Abstract
Monocyte chemoattractant protein 1 (MCP-1) is an important chemokine that induces monocyte recruitment in a number of different pathologies, including infection. To investigate the role of MCP-1 in protecting a host from a chronic interstitial polymicrobial infection, dental pulps of MCP-1−/− mice and controls were inoculated with six different oral pathogens. In this model the recruitment of leukocytes and the impact of a genetic deletion on the susceptibility to infection can be accurately assessed by measuring the progression of soft tissue necrosis and osteolytic lesion formation. The absence of MCP-1 significantly impaired the recruitment of monocytes, which at later time points was threefold higher in the wild-type mice than in MCP-1−/− mice (P < 0.05). The consequence was significantly enhanced rates of soft tissue necrosis and bone resorption (P < 0.05). We also determined that the MCP-1−/− mice were able to recruit polymorphonuclear leukocytes (PMNs) to a similar or greater extent as controls and to produce equivalent levels of Porphyromonas gingivalis-specific total immunoglobulin G (IgG) and IgG1. These results point to the importance of MCP-1 expression and monocyte recruitment in antibacterial defense and demonstrate that antibacterial defense is not due to an indirect effect on PMN recruitment or modulation of the adaptive immune response.
Monocyte chemoattractant protein 1 (MCP-1) is a C-C chemokine and a member of a subfamily containing five related molecules, MCP-1 through MCP-5 (11, 22, 27). MCP-1 is expressed in many pathological states, including atherosclerosis, asthma, pulmonary fibrosis, arthritis, delayed-type hypersensitivity reactions, sepsis, and chronic bacterial infections (1, 2, 5, 6, 15, 24, 33, 35-37). MCP-1 expression is also up regulated during normal developmental processes (23, 31). In vitro, MCP-1 stimulates recruitment of specific leukocyte subsets, including monocytes, memory T lymphocytes, and natural killer cells (27). However, an infiltrate induced by MCP-1 in vivo consists predominantly of mononuclear phagocytes (11, 34). Recent studies have demonstrated that MCP-1 expression shifts the immune response toward the production of Th2 cytokines and that targeted deletion of MCP-1 inhibits the development of a Th2 immune response (12).
MCP-1 activity is often necessary for maximal recruitment of monocytes even though there are multiple chemokines with overlapping functions (4, 13, 16-19). Genetic deletion of the MCP-1 gene significantly reduces the recruitment of monocytes following intraperitoneal thioglycolate administration or during delayed-type hypersensitivity reactions and impairs the response to infection by the intracellular bacterium Mycobacterium tuberculosis in mice (17). This is not due to an effect on the numbers of circulating leukocytes and resident macrophages, which are normal in such mice.
Experimental infections of the dental pulp provide an excellent model to study the spread of an interstitial anaerobic infection. After exposure of the dental pulp to a polymicrobial infection, an inflammatory response is stimulated that induces necrosis, which spreads from the exposed site to the root apex. The spread of infection can be measured by the rate at which the dental pulp becomes necrotic. Once the infection reaches the root apex, host cytokines are induced that cause osteolysis of the surrounding bone (28). A deficit in the host response renders the tissue more susceptible to the spread of infection, which is readily observed by an increased rate of necrosis and enhanced formation of an osteolytic lesion (7, 14). However, when the host response is enhanced, the progress of the infection and the resulting osteolysis is significantly reduced (29).
In experiments described here we examined the role of MCP-1 in protecting a host from a chronic polymicrobial infection using the model described above and mice with targeted disruption of the MCP-1 gene. The results indicate that the functional absence of MCP-1 significantly impairs monocyte recruitment and renders the host more susceptible to an interstitial infection. We also determined that MCP-1−/− mice were able to recruit polymorphonuclear leukocytes (PMNs) to a similar or greater extent as controls and to produce equivalent levels of Porphyromonas gingivals-specific total immunoglobulin G (IgG) and IgG1. These results point to the importance of MCP-1 expression and monocyte recruitment in antibacterial defense and demonstrate that antibacterial defense is not due to an indirect effect on PMN recruitment or antibody production.
MATERIALS AND METHODS
Reagents were purchased from Sigma Chemical Co. (St. Louis, Mo.) unless otherwise stated.
Bacteria.
Surgically exposed dental pulps were inoculated with six putative oral pathogens. The bacteria used were the facultatively anaerobic gram-positive coccus Streptococcus mutans ATCC 25175 and five anaerobic strains, including the gram-positive cocci Streptococcus intermedius ATCC 27335 and Peptostreptococcus micros ATCC 33270 and the gram-negative rods Porphyromonas gingivalis ATCC 33277, Prevotella intermedius ATCC 25611, and Fusobacterium nucleatum ATCC 49256. The amount of each bacterium was determined spectrophotometrically by using a standard curve established by colony formation on bacterial plates.
Inoculation of the dental pulp.
Anesthesia was obtained by intraperitoneal injection of ketamine (80 mg/kg) and xylazine (10 mg/kg) in sterile phosphate-buffered saline. The animals were then mounted on a jaw retraction board, and the dental pulps were exposed by removal of the mesial buccal cusps of the first mandibular molars. One hundred microliters of a viscous bacterial mixture containing 109 of each of the six bacteria described above was placed onto the tooth surface. Animals were monitored daily and showed no signs of pain-associated behavior. All of the animals continued to gain weight and behave in a manner similar to that of untreated animals.
Histologic analysis.
Experimental animals that were 10 to 12 weeks old and matched controls were sacrificed at zero time and 1, 3, 7, 14, or 21 days after pulp exposure by carbon dioxide overdose. The mandibles were resected and immediately placed in a 4% paraformaldehyde solution for overnight fixation at 4°C. After fixation, specimens were then decalcified for 2 to 4 weeks in a 15% glycerol-EDTA solution. Radiographic monitoring was performed to ensure complete decalcification. Specimens were dehydrated in 30% sucrose overnight and then snap frozen by immersion into prechilled 2-methylbutane (−80°C). All sectioning was done in a sagittal plane at a thickness of 5 μm. Every seventh slide was stained with hematoxylin and eosin. In these sections, the mesial root, periapical lesion, and surrounding peripheral bone could be observed. The osteolytic lesions were measured at the widest aspect of each lesion. Five to six specimens were examined for each data point.
Immunohistochemistry.
In order to quantify the recruitment of mononuclear phagocytes, frozen serial sections were incubated with rat monoclonal antibody F4/80 specific for murine CD68, which is a marker for peripheral monocytes and macrophages. Primary antibodies were localized with secondary antibody, followed by incubation with avidin-biotin-horseradish peroxidase complex (Vector Laboratories, Burlington, Calif.). Diaminobenzidine was used as a chromogen. Slides were counterstained with hematoxylin prior to mounting. The data are presented below as the increase in the number of monocytes over baseline levels to reflect recruitment of new cells.
Analysis.
All histologic slides were coded prior to analysis, and measurements were done in a double-blind manner. Immunohistochemical sections from each animal were examined at a magnification of ×500. Immunopositive cells with distinctly round and darkly stained nuclei were counted within the entire lesion area associated with the mesial root. The progress of necrosis down the mesial root canal was examined at a magnification of ×400 in hematoxylin- and eosin-stained sections. Necrosis was readily apparent since liquefaction occurs with clear destruction of both cells and connective tissue matrix. The length of the necrotic tissue in the mesial root canal was measured and divided by the entire length of the mesial canal to obtain the percentage that was necrotic. The size of each osteolytic lesion was calculated by subtracting the area of the normal periodontal ligament space from the area of the osteolytic lesion between the apical aspect of the dental root and the surrounding bone. This accurately represented osteolytic activity since the only mechanism by which this space increased was through osteoclastic bone resorption. Neutrophils were identified and counted in the same area by using their characteristic appearance in hematoxylin- and eosin-stained sections. Measurements were made with computer-assisted image analysis. Results were verified independently by a second examiner, and agreement was good.
Production of antibodies to P. gingivalis.
Mice were treated with P. gingivalis and Streptococcus sanguis (109 cells each) by intraperitoneal injection of formalin-fixed bacteria once each week for 4 weeks. The capacity of MCP-1−/− or wild-type mice to produce antibody to P. gingivalis was measured by using serum obtained from tail bleeds. P. gingivalis-specific total IgG and IgG1 were measured by an enzyme-linked immunosorbent assay by using 96-well plates precoated with formalin-fixed P. gingivalis. The data are presented below as the highest dilution that resulted in spectrophotometric absorbance greater than 1.5. Serial dilutions ranged from 1:25 to 1:25,000.
Statistical analysis.
Statistical significance was determined by one-way analysis of variance. Significance was established at a level of P < 0.05.
RESULTS
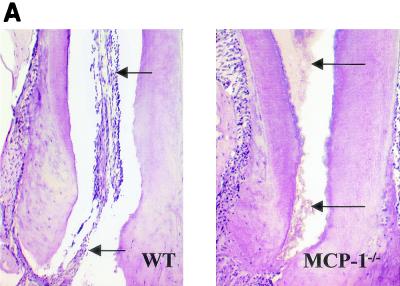
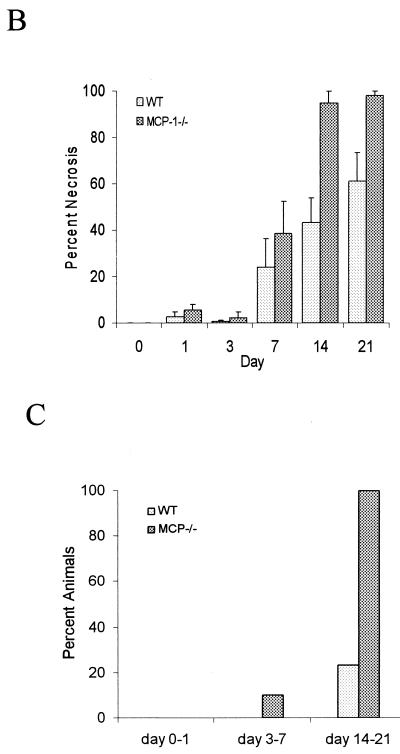
Exposure of the dental pulp to oral pathogens resulted in the spread of necrosis from the site of inoculation to the root apex. The percentage of the dental pulp that was necrotic was measured in MCP-1−/− and wild-type mice at each time point. At the early time points there was relatively little difference between the two groups. By day 14 necrosis of the dental pulp was clearly evident in the lower half of the mesial root in the MCP-1−/− mice, while in some of the wild-type mice there was no necrosis in this area (Fig. 1A). Quantitative analysis indicated that the amount of necrotic tissue in the MCP-1−/− group was 115% greater than the amount in the control group on day 14 and 60% greater on day 21 (Fig. 1B). Further analysis revealed that 100% of the MCP-1−/− mice exhibited necrosis of at least three-quarters of the dental pulp on days 14 and 21, while the same extent of necrosis was detected in only 20% of the wild-type mice (Fig. 1C).
FIG. 1.
Infection results in enhanced tissue necrosis in MCP-1−/− mice. Surgical pulp exposure followed by inoculation with six oral pathogens was carried out as described in Materials and Methods. Hematoxylin- and eosin-stained sections were examined for the presence of tissue necrosis in the dental pulp. (A) Lower half of the mesial root of the first molar. The arrows point to necrotic dental pulp in an MCP-1−/− mouse and normal dental pulp in a wild-type (WT) animal. (B) Percentages of necrosis. The length of necrotic tissue was compared to the total length of the dental pulp to calculate the percentage of necrosis. The means ± standard errors of the means (n = 5 for each time point) are shown. Significant differences (P < 0.05) were noted between the experimental and wild-type mice at 14 and 21 days following bacterial challenge. (C) Percentages of mice exhibiting greater than 75% necrosis of the dental pulp calculated for days 0 to 1, 3 to 7, and 14 to 21.
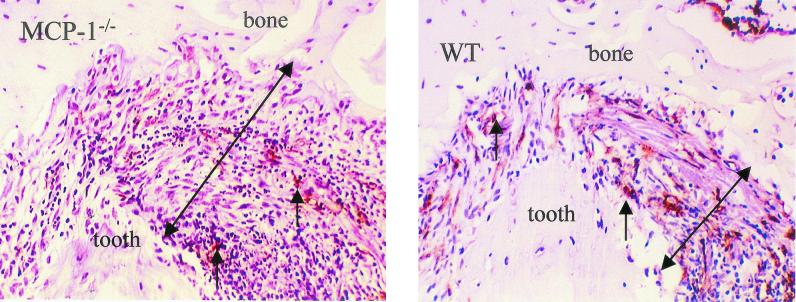
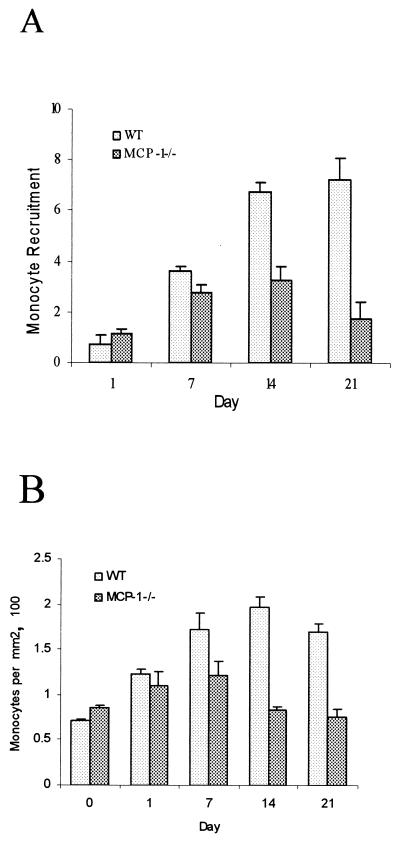
The histologic sections shown in Fig. 2 were immunostained with the F4/80 antibody to identify monocytes and macrophages and were counterstained with hematoxylin. The number of immunostained monocytes and macrophages was considerably lower in the MCP-1−/− mice than in the wild-type mice. Quantitative analysis was performed to measure the increase in the number of mononuclear phagocytes compared to the number at zero time (Fig. 3). At 1 and 7 days there were similar increases in the number of monocytes in both groups (Fig. 3A). However, the sustained recruitment of monocytes on days 14 and 21 was dependent upon MCP-1 expression. On day 14 the increase in the number of mononuclear phagocytes in the experimental group was one-half that of the control group, and on day 21 it was one-fourth; these differences were statistically significant (P < 0.05). The number of monocytes per area was also measured (Fig. 3B). The number of monocytes present per square millimeter in the wild-type group reached a peak at day 14, whereas in the MCP-1−/− mice the number reached a peak at day 7. The reductions in the experimental group on days 14 and 21 were likely due in part to large increases in the lesion area.
FIG. 2.
Monocyte recruitment and osteolytic lesion formation in MCP-1−/− and wild-type (WT) mice. The dental pulp was challenged with six oral pathogens. Animals sacrificed at day 14 were immunostained with F4/80 antibody specific for peripheral monocytes and macrophages. The small arrows indicate immunostained cells. The large arrows indicate the space between the apex of the root and the surrounding bone. This space is enhanced as a result of osteolysis in the MCP-1−/− mice.
FIG. 3.
MCP-1−/− mice have reduced monocyte recruitment in response to infection. The dental pulp was challenged with six oral pathogens. Monocytes and macrophages were identified by immunostaining with the F4/80 antibody, and the numbers were determined by computer-assisted image analysis. (A) Total number of F4/80-positive cells per lesion. (B) Number of F4/80-positive cells per square millimeter in each lesion. The means ± standard errors based on five or six specimens for each time point are shown. WT, wild type.
The formation of osteolytic lesions, as shown in Fig. 2, was greater in MCP-1−/− mice than in control mice. To assess differences in the extent of bone resorption in the periapical area, histomorphometric measurements of lesion size obtained for the two groups were compared (Fig. 4). Osteolytic lesions were larger in the MCP-1−/− mice than in the control mice on days 14 and 21 (P < 0.05). On day 14 the lesions in the MCP-1−/− animals were three times larger, and on day 21 they were nearly twice the size of the lesions in the wild-type mice (P < 0.05).
FIG. 4.
MCP-1−/− mice have enhanced osteolysis resulting from infection. The area of each lesion at the dental root apex was determined with a computer-assisted image analysis system at different times following exposure of the dental pulp and inoculation of bacteria. For each value the area at zero time representing the normal space between the root apex and bone was subtracted. The means ± standard errors based on five or six specimens are shown. WT, wild type.
It is possible that MCP-1−/− mice are more susceptible to infection because of secondary effects on PMN recruitment or antibody production. As shown in Table 1, when PMNs were counted, there were equivalent numbers per square millimeter in the experimental and control groups at the early time point (P > 0.05). However, on days 14 and 21 there were actually more PMNs in lesions of MCP-1−/− mice than in lesions of wild-type control mice. Experiments were undertaken to establish whether there was a difference in the capacity to produce antibodies by measuring IgG and IgG1 (Table 2). The results indicate that MCP-1 mice do not have an impaired capacity to produce anti-P. gingivalis total IgG or anti-P. gingivalis IgG1.
TABLE 1.
MCP-1−/− mice do not exhibit impaired recruitment of PMNs
| Day | PMNs/mm2 (mean ± SE)a
|
P value | |
|---|---|---|---|
| Wild-type mice | MCP-1−/− mice | ||
| 3 | 25.4 ± 8.95 | 26.4 ± 7.1 | 0.93 |
| 7 | 45.2 ± 7.54 | 42.72 ± 9.2 | 0.84 |
| 14 | 120.32 ± 18.4 | 200.8 ± 19.4 | 0.02 |
| 21 | 160 ± 31.2 | 391.5 ± 36.4 | 0.001 |
PMNs were measured in the periapical space between the root surface and bone. n = 5 or 6 for each group.
TABLE 2.
MCP-1−/− mice do not exhibit impaired production of total IgG or IgG1 specific for P. gingivalis
| Week | Total IgG dilutiona
|
IgG1 dilutiona
|
||||
|---|---|---|---|---|---|---|
| C57 mice | MCP-1−/− mice | P value | C57 mice | MCP-1−/− mice | P value | |
| 1 | 50 ± 0b | 50 ± 0 | 25 ± 0 | 25 ± 0 | ||
| 4 | 2,240 ± 391 | 1,800 ± 450 | 0.51 | 350 ± 134 | 175 ± 22 | 0.29 |
| 5 | 5,760 ± 639 | 7,200 ± 1,800 | 0.48 | 220 ± 49 | 750 ± 277 | 0.09 |
Mice were injected with P. gingivalis as described in Materials and Methods. The data are the highest dilutions that gave absorbance values greater than 1.5 in an enzyme-linked immunosorbent assay.
Mean ± standard error (n = 4 for each group).
DISCUSSION
Data presented here demonstrate that genetic deletion of MCP-1 significantly impairs sustained monocyte recruitment in response to a chronic polymicrobial infection. Given that there are over 50 different chemokines, many of which have overlapping functions, the extent to which MCP-1 deletion reduced the capacity of the host to form a monocytic infiltrate was striking. The data are even more striking when the capacity of bacterial products to stimulate recruitment of mononuclear phagocytes via formyl peptide receptors is taken into account. However, at early time points similar monocyte recruitment events occurred in both the control and MCP-1−/− mice. Thus, other chemotactic factors, including different chemokines or bacterial products, may be particularly important in the early induction of monocyte recruitment, while MCP-1 may play a critical role in sustained recruitment.
The reduction in monocyte recruitment caused by deletion of MCP-1 rendered the host more susceptible to infection. Although PMNs represent the principal type of cells that clear infection by bacteria that reside in an interstitial compartment, the significance of monocyte recruitment was demonstrated by the higher degree of tissue destruction in the experimental group. The importance of monocyte recruitment is underscored by the observation that PMN recruitment was greater in the MCP-1−/− group. This indicates that the enhanced susceptibility of the MCP-1−/− mice to infection is not due to a secondary effect of a decreased ability to attract PMNs. The fact that there was greater tissue destruction in the MCP-1−/− mice suggests that the infection was more severe, which could explain the enhanced recruitment of PMNs. However, the recruitment of the PMNs did not compensate for the loss of MCP-1 and monocyte recruitment. It is possible that the PMNs recruited in the MCP-1−/− mice were less effective since monocytes may be needed to fully activate antibacterial activities of PMNs through the production of cytokines, such as interleukin-1 or tumor necrosis factor (8, 9, 25). Additional studies are necessary to determine whether MCP-1 activity indirectly enhances bacterial killing by PMNs.
MCP-1 could potentially affect antibacterial defenses by modulating lymphocyte activity through development of a Th2 response. We examined whether functional deletion of MCP-1 caused a decrease in production of the Th2 immunoglobulin IgG1, which is specific for P. gingivalis, a pathogen commonly associated with tissue destruction during infection of the dental pulp (26). The results indicate that MCP-1−/− mice produce P. gingivalis-specific IgG1 at levels equivalent to the levels produced by wild-type controls, as well as P. gingivalis-specific total IgG. This suggests that the enhanced susceptibility of the MCP-1−/− mice to infection is not due to alteration of antibody production. In some cases inhibition of MCP-1 activity with antibodies has been reported to compromise the host response, due in part to secondary effects on the adaptive immune response or PMN recruitment. In the type of interstitial infection investigated here, the enhanced susceptibility could not be attributed to such specific secondary effects (13, 18).
Monocyte recruitment is induced by bacterial infection. The finding that MCP-1 plays an important role in protecting the host from bacterial infection is consistent with previous reports (13, 18, 19). In contrast to other reports, the results described here suggest that in a chronic polymicrobial infection, MCP-1 may play a more important role in the sustained recruitment of monocytic cells than other factors that may be more significant in the initial recruitment. Monocyte recruitment is also commonly found in osteolysis. Significantly enhanced monocyte recruitment is thought to play a central role in several conditions characterized by pathological bone loss, including arthritis, implant failures, and periodontal disease (10, 20, 38). Contrary to previous reports, the findings described here are novel because enhanced bone resorption occurred in the face of diminished recruitment of mononuclear phagocytes. Thus, enhanced recruitment of these cells is not a prerequisite for the cellular events that lead to pathological osseous resorption. In such cases, other cells, such as lymphocytes, may significantly contribute to bone resorption (3, 30). B lymphocytes under appropriate stimulation conditions can differentiate into osteoclasts, and T lymphocytes produce RANKL (receptor activator of NF-kB ligand) that stimulates osteoclastogenesis and osteoclast activity (21, 32).
Acknowledgments
This work was supported by grant DE07559 from the National Institute of Dental and Craniofacial Research.
We thank Keya Sau for help with the antibody studies and Alicia Ruff for assistance with preparing the manuscript.
Editor: R. N. Moore
REFERENCES
- 1.Alam, R., J. York, M. Boyars, S. Stafford, J. Grant, J. Lee, P. Forsythe, T. Sim, and N. Ida. 1996. Increased MCP-1, RANTES, and MIP-1alpha in bronchoalveolar lavage fluid of allergic asthmatic patients. Am. J. Respir. Crit. Care Med. 153:1398-1404. [DOI] [PubMed] [Google Scholar]
- 2.Antoniades, H., J. Neville-Golden, T. Galanopoulos, R. Kradin, M. Bravo, A. Valente, and D. Graves. 1992. Monocyte chemotactic protein 1 expression in idiopathic pulmonary fibrosis. Proc. Natl. Acad. Sci. USA 89:5371-5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker, P. J., M. Dixon, R. T. Evans, L. Dufour, E. Johnson, and D. C. Roopenian. 1999. CD4+ T cells and the proinflammatory cytokines gamma interferon and interleukin-6 contribute to alveolar bone loss in mice. Infect. Immun. 67:2804-2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boring, L., J. Gosling, S. W. Chensue, S. Kunkel, R. V. Farese, Jr., H. E. Broxmeyer, and I. F. Charo. 1997. Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J. Clin. Investig. 100:2552-2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bossink, A., L. Paemen, P. Jansen, C. Hack, L. Thijs, and J. Van Damme. 1995. Plasma levels of the chemokines monocyte chemotactic proteins-1 and -2 are elevated in human sepsis. Blood 86:3841-3847. [PubMed] [Google Scholar]
- 6.Botazzi, B., N. Polentarutti, R. Acero, A. Balsari, D. Boraschi, P. Ghezzi, M. Salmona, and A. Mantovani. 1983. Regulation of the macrophage content of neoplasms by chemoattractants. Science 8:210-212. [DOI] [PubMed] [Google Scholar]
- 7.Chen, C.-P., M. Hertzberg, J. Yanling, and D. T. Graves. 1999. IL-1 and TNF receptor signalling is not required for bacteria-induced osteoclastogenesis and bone loss but is essential for protecting the host from a mixed anaerobic infection. Am. J. Pathol. 6:2145-2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cross, A., L. Asher, M. Seguin, L. Yaun, N. Kelly, C. Hanumack, J. Sadoff, and P. Gemski. 1995. The importance of a lipopolysaccharide-initiated, cytokine-mediated host defense mechanism in mice against extraintestinally invasive Escherichia coli. J. Clin. Investig. 96:676-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrante, A. 1992. Activation of neutrophils by interleukins-1 and -2 and tumor necrosis factors. Immunol. Ser. 57:436.. [PubMed] [Google Scholar]
- 10.Fujikawa, Y., A. Sabokbar, S. Neale, and N. A. Athanason. 1996. Human osteoclast formation and bone resorption by monocytes and synovial macrophages in rheumatoid arthritis. Ann. Rheum. Dis. 55:816-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gu, L., B. Rutledge, J. Fiorillo, C. Ernst, I. Grewal, R. Flavell, R. Gladue, and B. Rollins. 1997. In vivo properties of monocyte chemoattractant protein-1. J. Leukoc. Biol. 62:577-580. [DOI] [PubMed] [Google Scholar]
- 12.Gu, L., S. Tseng, R. M. Horner, C. Tam, M. Loda, and B. J. Rollins. 2000. Control of TH2 polarization by the chemokine monocyte chemoattractant protein-1. Nature 404:407-411. [DOI] [PubMed] [Google Scholar]
- 13.Huffnagle, G., R. M. Strieter, T. J. Standiford, R. A. McDonald, M. D. Burdick, S. L. Kunkel, and G. B. Toews. 1995. The role of monocyte chemotactic protein-1 (MCP-1) in the recruitment of monocytes and CD4+ T cells during a pulmonary Cryptococcus neoformans infection. J. Immunol. 155:4790-4797. [PubMed] [Google Scholar]
- 14.Kawashima, N., and P. Stashenko. 1999. Expression of bone-resorptive and regulatory cytokines in murine periapical inflammation. Arch. Oral Biol. 44:55-66. [DOI] [PubMed] [Google Scholar]
- 15.Koch, A., S. Kunke, L. Harlow, B. Johnson, H. Evanoff, G. Haines, M. Burdick, R. Pope, and R. Strieter. 1992. Enhanced production of monocyte chemoattractant protein-1 in rheumatoid arthritis. J. Clin. Investig. 90:772-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurihara, T., G. Warr, J. Loy, and R. Bravo. 1997. Defects in macrophage recruitment and host defense in mice lacking the CCR2 chemokine receptor. J. Exp. Med. 186:1757-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu, B., B. Rutledge, L. Gu, J. Fiorillo, N. Lukacs, S. Kunkel, R. North, C. Gerard, and B. Rollins. 1998. Abnormalities in monocyte recruitment and cytokine expression in monocyte chemoattractant protein 1-deficient mice. J. Exp. Med. 187:601-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsukawa, A., C. Hogaboam, N. Lukacs, P. Lincoln, R. Strieter, and S. Kunkel. 2000. Endogenous MCP-1 influences systemic cytokine balance in a murine model of acute septic peritonitis. Exp. Mol. Pathol. 68:77-84. [DOI] [PubMed] [Google Scholar]
- 19.Nakano, Y., T. Kasahara, N. Mukaida, Y. C. Ko, M. Nakano, and K. Matsushima. 1994. Protection against lethal bacterial infection in mice by monocyte-chemotactic and -activating factor. Infect. Immun. 62:377-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neale, S., and N. Athanasou. 1999. Cytokine receptor profile of arthroplasty macrophages, foreign body giant cells and mature osteoclasts. Acta Orthop. Scand. 70:452-458. [DOI] [PubMed] [Google Scholar]
- 21.Nutt, S. L., B. Heavey, A. G. Rolink, and M. Busslinger. 1999. Commitment to the B-lymphoid lineage depends on the transcription factor Pax5. Nature 401:556-562. [DOI] [PubMed] [Google Scholar]
- 22.Proost, P., A. Wuyts, and J. Van Damme. 1996. Human monocyte chemotactic proteins-2 and -3: structural and functional comparison with MCP-1. J. Leukoc. Biol. 59:67-74. [DOI] [PubMed] [Google Scholar]
- 23.Que, B., and G. Wise. 1998. Tooth eruption molecules enhance MCP-1 gene expression in the dental follicle of the rat. Dev. Dyn. 212:346-351. [DOI] [PubMed] [Google Scholar]
- 24.Rahimi, P., C. Wang, P. Stashenko, S. Lee, J. Lorenzo, and D. Graves. 1995. MCP-1 expression and monocyte recruitment in osseous inflammation. Endocrinology 136:2752-2759. [DOI] [PubMed] [Google Scholar]
- 25.Rasmussen, L., Z. Konopski, P. Oian, and R. Slejelid. 1992. Killing of Escherichia coli by mononuclear phagocytes and neutrophils stimulated in vitro with beta-1,3-d-polyglucose derivatives. Microbiol. Immunol. 36:1188.. [DOI] [PubMed] [Google Scholar]
- 26.Siqueria, J. J., I. Rjcas, J. Oliveira, and K. Santos. 2001. Detection of putative oral pathogens in acute periradicular abscesses by 16S rDNA-directed polymerase chain reaction. J. Endod. 27:164-167. [DOI] [PubMed] [Google Scholar]
- 27.Sozzani, S., M. Locati, D. Zhou, M. Rieppi, W. Luini, G. Lamorte, G. Bianchi, N. Polentarutti, P. Allavena, and A. Mantovani. 1995. Receptors signal transduction and spectrum of action of monocyte chemotactic protein-1 and related chemokines. J. Leukoc. Biol. 57:788-794. [DOI] [PubMed] [Google Scholar]
- 28.Stashenko, P., R. Teles, and R. D'Souza. 1998. Periapical inflammatory responses and their modulation. Crit. Rev. Oral Biol. Med. 9:498-521. [DOI] [PubMed] [Google Scholar]
- 29.Stashenko, P., C. Wang, E. Riley, Y. Wu, G. Ostroff, and R. Niederman. 1995. Reduction of infection-stimulated periapical bone resorption by the biological response modifier PGG glucan. J. Dent. Res. 74:323-330. [DOI] [PubMed] [Google Scholar]
- 30.Teng, Y., H. Nguyen, X. Gao, Y. Kong, R. Gorczynski, B. Singh, R. Ellen, and J. Penninger. 2000. Functional human T-cell immunity and osteoprotegerin ligand control alveolar bone destruction in periodontal infection. J. Clin. Investig. 106:R59-R67. [DOI] [PMC free article] [PubMed]
- 31.Volejnikova, S., M. Laskari, S. Marks, and D. Graves. 1997. Monocyte recruitment and expression of monocyte chemoattractant protein-1 are developmentally regulated in remodeling bone in the mouse. Am. J. Pathol. 150:1711-1721. [PMC free article] [PubMed] [Google Scholar]
- 32.Yasuda, H., N. Shima, N. Nakagawa, K. Yamaguchi, M. Kinosaki, S. Mochizuki, A. Tomoyasu, K. Yano, M. Goto, A. Murakami, F. Tsuda, T. Morinaga, K. Higashio, N. Udagawa, N. Takahashi, and T. Suda. 1998. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc. Natl. Acad. Sci. USA 95:3597-3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yla-Herttuala, S., A. Lipton, M. Rosenfeld, T. Sarkioja, T. Yoshimura, E. Leonard, J. Witztum, and G. Steinber. 1991. Expression of monocyte chemoattractant protein 1 in macrophage-rich areas of human and rabbit atherosclerotic lesions. Proc. Natl. Acad. Sci. USA 88:5252-5256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoshimura, T., and E. Leonard. 1990. Identification of high affinity receptors for human monocyte chemoattractant protein-1 on human monocytes. J. Immunol. 145:292-297. [PubMed] [Google Scholar]
- 35.Yu, X., H. N. Antoniades, and D. T. Graves. 1993. Expression of monocyte chemoattractant protein 1 in human inflamed gingival tissues. Infect. Immun. 61:4622-4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu, X., R. Barnhill, and D. Graves. 1994. Expression of monocyte chemoattractant protein-1 (MCP-1) in delayed type hypersensitivity reactions in the skin. Lab. Investig. 71:226-235. [PubMed] [Google Scholar]
- 37.Yu, X., S. Dluz, D. Graves, L. Zhang, H. Antoniades, W. Hollander, W. Prusty, A. Valente, C. Scwartz, and G. Sonenshein. 1992. Elevated expression of monocyte chemoattractant protein-1 by vascular smooth muscle cells in hypercholesterolemic primates. Proc. Natl. Acad. Sci. USA 89:6953-6957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zappa, U., M. Reinking-Zappa, H. Graf, and D. Chase. 1992. Cell populations associated with active probing attachment loss. J. Periodontol. 63:748-752. [DOI] [PubMed] [Google Scholar]