence to intestinal epithelial cells, C. jejuni trigger the formation of a microtubule-based membrane extension that meets the invading bacterium (32). In contrast, phagocytosis of A. fumigatus by A549 cells involves both cytoskeletal components which is similar to M. tuberculosis entry into A549 cells (3) and C. albicans uptake by endothelial cells (18).
In summary, we have used several techniques to demonstrate that A. fumigatus conidia are internalized by lung epithelial and endothelial and murine macrophage cells in vitro. Examination of conidium uptake by A549 cells showed that the process was analogous to phagocytosis and utilized host microfilaments and microtubules. Internalization of conidia by nonprofessional phagocytes may be important in the development of aspergillosis in vivo, as sequestration inside these cells may allow the conidia to escape the immune response of the host. Future experiments will investigate the location of the conidium in the endosomal pathway and will determine whether uptake is a defense mechanism of the host or a virulence trait of the fungus.
Acknowledgments
This work was supported by a grant from the Natural Sciences and Engineering Research Council of Canada (NSERC) to M.M.M. J.A.W. was supported by an NSERC predoctoral scholarship.
We thank C. Breuil (Department of Wood Science, University of British Columbia) for the gift of the gGFP plasmid, Loekie van der Wal (Animal Care Facility, Simon Fraser University) for performing animal immunizations, and Wendy Olson for coordinating umbilical cord collections at Royal Columbian Hospital, New Westminster, British Columbia, Canada. We are grateful to Anna H. T. Gifford for critical reading of the manuscript.
Editor: T. R. Kozel
REFERENCES
- 1.Band, H., A. Bhattacharya, and G. P. Talwar. 1986. Mechanism of phagocytosis by Schwann cells. J. Neurol Sci. 75:113-119. [DOI] [PubMed] [Google Scholar]
- 2.Bennett, J. E. 1990. Antifungal agents, p. 1165-1181. In A. G. Gilman, T. W. Rall, A. S. Nies, and P. Taylor (ed.), Goodman and Gilman's the pharmacological basis of therapeutics, 8th ed. Pergamon Press Inc., Elmsford, N.Y.
- 3.Bermudez, L. E., and J. Goodman. 1996. Mycobacterium tuberculosis invades and replicates within type II alveolar cells. Infect. Immun. 64:1400-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biswas, D., K. Itoh, and C. Sasakawa. 2000. Uptake pathways of clinical and healthy animal isolates of Campylobacter jejuni into INT-407 cells. FEMS Immunol. Med. Microbiol. 29:203-211. [DOI] [PubMed] [Google Scholar]
- 5.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 6.Carrillo-Munoz, A. J., G. Quindos, C. Tur, M. T. Ruesga, Y. Miranda, O. del Valle, P. A. Cossum, and T. L. Wallace. 1999. In-vitro antifungal activity of liposomal nystatin in comparison with nystatin, amphotericin B cholesteryl sulphate, liposomal amphotericin B, amphotericin B lipid complex, amphotericin B desoxycholate, fluconazole and itraconazole. J. Antimicrob. Chemother. 44:397-401. [DOI] [PubMed] [Google Scholar]
- 7.Chiu, W., Y. Niwa, W. Zeng, T. Hirano, H. Kobayashi, and J. Sheen. 1996. Engineered GFP as a vital reporter in plants. Curr. Biol. 6:325-330. [DOI] [PubMed] [Google Scholar]
- 8.Cormack, B. 1998. Green fluorescent protein as a reporter of transcription and protein localization in fungi. Curr. Opin. Microbiol. 1:406-410. [DOI] [PubMed] [Google Scholar]
- 9.Couzinet, S., E. Cejas, J. Schittny, P. Deplazes, R. Weber, and S. Zimmerli. 2000. Phagocytic uptake of Encephalitozoon cuniculi by nonprofessional phagocytes. Infect. Immun. 68:6939-6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeHart, D. J., D. E. Agwu, N. C. Julian, and R. G. Washburn. 1997. Binding and germination of Aspergillus fumigatus conidia on cultured A549 pneumocytes. J. Infect. Dis. 175:146-150. [DOI] [PubMed] [Google Scholar]
- 11.Denning, D. W. 1996. Therapeutic outcome in invasive aspergillosis. Clin. Infect. Dis. 23:608-615. [DOI] [PubMed] [Google Scholar]
- 12.Denning, D. W. 2000. Early diagnosis of invasive aspergillosis. Lancet 355:423-424. [DOI] [PubMed] [Google Scholar]
- 13.Denning, D. W. 1998. Invasive aspergillosis. Clin. Infect. Dis. 26:781-803. [DOI] [PubMed] [Google Scholar]
- 14.De Pauw, B. 2000. Is there a need for new antifungal agents? Clin. Microbiol. Infect. 6(Suppl. 2):23-28. [DOI] [PubMed] [Google Scholar]
- 15.Dixon, D. M., M. M. McNeil, M. L. Cohen, B. G. Gellin, and J. R. La Montagne. 1996. Fungal infections: a growing threat. Public Health Rep. 111:226-235. [PMC free article] [PubMed] [Google Scholar]
- 16.Elsinghorst, E. A. 1994. Measurement of invasion by gentamicin resistance. Methods Enzymol. 236:405-420. [DOI] [PubMed] [Google Scholar]
- 17.Feldmesser, M., S. Tucker, and A. Casadevall. 2001. Intracellular parasitism of macrophages by Cryptococcus neoformans. Trends Microbiol. 9:273-278. [DOI] [PubMed] [Google Scholar]
- 18.Filler, S. G., J. N. Swerdloff, C. Hobbs, and P. M. Luckett. 1995. Penetration and damage of endothelial cells by Candida albicans. Infect. Immun. 63:976-983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finlay, B. B., and S. Falkow. 1997. Common themes in microbial pathogenicity revisited. Microbiol. Mol. Biol. Rev. 61:136-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Finlay, B. B., S. Ruschkowski, and S. Dedhar. 1991. Cytoskeletal rearrangements accompanying Salmonella entry into epithelial cells. J. Cell Sci. 99:283-296. [DOI] [PubMed] [Google Scholar]
- 21.Foster, K. A., M. Yazdanian, and K. L. Audus. 2001. Microparticulate uptake mechanisms of in-vitro cell culture models of the respiratory epithelium. J. Pharm. Pharmacol. 53:57-66. [DOI] [PubMed] [Google Scholar]
- 22.Francis, C. L., T. A. Ryan, B. D. Jones, S. J. Smith, and S. Falkow. 1993. Ruffles induced by Salmonella and other stimuli direct macropinocytosis of bacteria. Nature 364:639-642. [DOI] [PubMed] [Google Scholar]
- 23.Gigolashvili, T. 1999. Update on antifungal therapy. Cancer Pract. 7:157-159. [DOI] [PubMed] [Google Scholar]
- 24.Grassme, H. U., R. M. Ireland, and J. P. van Putten. 1996. Gonococcal opacity protein promotes bacterial entry-associated rearrangements of the epithelial cell actin cytoskeleton. Infect. Immun. 64:1621-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Griese, M., and D. Reinhardt. 1998. Smaller sized particles are preferentially taken up by alveolar type II pneumocytes. J. Drug Target. 5:471-479. [DOI] [PubMed] [Google Scholar]
- 26.Groll, A. H., P. M. Shah, C. Mentzel, M. Schneider, G. Just-Nuebling, and K. Huebner. 1996. Trends in the postmortem epidemiology of invasive fungal infections at a university hospital. J. Infect. 33:23-32. [DOI] [PubMed] [Google Scholar]
- 27.Haas, J., E. C. Park, and B. Seed. 1996. Codon usage limitation in the expression of HIV-1 envelope glycoprotein. Curr. Biol. 6:315-324. [DOI] [PubMed] [Google Scholar]
- 28.Hogan, L. H., B. S. Klein, and S. M. Levitz. 1996. Virulence factors of medically important fungi. Clin. Microbiol. Rev. 9:469-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ibrahim, A. S., S. G. Filler, M. S. Alcouloumre, T. R. Kozel, J. E. Edwards, Jr., and M. A. Ghannoum. 1995. Adherence to and damage of endothelial cells by Cryptococcus neoformans in vitro: role of the capsule. Infect. Immun. 63:4368-4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jaffe, E. A., R. L. Nachman, C. G. Becker, and C. R. Minick. 1973. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J. Clin. Investig. 52:2745-2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Káposzta, R., L. Marodi, M. Hollinshead, S. Gordon, and R. P. da Silva. 1999. Rapid recruitment of late endosomes and lysosomes in mouse macrophages ingesting Candida albicans. J. Cell Sci. 112:3237-3248. [DOI] [PubMed] [Google Scholar]
- 32.Kopecko, D. J., L. Hu, and K. J. Zaal. 2001. Campylobacter jejuni: microtubule-dependent invasion. Trends Microbiol. 9:389-396. [DOI] [PubMed] [Google Scholar]
- 33.Latgé, J. P. 1999. Aspergillus fumigatus and aspergillosis. Clin. Microbiol. Rev. 12:310-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manavathu, E. K., G. J. Alangaden, and P. H. Chandrasekar. 1998. In-vitro isolation and antifungal susceptibility of amphotericin B-resistant mutants of Aspergillus fumigatus. J. Antimicrob. Chemother. 41:615-619. [DOI] [PubMed] [Google Scholar]
- 35.Maor, R., M. Puyesky, B. A. Horwitz, and A. Sharon. 1998. Use of green fluorescent protein (GFP) for studying development and fungal-plant interaction in Cochliobolus heterostrophus. Mycol. Res. 102:491-496. [Google Scholar]
- 36.Martin, D. W., and C. D. Mohr. 2000. Invasion and intracellular survival of Burkholderia cepacia. Infect. Immun. 68:24-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maruta, K., M. Ogawa, H. Miyamoto, K. Izu, and S. I. Yoshida. 1998. Entry and intracellular localization of Legionella dumoffii in Vero cells. Microb. Pathog. 24:65-73. [DOI] [PubMed] [Google Scholar]
- 38.Mengaud, J., H. Ohayon, P. Gounon, R. M. Mege, and P. Cossart. 1996. E-cadherin is the receptor for internalin, a surface protein required for entry of L. monocytogenes into epithelial cells. Cell 84:923-932. [DOI] [PubMed] [Google Scholar]
- 39.Oelschlaeger, T. A., P. Guerry, and D. J. Kopecko. 1993. Unusual microtubule-dependent endocytosis mechanisms triggered by Campylobacter jejuni and Citrobacter freundii. Proc. Natl. Acad. Sci. USA 90:6884-6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Osherov, N., and G. May. 2000. Conidial germination in Aspergillus nidulans requires RAS signaling and protein synthesis. Genetics 155:647-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paris, S., E. Boisvieux-Ulrich, B. Crestani, O. Houcine, D. Taramelli, L. Lombardi, and J. P. Latgé. 1997. Internalization of Aspergillus fumigatus conidia by epithelial and endothelial cells. Infect. Immun. 65:1510-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peñalver, M. C., J. E. O'Connor, J. P. Martinez, and M. L. Gil. 1996. Binding of human fibronectin to Aspergillus fumigatus conidia. Infect. Immun. 64:1146-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pitt, J. I. 1994. The current role of Aspergillus and Penicillium in human and animal health. J. Med. Vet. Mycol. 32:17-32. [PubMed] [Google Scholar]
- 44.Puente, P., N. Fernandez, M. C. Ovejero, and F. Leal. 1992. Immunogenic potential of Aspergillus nidulans subcellular fractions and their polypeptide components. Mycoses 35:235-241. [DOI] [PubMed] [Google Scholar]
- 45.Rabinovitch, M. 1994. Professional and non-professional phagocytes: an introduction. Trends Cell Biol. 5:85-87. [DOI] [PubMed] [Google Scholar]
- 46.Rodriguez, E., F. Boudard, M. Mallie, J. M. Bastide, and M. Bastide. 1997. Murine macrophage elastolytic activity induced by Aspergillus fumigatus strains in vitro: evidence of the expression of two macrophage-induced protease genes. Can. J. Microbiol. 43:649-657. [DOI] [PubMed] [Google Scholar]
- 47.Sackett, D. L., and J. K. Varma. 1993. Molecular mechanism of colchicine action: induced local unfolding of beta-tubulin. Biochemistry 32:13560-13565. [DOI] [PubMed] [Google Scholar]
- 48.Sanchez, O., and J. Aguirre. 1996. Efficient transformation of Aspergillus nidulans by electroporation of germinated conidia. Fungal Genet. Newsl. 43:48-51. [Google Scholar]
- 49.Santerre Henriksen, A. L., S. Even, C. Muller, P. J. Punt, C. A. van den Hondel, and J. Nielsen. 1999. Study of the glucoamylase promoter in Aspergillus niger using green fluorescent protein. Microbiology 145:729-734. [DOI] [PubMed] [Google Scholar]
- 50.Schliwa, M. 1982. Action of cytochalasin D on cytoskeletal networks. J. Cell Biol. 92:79-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Skoufias, D. A., and L. Wilson. 1992. Mechanism of inhibition of microtubule polymerization by colchicine: inhibitory potencies of unliganded colchicine and tubulin-colchicine complexes. Biochemistry 31:738-746. [DOI] [PubMed] [Google Scholar]
- 52.Steffan, A. M., J. L. Gendrault, R. S. McCuskey, P. A. McCuskey, and A. Kirn. 1986. Phagocytosis, an unrecognized property of murine endothelial liver cells. Hepatology 6:830-836. [DOI] [PubMed] [Google Scholar]
- 53.Swanson, J. A., and S. C. Baer. 1995. Phagocytosis by zippers and triggers. Trends Cell Biol. 5:89-93. [DOI] [PubMed] [Google Scholar]
- 54.Tronchin, G., J. P. Bouchara, G. Larcher, J. C. Lissitzky, and D. Chabasse. 1993. Interaction between Aspergillus fumigatus and basement membrane laminin: binding and substrate degradation. Biol. Cell 77:201-208. [DOI] [PubMed] [Google Scholar]
- 55.Walsh, T. J., M. A. Viviani, E. Arathoon, C. Chiou, M. Ghannoum, A. H. Groll, and F. C. Odds. 2000. New targets and delivery systems for antifungal therapy. Med. Mycol. 38:335-347. [PubMed] [Google Scholar]
- 56.Wasylnka, J. A., and M. M. Moore. 2000. Adhesion of Aspergillus species to extracellular matrix proteins: evidence for involvement of negatively charged carbohydrates on the conidial surface. Infect. Immun. 68:3377-3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weidner, G., C. d'Enfert, A. Koch, P. C. Mol, and A. A. Brakhage. 1998. Development of a homologous transformation system for the human pathogenic fungus Aspergillus fumigatus based on the pyrG gene encoding orotidine 5′-monophosphate decarboxylase. Curr. Genet. 33:378-385. [DOI] [PubMed] [Google Scholar]
- 58.Zauner, W., N. A. Farrow, and A. M. Haines. 2001. In vitro uptake of polystyrene microspheres: effect of particle size, cell line and cell density. J. Control Release 71:39-51. [DOI] [PubMed] [Google Scholar]