Abstract
We identified Mycobacterium tuberculosis genes preferentially expressed during infection of human macrophages using a promoter trap adapted for this pathogen. inhA encodes an enoyl-acyl carrier protein reductase that is required for mycolic acid biosynthesis (A. Quemard et al., Biochemistry 34:8235-8241, 1995) and is a major target for isoniazid (INH) in mycobacterial species (A. Banerjee et al., Science 263:227-230, 1994). Since overexpression of inhA confers INH resistance in Mycobacterium smegmatis (Banerjee et al., Science 263:227-230, 1994), we designed a promoter trap based on this gene. A library of clones, containing small fragments of M. tuberculosis DNA cloned upstream of inhA in a plasmid vector, was electroporated into M. tuberculosis, and the resulting culture was used to infect the human monocytic THP-1 cell line. Selection was made for clones surviving INH treatment during infection but retaining INH sensitivity on plates. The DNA upstream of inhA was sequenced in each clone to identify the promoter driving inhA expression. Thirteen genes identified by this method were analyzed by quantitative reverse transcription-PCR (R. Manganelli et al., Mol. Microbiol. 31:715-724, 1999), and eight of them were found to be differentially expressed from cultures grown in macrophages compared with broth-grown cultures. Several of these genes are presumed to be involved in fatty acid metabolism; one potentially codes for a unique DNA binding protein, one codes for a possible potassium channel protein, and the others code for proteins of unknown function. Genes which are induced during infection are likely to be significant for survival and growth of the pathogen; our results lend support to the view that fatty acid metabolism is essential for the virulence of M. tuberculosis.
Virulence genes are generally defined as genes that are necessary for survival of the pathogen in a host and are involved in pathogenicity but are not necessary for growth in culture medium. Several virulence genes of Mycobacterium tuberculosis have been identified by comparing the pathogenicity of strains with mutations in the genes to the pathogenicity of isogenic strains carrying the wild-type alleles. These genes include katG, encoding a catalase peroxidase (35), hspX (acr), encoding a homologue of α-crystallin, (36), and erp, encoding an uncharacterized exported protein (2). Specific modifications of mycolic acids are essential for virulence since strains carrying disruptions in hma, required for the biosynthesis of oxygenated mycolic acids (8), and in pcaA, a gene coding for a mycolic acid cyclopropane synthetase (11), are attenuated in mice. Synthesis of the exported phthiocerol dimycocerosate (3, 6) is also required for virulence in mice. Fatty acid degradation is implicated in pathogenicity since a strain carrying a mutated aceA gene, which is required for synthesis of isocitrate lyase, was attenuated in mice (23).
Mahan et al. (19, 20) were the first workers to develop a promoter trap, known as an in vivo expression technology (IVET) system, to select for genes of Salmonella enterica serovar Typhimurium specifically induced in host tissues. This method has also been used to study pathogenesis in Staphylococcus aureus, Vibrio cholerae, and other bacteria (4). Using the same approach, we created an IVET method for M. tuberculosis to select for genes specifically upregulated during growth in macrophages derived from the human monocytic cell line THP-1. Our goal was to identify genes expressed during infection of macrophages as a means of analyzing the environmental conditions faced by the pathogen during infection, as well as to identify targets for diagnosis and treatment of tuberculosis. Selection was based upon increased expression of inhA, driven by promoters which are upregulated specifically during infection. inhA codes for an enoyl-ACP reductase, which is required for mycolic acid biosynthesis (26) and is a major target for isoniazid (INH) in M. tuberculosis. Overproduction of this enzyme confers resistance to INH in Mycobacterium smegmatis (1) and in M. tuberculosis (this communication). Specific induction of several candidate genes during growth in macrophages was confirmed by using real-time reverse transcription (RT)-PCR with molecular beacons (mbRT-PCR) (21).
MATERIALS AND METHODS
Construction of the promoter trap.
The plasmid promoter trap vector pJD32 was constructed by cloning a promoterless PCR-amplified M. smegmatis inhA gene containing an INH resistance mutation (1) into pYUB378, an Escherichia coli-Mycobacterium shuttle vector conferring kanamycin resistance in both organisms (9). This vector has a unique BamHI site upstream of inhA, and a derivative pJD33 plasmid was constructed with the hsp60 promoter from Mycobacterium bovis BCG cloned into this site. A library of small DNA fragments from M. tuberculosis was made by partial SauIIIA digestion and cloned into the BamHI site of pJD32. The plasmid library consisted of 1 × 106 clones in E. coli, and the minimum insertion frequency, measured by PCR, was 62%. Assuming that the average insertion size was 300 bp, as estimated by PCR, our library consisted of 192 Mb (6.4 × 105 clones × 0.3 kb) of DNA from M. tuberculosis. Since the size of the genome was 4.4 Mb, this library represented the entire genome reiterated about 43 times. We electroporated the plasmid library into M. tuberculosis H37Rv and selected for kanamycin resistance in broth culture. An aliquot of the transformation mixture was plated onto solid medium containing kanamycin, and a total of about 9 × 104 M. tuberculosis clones were obtained. With an insertion frequency of 62% and an average insertion size of 300 bp, this represented about 16.7 Mb of the 4.4-Mb genome and thus represented the entire genome about four times. A small percentage (0.01%) of the clones from the broth culture grew on plates containing INH (1 μg/ml), and these clones were presumed to contain a promoter upstream of inhA which was active when M. tuberculosis was grown on plates. The low incidence of INH-resistant clones in the library was presumably due to the low probability of a promoter fragment being inserted in the correct orientation upstream of inhA. Most of the small DNA inserts were expected to be internal fragments of various genes or sequences oriented in the wrong direction for transcription, and therefore the frequency of INH-resistant clones was expected to be low. In addition, since we plated the M. tuberculosis library on plates containing 1 μg of INH per ml, a rather high concentration, our estimate of the frequency of INH-resistant clones may have been low.
Growth of strains.
Cultures of M. tuberculosis H37Rv obtained from Barry Kreiswirth (TB Center, Public Health Research Institute) were grown in 7H9 broth supplemented with albumin, dextrose, and NaCl (ADN) and Tween 80, as previously described (17). Kanamycin was added when appropriate at a concentration of 10 μg/ml, and INH was added at a concentration of 0.5 μg/ml. Cultures in the exponential phase of growth were obtained by diluting logarithmically growing cultures at least 20-fold, followed by incubation on a rotator at 37°C.
Selection for INH resistance during infection of THP-1 cells.
THP-1 (= ATCC TIB-202) is a monocytic human cell line which can be induced with phorbol esters to a macrophage-like state (32). THP-1 cells were grown in RPMI 1640 medium (Gibco-BRL) supplemented with 10% fetal calf serum, 0.45% glucose, 0.15% sodium pyruvate, and 4 mM l-glutamine. Cultures were maintained at concentrations of 1 × 105 to 5 × 105 cells/ml, and the cells were induced to the macrophage-like state by 24 h of treatment with 40 nM 12-O-tetradecanoylphorbol-13-acetate (PMA) (32). The cells were infected with the M. tuberculosis library for 4 h at a multiplicity of infection of about 1; this was followed by washing with phosphate-buffered saline and replacement of the medium with fresh medium. The number of CFU was estimated based on the optical density of the culture, as previously determined experimentally. After 1 day, the medium was replaced with medium containing 0.5 μg of INH per ml, and the infected cultures were incubated five more days, with changing of the medium after 4 days. Most cells remained adherent throughout the infection. Intracellular bacteria were isolated after lysis of the macrophages with 0.05% sodium dodecyl sulfate (SDS), collection of the bacteria by centrifugation, and resuspension in fresh broth for reinfection of THP-1 cells. An aliquot of the culture was plated on plates containing kanamycin.
PCR primers and molecular beacons.
PCR primers were designed to anneal to their targets at the same temperature (60°C) and to amplify DNA fragments internal to the coding sequences of the genes. The molecular beacons, synthesized as previously described, were designed to hybridize to the relevant PCR products (33). The primer pair and the molecular beacon for sigA have been described previously (21), and the sequences of all primers and beacons are shown in Table 1.
TABLE 1.
Sequences of primers and beaconsa
| Primer or beacon | Sequence |
|---|---|
| Rv1171.rt | 5′GTCAGTAGCCACGCCCACAG3′ |
| Rv1171.up | 5′TGTCCTGGCCCTGTTGA3′ |
| Rv1171.down | 5′GGCACTGATCGGAAACGGAG3′ |
| Rv1171.beacon | 5′GCAGCC CGGTGAGGCATTGCTGT GGCTGC3′ |
| Rv3237c.rt | 5′AAACAAGCTACCCGCCCGGATG3′ |
| Rv3237c.up | 5′TGTATGGCCGCGATGA3′ |
| Rv3237c.down | 5′GGCGCACCCAGAATCTGAG3′ |
| Rv3237c.beacon | 5′GCCACC GTGAGCCGCAAACCG GGTGGC3′ |
| Rv3321c.rt | 5′TTCGATACCGCTCCAC3′ |
| Rv3321c.up | 5′GCGGGTGAAATCCTGTC3′ |
| Rv3321c.down | 5′CCATGAAAGGCGTCCTC3′ |
| Rv3321c.beacon | 5′GCAGCC ACCAACAGAATCCA GGCTGC3′ |
| nirA.rt | 5′TGGCGTAGATGTTTTCG3′ |
| nirA.up | 5′GAAGGAGAACCCCCAAT3′ |
| nirA.down | 5′CCTTCTTCAGCTCTTCGT3′ |
| nirA.beacon | 5′GCAGCC AAATGAGGGCCAGTGG GGCTGC3′ |
| Rv0977.rt | 5′TATCGCCGTCGTCCGTTTC3′ |
| Rv0977.up | 5′CCTCTTCCCCGAGTTTTCTGA3′ |
| Rv0977.down | 5′CATCAACCGGGTGTCGAAGGT3′ |
| Rv0977.beacon | 5′GCAGCC GTCGGCGGCGTCGGATCTGG GGCTGC3′ |
| Rv2224c.rt | 5′AGACCTCGACGGCTTTGG3′ |
| Rv2224c.up | 5′CGGGTGCGGGCAATGA3′ |
| Rv2224c.down | 5′AGTCGGCGGCAATAGTTGT3′ |
| Rv2224c.beacon | 5′GCAGCC GGCATTGGGGTCGACG GGCTGC3′ |
| echA19.rt | 5′ATGCCGAACTTCGCACTTTCACCG3′ |
| echA19.up | 5′ATCCGACATCCGTTGCT3′ |
| echA19.down | 5′GCTGCCGTCCTTGAAAGA3′ |
| echA19.beacon | 5′GCAGCC GCAACCCAGAAACCGCCG GGCTGC3′ |
| Rv1774c.rt | 5′GGCGAGGCAAGGGAGATGA3′ |
| Rv1774c.up | 5′AGGGCGGATACGGCTGGAA3′ |
| Rv1774c.down | 5′CGGCGTGATTGTCTGCGTC3′ |
| Rv1774c.beacon | 5′GCAGCC CAATCACGCTCTCGCAC GGCTGC3′ |
| fadA4.rt | 5′GCACCGTCGGAGATCTGT3′ |
| fadA4.up | 5′TTTGGACCACATGGCCTACG3′ |
| fadA4.down | 5′CTTTTGGTGGGACGCAGCC3′ |
| fadA4.beacon | 5′GCAGCC CAACGACGTCGACATGTTCACC GGCTGC3′ |
| aceA.rt | 5′CGCACCTGCTGGACGGCCA3′ |
| aceA.up | 5′GCGGAGCAGATCCAGCAGGT3′ |
| aceA.down | 5′GCGGCGGGCCAGCGTGTGCTC3′ |
| aceA.beacon | 5′GCGAGG AGGACGTCACCCGCACC CCTCGC3′ |
| fadA5.rt | 5′CGAACGCCTCGTTGATCT3′ |
| fadA5.up | 5′GATCGTCGCCCAGGCACT3′ |
| fadA5.down | 5′GATGTCGCCGATCTTCATGC3′ |
| fadA5.beacon | 5′GCAGCC CCTACTACCACCTGGACGGCC GGCTGC3′ |
| pckA.up | 5′ATACCGCGCCGACGAATCAC3′ |
| pckA.down | 5′ATCGCAGAGCCGCTGGAACTC3′ |
| pckA.beacon | 5′GGACGC GGGTGGTCTTCACTGACGG GCGTCC3′ |
| Rv2520c.up | 5′GGCTCCAACGTCCCGATCA3′ |
| Rv2520c.down | 5′CGCACCGCACCCACTCGTC3′ |
| Rv2520c.beacon | 5′CGGTCG GGGAAGGCCGCGAACA CGACCG3′ |
| hspX.up | 5′CCGAGCGCACCGAGCAGAAG3′ |
| hspX.down | 5′GGTGGCCTTAATGTCGTCCTCGTC3′ |
| hspX.beacon | 5′GGCTCC CCTTCGTTCGCACGGTGTC GGAGCC3′ |
| sigA.rt | 5′CGGACGAGACCATGGTGCGGC3′ |
| sigA.up | 5′GGCCAGCCGCGCACCCTTGAC3′ |
| sigA.down | 5′GTCCAGGTAGTCGCGCAGGACC3′ |
| sigA.beacon | 5′CCTCGC GTCGAAGTTGCGCCATCCGA GCGAGG3′ |
rt, RT primer; up, 5′ primer; down, 3′ primer. Where an RT primer is not indicated, the 3′ primer was used for RT. Beacon generic stems are indicated by boldface type. All beacons were synthesized with tetrachlorofluorescein attached to the 5′ end and dabcyl (quencher) attached at the 3′ end.
Preparation of RNA from M. tuberculosis growing in THP-1 cells .
THP-1 cells were differentiated with PMA as described above and seeded in 24-well tissue culture plates at a density of 7.5 × 105cells/well. After 24 h of PMA treatment, cells were infected with an exponentially growing broth culture of M. tuberculosis H37Rv at a multiplicity of infection of 0.5 to 1 CFU/macrophage. In several experiments larger volumes of macrophages were seeded into either 75- or 175-cm2 flasks pretreated with 0.2% gelatin overnight at 4°C. These macrophages were incubated with RPMI 1640 medium containing 20% fetal calf serum instead of 10% fetal calf serum. These conditions improved the ability of the differentiated THP-1 cells to adhere to the flasks (27). After 2 h of infection, the cells were washed twice with phosphate-buffered saline and incubated with fresh RPMI 1640 medium containing 50 μg of gentamicin per ml; at different time points the medium was removed, and each monolayer was lysed with an appropriate volume of TRI reagent (Molecular Research Center) mixed with polyacrylamide carrier provided by the manufacturer. The lysate was immediately transferred to a 2-ml screw-cap microcentrifuge tube with O-rings containing 0.5 ml of zirconia-silica beads (diameter, 0.1 mm; Biospec Products, Inc.), frozen in dry ice, and stored at −80°C. Aliquots of the rolling culture used for infection and bacteria incubated for 2 h in RPMI 1640 medium were collected by centrifugation, resuspended in 1 ml of TRI reagent with glass beads, and frozen as described above. The viability of intracellular bacteria was assayed in separate wells, infected as described above, by plating for CFU after lysis of the macrophages with 0.05% SDS. M. tuberculosis cells in the frozen samples were disrupted by two 1-min pulses in a miniBeadBeater; the samples were kept on ice for 2 min between the pulses. The liquid was removed from the beads, incubated at room temperature for 10 min, and centrifuged for 10 min at 12,000 × g. The supernatant was transferred to a clean tube, 100-μl portions of BCP reagent (Molecular Research Center) were added to 1-ml samples in TRI reagent, and then the tubes were shaken vigorously for 15 s, incubated for 10 min at room temperature, and then centrifuged for 15 min at 12,000 × g. The supernatant was recovered and precipitated with 600 μl of isopropanol. After the RNA was washed with 75% ethanol, it was resuspended in 30 to 100 μl of diethyl pyrocarbonate (DEPC)-treated H2O, 300 to 1,000 μl of TRI reagent containing polyacrylamide carrier was added, and the extraction procedure was repeated. The RNA was dissolved in 30 μl of DEPC-treated H2O, treated with RNase-free DNase (Ambion) as recommended by the manufacturer, precipitated again with 95% ethanol, washed with 75% ethanol, dissolved in 30 μl of DEPC-treated H2O, and stored at −80°C.
mbRT-PCR.
For RT, 2 μl of RNA was added to 1.2 μl of 10× PCR buffer II (Perkin-Elmer), 2.4 μl of MgCl2 (25 mM), 0.24 μl of dimethyl sulfoxide, and enough water to bring the volume to 6 μl. After denaturation at 95°C for 1.5 min, annealing between the RNA and the antisense primers was carried out for 3 min at 65°C and then for 5 min at 57°C. Subsequently, 5 μl of the annealing mixture was added to 2.5 μl of 10× PCR buffer II (Perkin-Elmer), 5 μl of MgCl2 (25 mM), 1 μl of a deoxynucleoside triphosphate mixture (25 mM each), 1.5 μl (6 U) of C. therm RT polymerase (Roche), 1.3 μl of dimethyl sulfoxide, 1.3 μl of dithiothreitol (100 mM), and enough water to bring the volume to 20 μl. Samples were incubated for 1 h at 60°C, heated to 95°C for 1 min, and then chilled on ice. Control samples that were not treated with C. therm RT polymerase were also prepared. These template samples for PCRs were then diluted with 70 μl of H2O and stored at −20°C. The PCR conditions were identical for all reactions. Each 25-μl reaction mixture consisted of 1× DNA polymerase buffer, 4 mM MgCl2, each deoxynucleoside triphosphate at a concentration of 0.25 mM, 1.25 U of AmpliTaq Gold polymerase (Perkin-Elmer), each primer at a concentration of 1 μM, 50 ng of the appropriate molecular beacon, and 10 μl of template. After incubation for 10 min at 94°C to activate the DNA polymerase, the first set of 10 cycles was run with an annealing temperature of 65°C. The denaturation step was 30 s at 94°C, and the extension step was 30 s at 72°C. In the second set of 30 cycles, denaturation was at 94°C for 30 s, annealing was at 60°C for 30 s, and extension was at 72°C for 30 s. The reactions were carried out in sealed tubes in an Applied Biosystems 7700 Prism spectrofluorometric thermal cycler (Perkin-Elmer). Fluorescence was measured during the annealing step of the second set of cycles and was plotted automatically for each sample. Quantitative analysis of the data was performed as previously described (21). In order to obtain standard curves for the mbRT-PCR, a PCR was performed with each primer-beacon set using different amounts of H37Rv chromosomal DNA, and these reactions were performed at the same time as the mbRT-PCR. The standard curves (data not shown) were used to calculate the amount of cDNA for each gene present in the different samples. All values were normalized to the amount of sigA mRNA. The values obtained with the RNA sample from the bacteria incubated in 7H9 medium were used as arbitrary standards for the calculations. RNAs from bacterial cultures initially grown in 7H9 medium and then incubated for 2 h in RPMI 1640 medium were also analyzed by mbRT-PCR.
RESULTS
Construction of a promoter trap for M. tuberculosis.
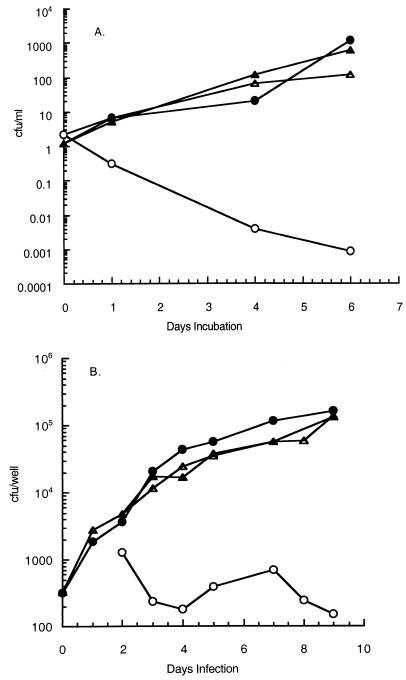
Drug resistance can result from a decreased ratio of drug to target (7), and this mechanism explains the observation that overexpression of inhA confers INH resistance to M. smegmatis (1). InhA, in the presence of NADH, binds INH (25), consistent with its role as a major target for this antibiotic. These considerations led us to predict that inhA would be suitable for construction of a promoter trap which could allow selection (INH resistance) for promoters driving inhA expression in M. tuberculosis. We cloned the inhA gene from M. smegmatis (1) without its promoter into the shuttle vector pYUB378 (9) to form the vector pJD32. To test if increased expression of inhA could confer INH resistance in M. tuberculosis, we cloned the hsp60 promoter (Phsp60) (30) upstream of inhA in pJD32 to form pJD33. This promoter is expressed constitutively at high levels when it is present on a multicopy plasmid (30). The inhA promoterless plasmid (pJD32) and the plasmid containing Phsp60 driving inhA (pJD33) were electroporated into M. tuberculosis H37Rv, and the resulting strains were tested for INH resistance during growth in broth and during infection of human monocytic cell line THP-1 (32) after differentiation into macrophage-like cells with phorbol ester. The strain with Phsp60 driving inhA expression conferred INH resistance under both growth conditions, whereas the strain carrying the promoterless inhA gene remained relatively sensitive to INH (Fig. 1). These results show that as previously reported for M. smegmatis (1), overproduction of InhA confers INH resistance to M. tuberculosis. We concluded that a promoter trap based upon INH resistance caused by overexpression of inhA should be suitable for identification of M. tuberculosis promoters induced during infection. We should note that M. tuberculosis carrying the plasmid with no promoter driving inhA is more sensitive to INH in broth culture (Fig. 1A) than in THP-1 cells (Fig. 1B). This could be due to lower actual levels of INH in the macrophages than in broth or to decreased INH sensitivity of the macrophage-grown M. tuberculosis. Thus, it is possible that clones with a weak, constitutively expressed promoter might appear to be INH resistant during infection of THP-1 cells while remaining INH sensitive in broth. Therefore, all clones identified by this system must be further evaluated to eliminate false positives (see below).
FIG. 1.
INH sensitivity of M. tuberculosis H37Rv carrying pJD32 (promoterless inhA) or pJD33 (inhA driven by Phsp60). (A) Growth in broth culture. Exponentially growing cultures of M. tuberculosis H37Rv were diluted to a concentration of about 106 CFU/ml, and INH (0.5 μg/ml) was added to one sample of each strain. The cultures were incubated in a 24-well plate at 37°C, and samples were assayed to determine the number of CFU per milliliter at timed intervals. (B) Growth in THP-1 cells. Exponentially growing cultures of M. tuberculosis H37Rv were diluted to a concentration of about 5 × 103 CFU/ml with tissue culture medium, and 0.1-ml portions of the diluted cultures (5 × 102 CFU per well) were used to infect differentiated THP-1 macrophages (2 × 104 cells per well) in 96-well plates. After 1 day of incubation, the medium was replaced with fresh medium with or without 0.5 μg of INH per ml. Extracellular and intracellular bacteria were assayed to determine the number of CFU at different times. Symbols: ○, H37Rv(pJD32) with INH; •, H37Rv(pJD32) without INH; ▵, H37Rv(pJD33) with INH; ▴, H37Rv(pJD33) without INH.
Selection of M. tuberculosis INH-resistant clones during infection of THP-1 cells.
We constructed a library using small (100- to 500-bp) M. tuberculosis DNA fragments cloned upstream of inhA in the pJD32 vector. This plasmid library was electroporated into M. tuberculosis, with selection for kanamycin resistance; 0.01% of the clones resulting from the electroporation procedure were INH resistant prior to further selection. This low frequency was presumably due to the fact that most clones contained small DNA fragments with no promoter activity at all; pos sibly there were also some promoters that conferred resistance to levels of INH lower than that used in the original selection. A culture from the library was used to infect THP-1 cells that had been differentiated into macrophage-like cells by the addition of phorbol esters. Treatment with INH was imposed after 1 day of infection, and clones that survived a 5-day treatment with 0.5 μg of INH per ml were selected. Surviving clones were isolated from the macrophages, diluted into broth, and used to reinfect THP-1 cells, again with treatment with INH for 5 days. Passaging of the library in this manner was repeated four times. Each time, an aliquot of the culture containing survivors of the INH treatment was plated on medium with no INH, and single colonies were picked and tested individually for INH resistance by streaking on plates containing INH (0.5 μg/ml). Clones completely sensitive to this level of INH were analyzed by PCR for the presence of a cloned insert by using primers for inhA and vector sequences that flanked the insert.
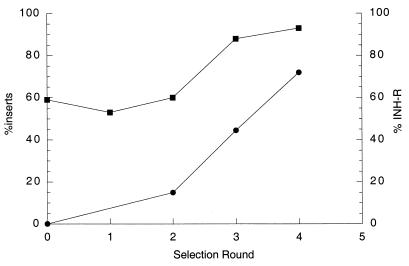
Clones were analyzed for the frequency of inserts and the frequency of INH resistance before and after selection in macrophages in order to determine if INH selection was working during growth in macrophages. It was expected that the frequency of clones resistant to INH on plates, as well as the frequency of inserts in INH-sensitive clones, would increase with each selection round. The presence of an insert was determined by PCR analysis with heat-killed bacteria by using primers flanking the cloning site, and INH resistance was assayed by streaking on plates with 0.5 μg of INH per ml. The enrichment of the frequency of INH-resistant clones and INH-sensitive clones containing inserts is shown in Fig. 2. Only 59% of the INH-sensitive clones from the original library had inserts, whereas after four passages with selection with INH in macrophages, 93% of the INH-sensitive clones had inserts and 72% were INH resistant.
FIG. 2.
Enrichment for INH-resistant (INH-R) clones and clones containing DNA inserts after multiple rounds of selection in THP-1 cells. Individual colonies surviving the infection were picked from plates with kanamycin and tested for INH resistance by streaking on plates with and without INH. Colonies were also tested for the presence of a DNA insert by PCR analysis of heat-killed bacteria using primers flanking the site of insertion. Symbols: •, percentage of INH-resistant clones; ▪, percentage of clones with inserts.
Identification of promoters driving inhA in clones surviving INH treatment during macrophage infection.
Plasmid DNAs from individual M. tuberculosis clones that survived INH treatment during growth in THP-1 cells, that were INH sensitive on plates, and that carried DNA inserts upstream of inhA, as determined by direct PCR analysis of the M. tuberculosis colonies, were isolated from M. tuberculosis cultures and used to transform E. coli. Plasmid DNAs were then prepared from the E. coli kanamycin-resistant transformants, and the sequences of the inserts upstream of inhA were determined by using a primer specific for the adjacent 5′ region of M. smegmatis inhA. The DNA sequences of the inserts were then compared to the M. tuberculosis H37Rv genome database (5). Whereas only 24% (4 of 17) of the INH-sensitive clones from the second passage contained sequences 5′ to an open reading frame in the correct orientation for transcription of inhA, 41% (12 of 41) of the clones from the third passage and 69% (45 of 65) of the comparable clones from the fourth passage contained such sequences. Our initial conclusion was that these sequences contained promoters that drove expression of inhA during M. tuberculosis infection of macrophages, resulting in overexpression of InhA and conferring INH resistance during infection. A list of the open reading frames found downstream from these putative promoter sequences is shown in Table 2.
TABLE 2.
Genes identified by INH selection in macrophages
| Category | Gene | Rv no.a | Comment |
|---|---|---|---|
| Fatty acid degradation | aceA | Rv0467 | Isocitrate lyase |
| fadA4 | Rv1323 | Acetyl-coenzyme A acetyltransferase | |
| echA19 | Rv3516 | Enoyl-coenzyme A hydratase | |
| fadA5 | Rv3546 | Acetyl-coenzyme A acetyltransferase | |
| Possible fatty acid metabolism | ephF | Rv0134 | Epoxide hydrolase |
| Rv0610c | Monooxygenase | ||
| Rv1144 | Alcohol dehydrogenase | ||
| Rv1774 | Oxidoreductase | ||
| Cell envelope | Rv0102 | Membrane | |
| Rv1171 | Hydrophobic protein | ||
| Rv2120c | Membrane | ||
| lppM | Rv2171 | Lipoprotein | |
| Rv3237c | Potassium channel | ||
| Rv3524 | Membrane, sensor | ||
| Rv3717 | N-Acetyl-muramoyl-L-alanine amidase | ||
| Intermediary metabolism | pckA | Rv0211 | Phosphoenol carboxykinase |
| eno | Rv1023 | Enolase | |
| PPE/PEPGRS | Rv0977 | PE/PGRS | |
| Rv1361c | PPE | ||
| Rv1840c | PE/PGRS | ||
| Putative transcriptional regulators | Rv0549c | Helix-turn-helix motif | |
| Rv2009 | Helix-turn-helix motif | ||
| Rv3321c | Helix-turn-helix motif | ||
| Miscellaneous | fusA2 | Rv0120c | Elongation factor G |
| proC | Rv0500 | Proline biosynthesis | |
| uvrC | Rv1420 | Exonuclease ABC | |
| infC | Rv1641 | Initiation factor 3 | |
| Rv2224c | Exported protease | ||
| nirA | Rv2391 | Nitrite reductase | |
| Rv2520c | Transmembrane domain | ||
| dut | Rv2697c | Deoxyuridine triphosphatase | |
| Rv3225c | Aminoglycoside 3′ phosphotransferase | ||
| Unknown | Rv0036c, Rv0406c, Rv0811c, Rv1778c, Rv2273, Rv2468c, Rv2632c, Rv2717c, Rv3427c, Rv3493c, Rv3717 |
The Rv number is the number assigned to the open reading frame, and the genes are annotated as described by the Pasteur Institute at the TUBERCULIST website (http://genolist.pasteur.fr/TubercuList).
Differential expression of selected genes measured by RT-PCR.
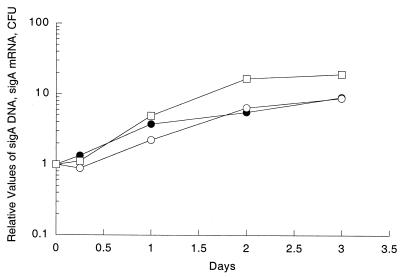
Since some of the promoters identified by our system could be false positives, selected on the basis of artifactual increased expression from a multicopy plasmid or because they were low-level constitutive promoters, as discussed above, we set out to verify whether they were indeed induced during growth of wild-type M. tuberculosis in macrophages. We determined the ratio of specific mRNAs from M. tuberculosis H37Rv grown in broth to specific mRNAs found in bacteria infecting THP-1 cells by using mbRT-PCR (21). Normalization is necessary for quantitative analysis by RT-PCR, and since initial experiments had shown that the sigA mRNA/total bacterial RNA ratio was the same in log-phase broth-grown and macrophage-grown cultures (data not shown), we performed an experiment to verify that sigA mRNA could be used for normalization of the mRNAs of the genes identified by the promoter trap selection procedure. We monitored the relative levels of sigA DNA by PCR and the levels of sigA mRNA by RT-PCR with samples prepared during growth of M. tuberculosis in THP-1 cells. At the same time, we measured the CFU. The levels of sigA mRNA increase in parallel with the levels of sigA DNA and the CFU (Fig. 3). This shows that as expected for a housekeeping gene, sigA mRNA accumulates in proportion to DNA levels and bacterial replication under conditions of growth in THP-1 macrophages, validating the use of sigA mRNA to normalize the mRNA levels of the genes analyzed.
FIG. 3.
Levels of sigA DNA and sigA mRNA and growth of M. tuberculosis H37Rv in THP-1 cells. The numbers of CFU (□), the levels of sigA mRNA (○), and the levels of sigA DNA (•) during growth of M. tuberculosis H37Rv in THP-1 cells were determined. The values were normalized to the values obtained after 1 h of infection in THP-1 cells.
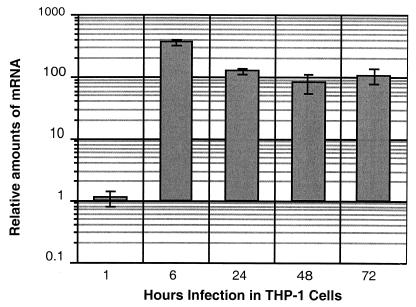
Next, we tested if our methods would work for a gene whose expression was known to be induced in M. tuberculosis during growth in macrophages. hspX, coding for the α-crystallin-like protein, had previously been shown to be highly induced (36), as was the protein itself (24). Therefore, we measured the relative levels of hspX mRNA during infection and during growth in broth. As expected, hspX was induced very dramatically (maximum, 366-fold) in bacteria grown in THP-1 cells after only 6 h of incubation (Fig. 4). This result validated the mbRT-PCR method for quantitating mRNA levels in bacteria grown in macrophages. We chose 13 of the 43 genes identified by the selection system for validation by mbRT-PCR (21). We made the selection based upon genes which could be involved in a variety of functions. Some of these genes encoded proteins postulated to be involved in fatty acid degradation, others could be involved in export or import, others with DNA binding motifs are potential transcriptional activators, and others encoded proteins whose functions are completely unknown. We determined the ratios of the mRNAs of these genes in broth-grown and THP-1-grown cultures of M. tuberculosis. In order to be sure that our RNA preparations were not contaminated with RNA from a small population of extracellular bacteria which had not been removed by washing, we added gentamicin (50 μg/ml) to the medium after the infected monolayer was washed, and the antibiotic was present throughout the infection. This treatment did not significantly affect the yield of intracellular bacteria but was effective in killing extracellular bacteria (data not shown). In an earlier series of experiments, we infected the cells in the absence of gentamicin and obtained the same results with mbRT-PCR (R. Manganelli, E. Dubnau, and I. Smith, unpublished data). Eight of the 13 genes tested (Rv2224c, Rv3237c, Rv3321c, Rv2520c, pckA, echA19, fadA4, and aceA) were upregulated more than twofold during growth in THP-1 cells relative to growth in broth culture (Table 3). Similar data were obtained with two separate infections and, in the case of Rv2520c and aceA, with three separate infections. Therefore, 61% (8 of 13) of the genes tested were found to be actually induced during growth in THP-1 cells. In order to control for differential expression of bacterial genes in response to the tissue culture medium (RPMI 1640 medium) during the 2 h of incubation allowed for uptake of the bacteria, we also compared the levels of mRNAs from bacteria incubated in RPMI 1640 medium for 2 h with the levels of RNAs from bacteria grown in broth culture. Most of the genes showed no significant differences in RNA levels, but aceA and echA19 were induced by this treatment (9.5- and 4.2-fold, respectively), albeit at lower levels than during growth in THP-1 cells (85.4- and 25-fold, respectively) (Table 3). This induction may have been due to the high levels of fetal calf serum (20%) added to the RPMI 1640 medium. We expected that this component would be very rich in fatty acids and could cause induction of genes postulated to be involved in fatty acid metabolism. We are currently investigating the induction patterns of these genes in various media.
FIG. 4.
Changes in hspX mRNA levels during growth in THP-1 cells. The results are expressed as the ratios of the numbers of RNA copies detected in RNA samples taken at various times (shown on the x axis and expressed in hours) from cultures of M. tuberculosis H37Rv to the numbers of RNA copies detected in samples obtained from bacteria growing in 7H9 medium. The values were normalized to the sigA RNA value. Each measurement was obtained at least twice using independent RNA preparations.
TABLE 3.
M. tuberculosis genes induced during macrophage infection
| Gene | Induction ratiosa
|
||
|---|---|---|---|
| RPMI 1640 mediumb | 7H9 medium
|
||
| 24 h after infectionc | 72 h after infectionc | ||
| Induced genes | |||
| Rv2224c | 0.9 ± 0.1 | 4.0 ± 0.8 | 3.5 ± 0.7 |
| Rv3237c | 0.6 ± 0.0 | 3.6 ± 1.8 | 4.0 ± 1.2 |
| Rv3321c | 1.3 ± 0.2 | 2.3 ± 0.0 | 2.0 ± 0.2 |
| echA19 | 4.2 ± 0.4 | 25.0 ± 14.5 | 35.0 ± 13.5 |
| aceA | 9.5 ± 1.3 | 85.4 ± 3.1 | 88.4 ± 8.6 |
| fadA4 | 1.0 ± 0.3 | 2.6 ± 1.0 | 4.0 ± 1.5 |
| pckA | 2.2 ± 0.1 | 7.0 ± 2.0 | 6.1 ± 0.9 |
| Rv2520c | 1.1 ± 0.1 | 2.2 ± 1.3 | 0.9 ± 0.5 |
| Genes not induced | |||
| fadA5 | 1.5 ± 0.4 | 0.3 ± 0.1 | 0.5 ± 0.3 |
| Rv1171 | 1.0 ± 0.1 | 0.1 ± 0.1 | 0.2 ± 0.1 |
| Rv0977 | 1.0 ± 0.0 | 0.5 ± 0.1 | 1.5 ± 0.3 |
| Rv1774 | 0.4 ± 0.1 | 0.1 ± 0.2 | 0.7 ± 0.0 |
| nirA | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.1 |
The values are means ± standard errors based on triplicate samples.
The values are the relative amounts of M. tuberculosis mRNA obtained after 2 h of incubation in RPMI 1640 medium compared to the amounts of mRNA obtained after growth in broth. The values are normalized to sigA mRNA.
The values are the relative amounts of M. tuberculosis mRNA obtained 24 and 72 h after infection in THP-1 cells compared to the amounts of mRNA obtained after growth in broth. The values are normalized to sigA mRNA.
Several genes identified by our selection system, (Rv0977, Rv1774, Rv1171, fadA5, and nirA) were not upregulated, and in fact, two of these genes (Rv1171 and Rv1774) seemed to be actually repressed in macrophages, suggesting that our system does identify some false positives. nirA appears to be downregulated both in RPMI 1640 medium and in macrophages. Therefore, the other genes listed in Table 2 must be considered candidate genes prior to further validation.
DISCUSSION
The virulence of any pathogen is determined by its ability to adapt to the host environment. Our approach to describe adaptation by M. tuberculosis to the macrophage environment, in which a promoter trap screen was used, resulted in identification of 43 genes, 9 of which may code for proteins involved directly or indirectly in fatty acid metabolism: aceA, echA19, fadA4, pcKA, ephF, Rv0610c, Rv1144, fadA5, and Rv1774. It is expected that microarray analysis of gene expression in macrophages should produce a far more extensive list of genes that are transcriptionally upregulated during growth in macrophages, and several laboratories, as well as our laboratory, are currently doing this sort of analysis. Our survey was certainly not extensive enough to detect all M. tuberculosis genes upregulated in macrophages since various genes, including iron-regulated genes, sigE, sigH, hspX, and fbpB, known to be upregulated in macrophages (12, 18, 28, 36), were not identified by our screening procedure. None of the genes identified as upregulated during growth in the presence of INH (34), several of which are also involved in fatty acid metabolism, were identified by our method. Obviously, the main reason for this is the fact that we selected against this group of genes by choosing only clones which are sensitive to INH on plates. Promoters induced by INH would confer resistance to INH.
Thirteen genes were selected for quantitative analysis by mbRT-PCR from RNAs prepared from broth-grown cultures and THP-1-grown cultures. It is important to emphasize that this analysis was done with wild-type M. tuberculosis H37Rv and not with strains carrying plasmids containing inhA under the control of cloned promoters. Thus, the measurements of mRNA were based upon transcription from the native promoters of the genes in their normal chromosomal location. hspX was highly induced under our conditions, validating our methods, since it has been shown previously that this gene is upregulated in macrophages (24, 36). Some workers reported high levels of hspX mRNA in log-phase cultures of M. tuberculosis (16), but we found this molecule to be present at very low levels in such cultures and we assume that the different results arose from differences in bacterial growth conditions.
Expression of eight genes was shown to be induced during growth in THP-1 cells. Five genes were not found to be induced, and therefore it is important to stress that the genetic screening method does pick up false positives. There are several different sources of false positives: (i) promoter activity due to multicopy effect, (ii) low-activity promoters leading to survival in vivo and sensitivity in vitro, (iii) lower sensitivity to INH in vivo because of lower levels of INH in the macrophages, decreased levels of catalase, or other unknown reasons, and (iv) induced expression which is below the detection limit of the RT-PCR. Although most of the genes which we identified coded for unknown functions, 9 of the 43 genes are predicted to be involved in fatty acid metabolism. The genome of M. tuberculosis has approximately 250 open reading frames which are annotated to be involved in fatty acid metabolism, out of a total of about 4,000 open reading frames. The probability of detecting 9 of these 250 open reading frames in 43 genes simply by chance is very low (P < 0.001, as determined by chi-square test).
The genes identified by our selection procedure include fadA4 and echA19, which are annotated as genes involved in β-oxidation of fatty acids, as well as aceA (icl) coding for isocitrate lyase, an enzyme of the glyoxylate shunt pathway. This pathway is required to replenish substrates for the Krebs cycle during growth on fatty acids. The fact that aceA appeared among the selected genes validated our approach since it was shown previously that aceA was upregulated during infection of macrophages (13, 23) and that the levels of isocitrate lyase increase during infection (15, 31). Significantly, this gene is necessary for virulence in mice (23). pckA, although not directly involved in fatty acid metabolism, is required to produce phosphoenolpyruvate from the tricarboxylic acid cycle during metabolism of acetate, the product of fatty acid β-oxidation. Other genes identified by our selection procedure which may be involved in fatty acid metabolism include ephF, encoding an annotated epoxide hydrolase, Rv0610c, encoding an annotated monooxygenase, and Rv1144, encoding an annotated short-chain alcohol dehydrogenase. This group of genes has not yet been validated. Compared with the 50 genes in E. coli, the number of genes presumed to be involved in β-oxidation of fatty acids in M. tuberculosis is astounding (5). There are 36 fadD, 36 fadE, 21 echA, 5 fadB, and 6 fadA paralogs in M. tuberculosis, whereas there is only one copy of each of these genes in E. coli. This, together with biochemical data on the differences in metabolism of M. tuberculosis growing in mouse lungs and in broth (29), has led to a consensus that fatty acid degradation may provide a major source of energy during infection.
It is interesting that pckA, aceA, and seven fad genes are also upregulated in M. tuberculosis by treatment with SDS (22), a stress condition which may cause cell envelope damage and require remodeling of the cell envelope. S. enterica serovar Typhimurium modifies the structure of lipid A during growth in macrophages; the modifications require phoP-phoQ, a two-component system activated in macrophages, and are postulated to function by attenuating the host cell innate immune response (10, 14). It is possible that M. tuberculosis also remodels its cell envelope upon entry into macrophages, a process that would require degradation followed by resynthesis of various lipids in the cell envelope. Consistent with this hypothesis is the fact that our search identified Rv3717, encoding N-acetyl-muramyl-l-alanine amidase.
There are several other interesting genes which are also induced in THP-1 macrophages. Rv3321c is a member of a subfamily of genes with helix-turn-helix motifs typical of DNA binding proteins (http://cbcsrv.watson.imb.com/servlets/Utility); all of these motifs are upstream of a family of open reading frames with some similarity to one another. Rv3237c is annotated as a possible potassium channel protein gene.
Our findings highlight the significance of fatty acid metabolism for M. tuberculosis during growth in macrophages. It is interesting that fadB, coding for an enzyme required for β-oxidation of fatty acids, was upregulated in S. enterica serovar Typhimurium during infection of mice, and this in vivo induction was thought to be due to the high concentration of fatty acids encountered by the pathogen during infection (19). Lipids have long been postulated to be an energy source for M. tuberculosis during infection, but it is also possible that lipid biosynthesis and degradation may be important for the remodeling of the cell envelope upon entry into the macrophage. Ultimately, we are interested in defining new M. tuberculosis drug targets, and we are currently conducting experiments to determine if any of the genes identified by our approach are required for virulence. This is being done by assaying the virulence in macrophages and mice of mutant strains inactivated for the various genes.
Acknowledgments
We express our deep appreciation to Leonard Mindich, Marcela Rodriguez, Salvatore Marras, David Dubnau, and Benjamin Gold for helpful discussions and critical reading of the manuscript. We thank Juliano Timm for designing the primers and beacons for aceA, fadA4, and fadA5.
This work was supported by NIH grant AI 44856 awarded to I.S.
Editor: S. H. E. Kaufmann
Footnotes
Publication no. 71 from the TB Center.
REFERENCES
- 1.Banerjee, A., E. Dubnau, A. Quemard, V. Balasubramanian, K. S. Um, T. Wilson, D. Collins, G. de Lisle, and W. R. Jacobs, Jr. 1994. inhA, a gene encoding a target for isoniazid and ethionamide in Mycobacterium tuberculosis. Science 263:227-230. [DOI] [PubMed] [Google Scholar]
- 2.Berthet, F. X., M. Lagranderie, P. Gounon, C. Laurent-Winter, D. Ensergueix, P. Chavarot, F. Thouron, E. Maranghi, V. Pelicic, D. Portnoi, G. Marchal, and B. Gicquel. 1998. Attenuation of virulence by disruption of the Mycobacterium tuberculosis erp gene. Science 282:759-762. [DOI] [PubMed] [Google Scholar]
- 3.Camacho, L. R., D. Ensergueix, E. Perez, B. Gicquel, and C. Guilhot. 1999. Identification of a virulence gene cluster of Mycobacterium tuberculosis by signature-tagged transposon mutagenesis. Mol. Microbiol. 34:257-267. [DOI] [PubMed] [Google Scholar]
- 4.Chiang, S. L., J. J. Mekalanos, and D. W. Holden. 1999. In vivo genetic analysis of bacterial virulence. Annu. Rev. Micrbiol. 53:129-154. [DOI] [PubMed] [Google Scholar]
- 5.Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, K. Eiglmeier, S. Gas, C. E. Barry 3rd, F. Tekaia, K. Badcock, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. Davies, K. Devlin, T. Feltwell, S. Gentles, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, B. G. Barrell, et al. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. [DOI] [PubMed] [Google Scholar]
- 6.Cox, J. S., B. Chen, M. McNeil, and W. R. Jacobs, Jr. 1999. Complex lipid determines tissue-specific replication of Mycobacterium tuberculosis in mice. Nature 402:79-83. [DOI] [PubMed]
- 7.Davies, J. 1994. Inactivation of antibiotics and the dissemination of resistance genes. Science 264:375-382. [DOI] [PubMed] [Google Scholar]
- 8.Dubnau, E., J. Chan, C. Raynaud, V. P. Mohan, M. A. Laneelle, K. Yu, A. Quemard, I. Smith, and M. Daffe. 2000. Oxygenated mycolic acids are necessary for virulence of Mycobacterium tuberculosis in mice. Mol. Microbiol. 36:630-637. [DOI] [PubMed] [Google Scholar]
- 9.Dubnau, E., S. Soares, T. J. Huang, and W. R. Jacobs, Jr. 1996. Overproduction of mycobacterial ribosomal protein S13 induces catalase/peroxidase activity and hypersensitivity to isoniazid in Mycobacterium smegmatis. Gene 170:17-22. [DOI] [PubMed] [Google Scholar]
- 10.Ernst, R. K., T. Guina, and S. I. Miller. 1999. How intracellular bacteria survive: surface modifications that promote resistance to host innate immune responses. J. Infect. Dis. 179(Suppl. 2):S326-S330. [DOI] [PubMed]
- 11.Glickman, M. S., J. S. Cox, and W. R. Jacobs, Jr. 2000. A novel mycolic acid cyclopropane synthetase is required for coding, persistence, and virulence of Mycobacterium tuberculosis. Mol. Cell 5:717-727. [DOI] [PubMed] [Google Scholar]
- 12.Gold, B., G. M. Rodriguez, S. A. E. Marras, M. Pentecost, and I. Smith. 2001. The Mycobacterium tuberculosis IdeR is a dual functional regulator that controls transcription of genes involved in iron acquisition, iron storage and survival in macrophages. Mol. Microbiol. 42:851-865. [DOI] [PubMed] [Google Scholar]
- 13.Graham, J. E., and J. E. Clark-Curtiss. 1999. Identification of Mycobacterium tuberculosis RNAs synthesized in response to phagocytosis by human macrophages by selective capture of transcribed sequences (SCOTS). Proc. Natl. Acad. Sci. USA 96:11554-11559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo, L., K. B. Lim, J. S. Gunn, B. Bainbridge, R. P. Darveau, M. Hackett, and S. I. Miller. 1997. Regulation of lipid A modifications by Salmonella typhimurium virulence genes phoP-phoQ. Science 276:250-253. [DOI] [PubMed] [Google Scholar]
- 15.Honer zu Bentrup, K., A. Miczak, D. L. Swenson, and D. G. Russell. 1999. Characterization of activity and expression of isocitrate lyase in Mycobacterium avium and Mycobacterium tuberculosis. J. Bacteriol. 181:7161-7167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu, Y., and A. R. Coates. 1999. Transcription of the stationary-phase-associated hspX gene of Mycobacterium tuberculosis is inversely related to synthesis of the 16-kilodalton protein. J. Bacteriol. 181:1380-1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacobs, W. R., Jr., G. V. Kalpana, J. D. Cirillo, L. Pascopella, S. B. Snapper, R. A. Udani, W. Jones, R. G. Barletta, and B. R. Bloom. 1991. Genetic systems for mycobacteria. Methods Enzymol. 204:537-555. [DOI] [PubMed] [Google Scholar]
- 18.Jensen-Cain, D. M., and F. D. Quinn. 2001. Differential expression of sigE by Mycobacterium tuberculosis during intracellular growth. Microb. Pathog. 30:271-278. [DOI] [PubMed] [Google Scholar]
- 19.Mahan, M. J., J. W. Tobias, J. M. Slauch, P. C. Hanna, R. J. Collier, and J. J. Mekalanos. 1995. Antibiotic-based selection for bacterial genes that are specifically induced during infection of a host. Proc. Natl. Acad. Sci. USA 92:669-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahan, M. J., J. M. Slauch, and J. J. Mekalanos. 1993. Selection of bacterial virulence genes that are specifically induced in host tissues. Science 259:686-688. [DOI] [PubMed] [Google Scholar]
- 21.Manganelli, R., E. Dubnau, S. Tyagi, F. R. Kramer, and I. Smith. 1999. Differential expression of 10 sigma factor genes in Mycobacterium tuberculosis. Mol. Microbiol. 31:715-724. [DOI] [PubMed] [Google Scholar]
- 22.Manganelli, R., M. I. Voskuil, G. K. Schoolnik, and I. Smith. 2001. The Mycobacterium tuberculosis ECF sigma factor σ E: role in global gene expression and survival in macrophages. Mol. Microbiol. 41:423-437. [DOI] [PubMed] [Google Scholar]
- 23.McKinney, J. D., K. Honer zu Bentrup, E. J. Munoz-Elias, A. Miczak, B. Chen, W. T. Chan, D. Swenson, J. C. Sacchettini, W. R. Jacobs, Jr., and D. G. Russell. 2000. Persistence of Mycobacterium tuberculosis in macrophages and mice requires the glyoxylate shunt enzyme isocitrate lyase. Nature 406:735-738. [DOI] [PubMed] [Google Scholar]
- 24.Monahan, I. M., J. Betts, D. K. Banerjee, and P. D. Butcher. 2001. Differential expression of mycobacterial proteins following phagocytosis by macrophages. Microbiology 147:459-471. [DOI] [PubMed] [Google Scholar]
- 25.Quémard, A., A. Dessen, M. Sugantino, W. R. Jacobs, Jr., J. C. Sacchettini, and J. S. Blanchard. 1996. Binding of catalase-peroxidase-activated isoniazid to wild-type and mutant Mycobacterium tuberculosis enoyl-ACP reductases. J. Am. Chem. Soc. 118:1561-1562. [Google Scholar]
- 26.Quemard, A., J. C. Sacchettini, A. Dessen, C. Vilcheze, R. Bittman, W. R. Jacobs, Jr., and J. S. Blanchard. 1995. Enzymatic characterization of the target for isoniazid in Mycobacterium tuberculosis. Biochemistry 34:8235-8241. [DOI] [PubMed] [Google Scholar]
- 27.Rafii, S., F. Shapiro, R. Pettengell, B. Ferris, R. L. Nachman, M. A. Moore, and A. S. Asch. 1995. Human bone marrow microvascular endothelial cells support long-term proliferation and differentiation of myeloid and megakaryocytic progenitors. Blood 86:3353-3363. [PubMed] [Google Scholar]
- 28.Ramakrishnan, L., N. A. Federspiel, and S. Falkow. 2000. Granuloma-specific expression of Mycobacterium virulence proteins from the glycine-rich PE-PGRS family. Science 288:1436-1440. [DOI] [PubMed] [Google Scholar]
- 29.Segal, W., and H. Bloch. 1956. Biochemical differentiation of Mycobacterium tuberculosis grown in vivo and in vitro. J. Bacteriol. 72:132-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stover, C. K., V. F. de la Cruz, T. R. Fuerst, J. E. Burlein, L. A. Benson, L. T. Bennett, G. P. Bansal, J. F. Young, M. H. Lee, G. F. Hatfull, et al. 1991. New use of BCG for recombinant vaccines. Nature 351:456-460. [DOI] [PubMed] [Google Scholar]
- 31.Sturgill-Koszycki, S., P. L. Hadddix, and D. G. Russell. 1997. The interaction between Mycobacerium and the macrophage analyzed by two-dimensional polyacrylamide gel electrophoresis. Electrophoresis 18:2558-2565. [DOI] [PubMed] [Google Scholar]
- 32.Tsuchiya, S., Y. Kobayashi, Y. Goto, H. Okumura, S. Nakae, T. Konno, and K. Tada. 1982. Induction of maturation in cultured human monocytic leukemia cells by a phorbol diester. Cancer Res. 42:1530-1536. [PubMed] [Google Scholar]
- 33.Tyagi, S., and F. R. Kramer. 1996. Molecular beacons: probes that fluoresce upon hybridization. Nat. Biotechnol. 14:303-308. [DOI] [PubMed] [Google Scholar]
- 34.Wilson, M., J. DeRisi, H. H. Kristensen, P. Imboden, S. Rane, P. O. Brown, and G. K. Schoolnik. 1999. Exploring drug-induced alterations in gene expression in Mycobacterium tuberculosis by microarray hybridization. Proc. Natl. Acad. Sci. USA 96:12833-12838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson, T. M., G. W. de Lisle, and D. M. Collins. 1995. Effect of inhA and katG on isoniazid resistance and virulence of Mycobacterium bovis. Mol. Microbiol. 15:1009-1015. [DOI] [PubMed] [Google Scholar]
- 36.Yuan, Y., D. D. Crane, R. M. Simpson, Y. Q. Zhu, M. J. Hickey, D. R. Sherman, and C. E. Barry 3rd. 1998. The 16-kDa alpha-crystallin (Acr) protein of Mycobacterium tuberculosis is required for growth in macrophages. Proc. Natl. Acad. Sci. USA 95:9578-9583. [DOI] [PMC free article] [PubMed] [Google Scholar]