Abstract
In areas of intense Plasmodium falciparum transmission, protective immunity is acquired during childhood in parallel with acquisition of agglutinating antibodies to parasite-encoded variant surface antigens (VSA) expressed on parasitized red blood cells. In a semi-immune child in such an area, clinical disease is caused mainly by parasites expressing VSA not recognized by preexisting VSA-specific antibodies in that child. Such malaria episodes are known to cause an increase in agglutinating antibodies specifically recognizing VSA expressed by the parasite isolate causing the illness, whereas antibody responses to other parasite isolates are relatively unaffected. However, the detailed kinetics of this VSA antibody acquisition are unknown and hence were the aim of this study. We show that P. falciparum malaria in Ghanaian children generally caused a rapid and sustained increase in variant-specific VSA antibody levels, while more transient and limited increases in levels of antibodies to VSA expressed by other parasite isolates were also seen. Plasma VSA antibody levels were positively correlated with the age of the healthy plasma donors but negatively correlated with the age of the parasite donors (the malaria patient). The data from this first detailed longitudinal study of acquisition of VSA antibodies support the hypothesis that naturally acquired protective immunity to P. falciparum malaria is mediated, at least in part, by VSA-specific antibodies.
Plasmodium falciparum malaria remains one of the leading health problems of the world. In areas where malaria is endemic, substantial clinical protection is acquired during the first decade of life and the majority of malaria-related morbidity and mortality are concentrated in young children (reviewed in reference 27). This acquisition of protective immunity is paralleled by increases in levels of antibodies capable of agglutinating red blood cells (RBC) infected by late developmental stages of malaria parasites (20, 21). The agglutination is mediated by antibodies recognizing variant surface antigens (VSA) inserted by the parasites into the RBC membrane (22, 31). The best characterized of these VSA is P. falciparum erythrocyte membrane protein 1, which mediates adhesion of parasitized RBC to a number of specific receptors in the host vasculature (3, 4, 7, 23-25, 28-30). This in vivo adhesion, termed sequestration, is thought to be an important parasite survival strategy and a key element in the pathogenesis of P. falciparum malaria (18). Previous studies have shown that P. falciparum parasites causing clinical disease in semi-immune children express VSA not recognized by preexisting variant-specific antibodies and that malaria episodes cause an increase in antibodies specifically recognizing VSA expressed by the parasite isolate causing illness (10, 15, 20). However, the detailed kinetics of changes in VSA antibody levels in relation to clinical episodes are not known, particularly during the period shortly after the clinical episode, and the aim of the present study was to provide such information. To this end, we conducted a community-based study in which a cohort of 108 Ghanaian children was monitored over a period of 10 months with regular collection of plasma samples. Twenty-five of these children had P. falciparum malaria during the period of surveillance. The parasite isolates obtained from 12 of these malaria patients were used to evaluate levels of plasma antibodies specifically recognizing VSA expressed by the isolate causing disease in a given child (homologous responses) and by isolates obtained from other children in the cohort (heterologous responses).
MATERIALS AND METHODS
Study area and study population.
The study was conducted in Dodowa, a town situated in the Dangbe West district of Greater Accra region, Ghana. Malaria transmission in the area is perennial, with marked seasonal variation. Peak transmission occurs during and after the rainy season (May to October), and residents are exposed to approximately 20 infective bites per year (1). Parasite prevalence in Dodowa peaks (∼70%) before 10 years of age, and high parasite densities are found mainly in children <5 years old (2).
From this population, 150 healthy children between 1 and 11 years old were recruited in early 1998. Of these, 108 sickle-cell-trait (HbAS)-negative children were admitted to the study. Informed consent was obtained from all study participants, and the study was approved by the Ghanaian Ministry of Health.
Clinical surveillance and collection of blood samples.
The children were monitored by active and passive case detection from February to October 1998. At the beginning (preseason) and the end of the study (postseason), two 5-ml venous blood samples were collected in a heparinized tube and a CPD-adenine tube (BD PharMingen, San Diego, Calif.). In addition, monthly finger-prick blood samples (250 to 500 μl) were collected in heparinized microtubes (BD PharMingen). In case of a malaria episode, additional 5-ml samples were collected at diagnosis (day 0) and 3 and 7 days later. A malaria episode was defined as an axillary temperature of >37.5°C in the presence of >5,000 asexual P. falciparum parasites per μl of blood in the absence of any differential diagnosis. All malaria cases were treated with a standard regimen of chloroquine (25 mg/kg of body weight, divided in two daily doses of 10 mg/kg, followed by a 5-mg/kg dose on the third day). All patients recovered completely.
A plasma pool of samples collected previously from clinically immune Ghanaian adult residents of the same region was used as a positive control. Plasma samples from seven Danish adults without known prior exposure to P. falciparum parasites were used as negative controls.
Sample preparation.
Plasma was separated by centrifugation (500 × g, 10 min) of heparinized blood samples and stored at −40°C. In the case of P. falciparum parasitemia detectable by microscopy, RBC were isolated from CPD-adenine tubes and cryopreserved in liquid N2 (16).
Parasites, in vitro culture, and preparation of late-stage-infected RBC.
All the parasite isolates used in the study were typed by PCR on merozoite surface protein 1 (MSP-1), merozoite surface protein 2 (MSP-2), and glutamate-rich protein, as described previously (32). In vitro P. falciparum cultures were established from cryopreserved infected RBC (iRBC) (15). To minimize the risk of unwanted changes in VSA expression as a result of antigenic switching during prolonged in vitro culture, only the 12 of 25 isolates where sufficient late-stage iRBC were available for the assays within 20 days were used for the study. Among these isolates there was no significant correlation between time in culture before assay and subsequent recognition of the isolates by plasma VSA antibodies. Immediately before assaying, we labeled the cultures with ethidium bromide (40 μg/ml) and purified late-stage iRBC (hemozoin-containing trophozoites and schizonts) by exposure to a high-gradient magnetic field (33).
Measurement of plasma antibodies to variant surface antigens by flow cytometry.
Following magnet purification, the purified, ethidium bromide-labeled iRBC (2 × 105 RBC, now at a parasitemia of 60 to 80%) were sequentially incubated with 5 μl of plasma (10 min), 0.4 μl of goat anti-human immunoglobulin G (Dako, Glostrup, Denmark) (30 min), and 4 μl of fluorescein isothiocyanate-conjugated rabbit anti-goat immunoglobulin G (Dako) (30 min). All incubations were done at room temperature with extensive washing between each incubation step. Agglutination does not occur under these experimental conditions (∼0.02% hematocrit). In each experiment, the VSA antibody reactivity to a given isolate was always determined consecutively in all plasma samples and on the same parasite preparation. A minimum of 10,000 events were collected on a FACScan flow cytometer (BD PharMingen), using a live gate on ethidium bromide-positive (and thus infected) RBC. Appropriate control samples, such as uninfected RBC from the same samples (gated on ethidium bromide-negative RBC), iRBC exposed to serial fivefold dilutions of a plasma pool, and plasma samples from nonexposed control donors were always included. Flow cytometry data were analyzed by means of WinList 4.0 software (Verity, Topsham, Maine), and results were expressed as mean fluorescence index (MFI) (also called mean channel number).
Statistical analysis and data presentation.
We calculated Pearson product moment correlation coefficients (r) to examine associations between age and VSA antibody reactivity, and Student's t test (t) for intergroup comparisons. Differences in proportions and relative risk were analyzed by the χ2 test with Yates' correction.
RESULTS
Clinical surveillance.
The prevalence of microscopically detectable, asymptomatic P. falciparum infection at the beginning of the study (preseason) was 70% (76 of 108 children), in agreement with previous data from the same town (2, 12). The mean ages of children with (7.0 years, 95% confidence interval [CI]: 6.3 to 7.6 years) and without (6.3 years, 95% CI: 5.4 to 7.3) asymptomatic preseason parasitemia were similar (P[t] = 0.29). During the 10-month period of clinical surveillance, 23% (25 of 108) had clinical P. falciparum malaria episodes. Most of these had only one episode, but three children had two, and one child had three attacks. Fewer children (13 of 76) with asymptomatic preseason infections subsequently developed malaria than did children without detectable preseason parasitemia (12 of 32). This corresponds to a relative risk of acquiring malaria during the surveillance period of 0.75 (95% CI: 0.57 to 1.00) (P[χ2] = 0.04), suggesting that asymptomatic infection can protect against subsequent clinical attacks in semi-immune children.
Parasite genotyping.
All the parasite isolates were genotypically distinct according to PCR typing on MSP-1, MSP-2, and glutamate-rich protein (data not shown). Minimal clone number estimates ranged from one to three, with only 2 of 12 isolates being monoclonal. There was no significant correlation between the estimated number of clones on the one hand and either VSA antibody reactivity or parasite donor age on the other (P > 0.22 in both cases).
Levels of preseason VSA plasma antibodies increase with increasing age of the plasma donor.
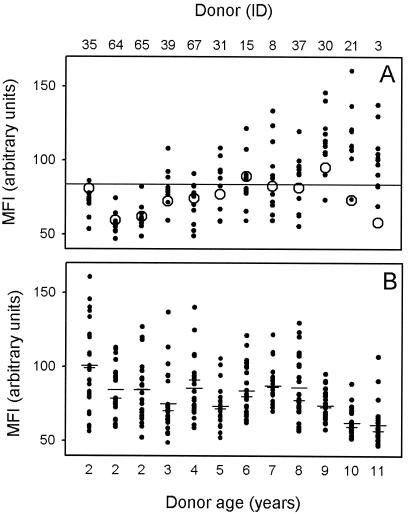
Levels of preseason VSA antibodies in the plasma, measured as the MFI, were positively correlated with the age of the plasma donor (n = 144; r = 0.63; P < 10−5), indicating age-dependent acquisition of VSA-specific antibodies (Fig. 1A). Although these data points are not strictly independent, analysis of plasma donor-specific means overcomes this problem, yielding a similar result (n = 12; r = 0.90; P < 0.0001). More interestingly, levels of antibody to 10 of 12 homologous isolates (Fig. 1) were below the overall mean VSA reactivity (MFI, 83.9), and 8 of 12 were below the overall median reactivity (MFI, 80.0). Malaria in these semi-immune individuals thus tended to be caused by parasite isolates expressing VSA to which the patient had relatively low levels of specific antibodies, in line with the “hole-in-the-VSA-antibody-repertoire” hypothesis of Bull et al. (9). However, the mean level (MFI, 84.7) of VSA antibodies to heterologous isolates (Fig. 1) was not significantly different from that for homologous isolates (MFI, 75.4) (t test; estimated difference, 9.5; 95% CI: −4.5 to 22.9; P = 0.19). Furthermore, in most cases a given individual had a weaker VSA antibody reactivity to some of the heterologous parasite isolates than to the isolate causing disease in that individual (Fig. 1A). It thus appears that although the children evidently had acquired some anti-VSA immune responses, they would have been susceptible to many of the heterologous parasite isolates had they been infected by them. In other words, the data indicate that these children still had many remaining “holes” in their VSA antibody repertoires, and this was particularly so among the younger children.
FIG. 1.
Levels of VSA antibodies in plasma obtained at the beginning of the study (preseason), expressed as MFI values. (A) Increasing levels of VSA-specific plasma antibodies with increasing age of the plasma donor. For each plasma donor, the levels of plasma antibodies specifically recognizing VSA expressed by the isolate causing malaria in that individual (homologous isolate [○]) and by the 11 isolates causing malaria in the other children (heterologous isolates [•]) are shown. The overall mean VSA antibody reactivity is indicated by a horizontal line. (B) Decreasing VSA-specific plasma antibody recognition of parasites with increasing age of the parasite donor (malaria patient). For each parasite isolate, the levels of isolate-specific VSA antibodies in plasma from each of the 25 children who suffered malaria attacks during the study period, as well as means (long bars) and medians (short bars), are shown. The donor ID and age of the donors of the plasma samples (A) or parasite isolates (B) are indicated at the top and bottom of the figure, respectively.
Levels of plasma antibodies recognizing VSA expressed by a specific parasite isolate are inversely correlated with the age of the donor of that parasite isolate.
In addition to the age of the plasma donor (see above), the available evidence suggests that the plasma antibody recognition of VSA also depends on the age of the donor of the parasite isolate (8, 9). To investigate this hypothesis, we next measured VSA antibody reactivity to each of the 12 parasite isolates in the preseason plasma samples from all the 25 children who had malaria during the study period. The VSA-specific MFI correlated inversely with the age of the parasite donor (malaria patient) (n = 300; r = −0.35; P < 10−5), supporting the hypothesis of a relationship between VSA antibody reactivity and the age of the donor of the VSA-expressing parasite isolate (Fig. 1B). Again, the data points are not strictly independent, but as before, analysis of parasite donor-specific means yielded a similar result (n = 12; r = −0.68; P < 0.01).
P. falciparum malaria causes a strong and sustained variant-specific antibody response.
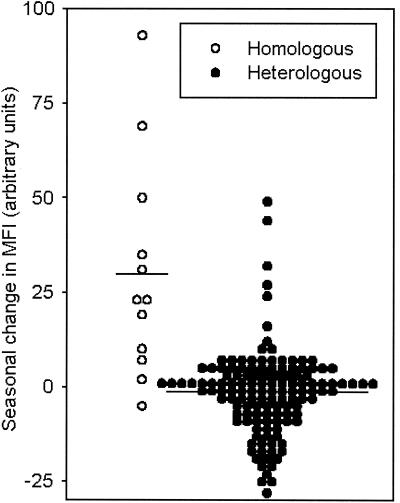
The main aim of the present study was to provide comprehensive and detailed information regarding the impact of malaria episodes on levels and specificity of VSA antibodies. As a first approach, we compared VSA antibody levels in matched plasma collected before (preseason samples) and after (postseason samples) the malaria episode in the 12 children from whom the infecting parasite isolates were available for study. As shown in Fig. 2, the postseason antibody levels were almost always higher than preseason levels with respect to antibodies specifically recognizing VSA expressed by the parasite causing disease (homologous response) (n = 12; P[t] = 0.004). In contrast, seasonality was not observed for most of the heterologous responses (n = 132; P[t] = 0.17). This finding shows that P. falciparum malaria causes a sustained increase in the levels of VSA-specific antibodies expressed by the P. falciparum isolate causing the malaria attack, which is rarely seen in levels of VSA antibodies with other specificities.
FIG. 2.
Seasonal change in antibodies specifically recognizing VSA expressed by the parasite isolate causing clinical disease (homologous response [○]) and by 11 other parasites (heterologous responses [•]). For each child-parasite combination, the difference between postseason and preseason MFI is shown. In addition, the overall means are indicated by horizontal bars.
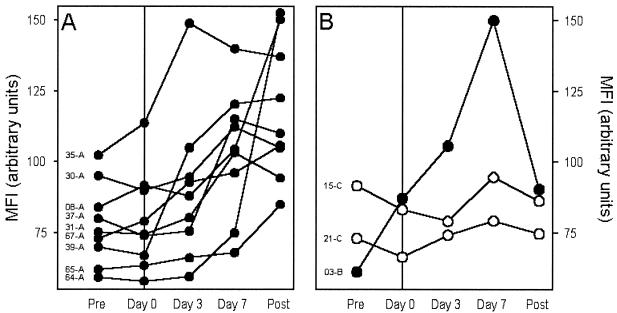
We next measured antibody directed against VSA expressed by each of the parasite isolates in plasma samples obtained from the patient at the beginning of the study, at the diagnosis of P. falciparum malaria (day 0), 3 and 7 days after diagnosis, and at the end of the study. The aim of this part of the study was to identify the broad patterns of disease-induced changes in VSA antibody reactivity. The most common pattern (9 of 12 isolates [Fig. 3A ]) was an abrupt increase in the levels of homologous VSA antibodies within the first week of clinical symptoms and sustained high levels at the end of the study. The second pattern (1 of 12 isolates) was like the first, except that levels had declined by the end of the study, indicating a more transient response (Fig. 3B). The remaining two patients showed only minimal changes in homologous VSA antibody levels in response to the malaria attack (Fig. 3B). For each parasite isolate, the levels of VSA antibodies in heterologous plasma were similar at the beginning and at the end of the study (data not shown).
FIG. 3.
Temporal changes in plasma levels of antibodies recognizing VSA expressed by the causative (homologous) parasite isolate relative to the time of the P. falciparum malaria episode. Plasma VSA antibody levels (expressed as MFI values) at the beginning of the study (Pre), at diagnosis (Day 0), during convalescence (Day 3 and Day 7), and at the end of the study (Post) for individual patients are indicated. Donor identification codes are indicated in the left side of each panel. (A) Changes in individuals with rapid, marked, and sustained disease-induced increases in VSA antibody levels. (B) Changes in individuals with transient (•) or without conspicuous (○) changes in VSA antibody levels.
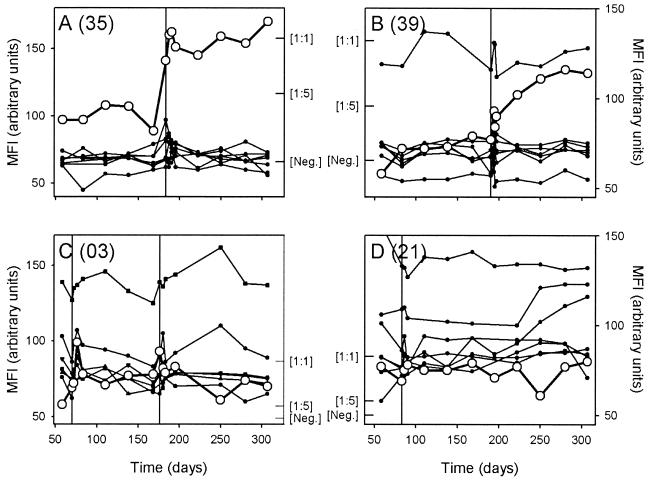
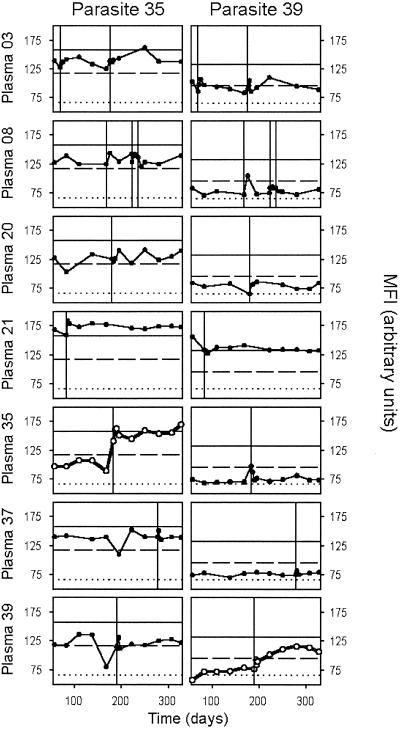
To further resolve these changes in VSA antibody reactivity and to compare responses to homologous and heterologous parasite isolates, we conducted an additional series of experiments. In these experiments, we measured levels of VSA antibodies to both the homologous and the heterologous isolates in all the plasma samples obtained at monthly intervals throughout the study, in addition to the samples mentioned above. For these experiments we used parasites brought into culture from another tube of the material frozen at the time of the disease episode. To allow a comparison of parasite isolates causing clinical and asymptomatic infections, we also studied two parasite isolates, obtained at the beginning and at the end of the study, from a cohort member who was asymptomatically infected at these time points. Levels of antibodies recognizing VSA on the homologous parasite increased within the first week following clinical disease in 10 of 12 cases, whereas levels of VSA antibodies recognizing heterologous isolates were relatively unaffected. In the same nine of these cases identified by the initial analysis (Fig. 3), the levels of antibodies recognizing the homologous VSA remained high or even continued to increase until the end of the study (Fig. 4A and B). A more limited and transient response was seen in the single case (Fig. 4C) where a short-lived response was indicated by the initial analysis (Fig. 3B). In the remaining two isolates, which showed minimal VSA antibody responses in the first set of experiments (Fig. 3B), the nonreactivity was confirmed by the detailed assay (Fig. 4D). A similar absence of change in VSA antibody levels with time was seen in the individual who was asymptomatically infected at both ends of the study, regardless of whether the parasite isolate obtained at the beginning or the end of the study was examined (data not shown).
FIG. 4.
Detailed analysis of temporal changes in levels of VSA antibodies in plasma obtained at monthly intervals throughout the study, expressed as MFI values. Antibody responses to the homologous isolate (○) and heterologous isolates (•) are shown, and the timings of P. falciparum malaria episodes in the children are indicated by vertical lines. In all the panels, MFI values obtained using an undiluted ([1:1]) and 5-fold-diluted ([1:5]) hyperimmune plasma pool have been indicated by inward ticks to allow quantitative evaluation of the changes in antibody levels. The mean MFI + 2 standard deviations obtained from seven nonexposed donors is similarly indicated ([Neg.]). (A and B) Changes in two individuals (A, 35; B, 39) in whom the malaria episode caused rapid and sustained increases in VSA antibodies specific for the homologous isolate. (C) Changes in an individual (03) in whom the malaria episode caused limited and transient increases in VSA antibodies specific for the homologous isolate from the first of two malaria attacks after each of two clinical episodes with this individual (note marked disease-induced changes in levels of antibodies specific for VSA expressed by several heterologous isolates in this individual). (D) Changes in an individual (21) in whom the malaria episode caused no changes in VSA antibodies specific for the homologous isolate.
P. falciparum malaria causes a transient antibody response to heterologous VSA.
The experiments discussed above and illustrated in Fig. 4 indicated that although clinical episodes mainly cause an antibody response to the VSA expressed by the causative isolate, disease-associated responses to other isolates can also be seen (e.g., Fig. 4C). The design of our experiments allowed us to study this in more detail. Our analysis confirmed that malaria episodes generally cause only minor changes in levels of antibodies recognizing VSA other than those expressed by the parasite causing the episode (Fig. 5). Although such responses could be detected in some cases, they were generally weak and transient compared to the homologous response. As examples, consider the increase in parasite 35-specific VSA antibody levels in donor 03 after two episodes (caused by parasite isolates 03 and 03-2, respectively), and in donor 39 at the time of malaria (caused by parasite 39) (Fig. 5). Such responses can be taken as evidence of overlapping VSA repertoires between isolates, similar to what has been reported in other studies (15). However, unexplained fluctuations were also seen (Fig. 5), cautioning against drawing too-firm conclusions on this issue based on the data available here.
FIG. 5.
Detailed analysis of temporal changes in levels of VSA antibodies in plasma obtained at monthly intervals throughout the study, expressed as MFI values. Temporal changes in antibody responses to VSA expressed by two parasite isolates (35 [left panels] and 39 [right panels]) as seen in seven representative individuals (rows) are shown. The timing of P. falciparum malaria episodes in individual children is indicated by vertical lines. In all panels, the MFI obtained using a hyperimmune plasma pool undiluted (1:1) (horizontal solid line) and diluted 1:5 (dashed line), and the mean MFI + 2 standard deviations using plasma from seven nonexposed donors (dotted line) are shown.
DISCUSSION
P. falciparum parasites can adhere to several host molecules, including CD31 (PECAM-1), CD36 (platelet glycoprotein IV), CD54 (ICAM-1), CD62E (E-selectin), CD106 (INCAM-110, VCAM-1), thrombospondin, and chondroitin sulfate A (3, 4, 7, 23-25, 28-30, 36). This adhesion is mediated through parasite-encoded VSA on late-stage (mature trophozoites and schizonts)-infected RBC, of which the best characterized is P. falciparum erythrocyte membrane protein 1 (5, 19). VSA-mediated adhesion in the host vasculature (sequestration) is thought to be an important parasite survival strategy and a key element in the pathogenesis of P. falciparum malaria (18). In line with this, acquisition of protective immunity to the disease in populations to which it is endemic is associated with the development of VSA-specific agglutinating antibodies (11, 15, 20, 21). Furthermore, parasite isolates causing clinical disease in such a setting express VSA that correspond to a hole in the VSA antibody repertoire in the patient, and the disease episode results in an increase in levels of antibody to VSA expressed by the infecting isolate, i.e., in a “closing of the hole” (9, 11, 15, 20). Potential mechanisms by which VSA-specific plasma antibodies can protect include opsonization and interference with VSA-specific adhesion (14, 26, 37).
In the present longitudinal cohort study, we studied the impact of P. falciparum malaria on the development of antibodies recognizing VSA expressed by the parasite isolate causing the disease episode (homologous response) as well as by other parasite isolates (heterologous responses). At the beginning of the study (preseason), most children in the study cohort had high levels of antibodies to VSA expressed by some but not all of the parasite isolates causing malaria among cohort members during the period of clinical surveillance. As expected, the overall VSA antibody levels increased with increasing plasma donor age, in all likelihood reflecting exposure (and age)-related acquisition of immunity (20). Levels of antibodies specific for VSA expressed by the parasite causing disease in individual children (the homologous isolates) tended to be lower than average, consistent with the hole-in-the-acquired-antibody-repertoire hypothesis (9, 10). However, a substantial proportion of the children had low levels of VSA antibodies to isolates other than the ones causing their malaria episodes. It is thus tempting to postulate that these children would have been as susceptible to those isolates as to the one actually causing their malaria attack, had they been exposed to them.
Interestingly, we found a highly significant inverse relationship between antibody recognition of VSA and the age of the donor of the parasite (the malaria patient), in line with data from similar studies in Kenya (8, 9). These findings suggest that acquisition of protective immunity operates, in part at least, by gradually restricting the repertoire of VSA compatible with parasite survival in the semi-immune host. It is interesting in this context that parasite isolates expressing commonly recognized VSA have been associated with severe disease (8, 9) and that immunological protection against severe malaria is acquired earlier than that against uncomplicated disease (17).
Comparison of plasma levels of VSA antibodies at the beginning and at the end of the study clearly showed that P. falciparum malaria was associated with a specific and sustained antibody response to VSA expressed by the parasite isolate causing disease, in agreement with data from previous studies (10, 20). Detailed analysis confirmed this observation and demonstrated that the increase in antibody specific for the VSA expressed by the disease-causing isolate slightly precedes or occurs within the first week after the development of clinical symptoms. Similar, but weaker and transient, responses to heterologous VSA were also detected, indicating a degree of cross-reactivity between VSA expressed by different isolates, in accord with results of previous studies (15, 16).
Of note, children who carried an asymptomatic infection at the beginning of the study were less likely to develop malaria during the period of surveillance than were children without detectable parasitemia at the beginning of the study. This suggests that subclinical parasitemia can protect against clinical disease in semi-immune children, with this protection perhaps mediated—at least in part—by VSA antibodies (34). However, the present study was not designed to address this possibility, which has considerable implications for treatment policies in endemic countries.
Our study is the first geographically and methodologically independent confirmation of earlier studies from Kenya, where agglutination assays were used to demonstrate the hole in the acquired antibody response (10) and the relationship between the age of the parasite donor (patient) and antibody recognition of the parasites causing disease (8). We have used a flow-cytometric technique that allows efficient, unbiased, and quantitative analysis of large combinations of parasite isolates and plasma samples but which necessitates in vitro cultivation of the isolates studied. However, in our hands parasite isolates from patients retain their VSA expression profile as measured by plasma antibody recognition for long periods of time, with assays run several weeks apart yielding results that correlate tightly and very significantly (our unpublished data). This observation is in line with earlier data showing a stable agglutination profile of a patient isolate after prolonged in vitro culture (13). As mentioned, agglutination that could affect the flow-cytometric analysis does not occur under our experimental conditions. In fact, we have previously shown concordance of results obtained by traditional agglutination assays and flow cytometry, although the latter assay has higher sensitivity (16). These data suggest that the assays are measuring the same type of antibodies and can generally be used interchangeably. The exception is studies of parasites involved in pregnancy-associated malaria, which cannot be agglutinated (6, 14), and where the flow cytometry assay is thus clearly superior (26, 35). Eventually, the choice between these assays depends on their relative merits and the specific research question being asked.
In conclusion, we have shown that P. falciparum malaria causes a strong antibody response specifically directed against VSA expressed by the parasite population causing the clinical disease, whereas VSA antibodies specific for unrelated parasites are only marginally affected. This variant-specific antibody response is usually maintained for several weeks to months. In combination with previously published evidence, our data support the hypothesis that acquisition of a broad repertoire of VSA antibodies is an important element in the acquisition of protective immunity to P. falciparum malaria.
Acknowledgments
This study was financially supported by the Enhancement of Research Capacity in Developing Countries (ENRECA) program of the Danish International Development Assistance (Danida), grant no. 14.Dan.8.L.306, by the International Co-operation with Developing Countries (INCO-DC) program of the European Commission, grant no. IC18-CT970238, and by the Danish Medical Research Council (SSVF), grant no. 9702273. L.H. is supported by SSVF grant no. 9802405 and by the Danish Research Council for Development Research (RUF), grant no. 90900.
The support and collaboration of all the study cohort members, and of the Noguchi Memorial Institute for Medical Research field staff, is gratefully acknowledged. We thank Kirsten Pihl for the parasite genotyping by PCR.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Afari, E. A., M. Appawu, S. Dunyo, A. Baffoe-Wilmot, and F. K. Nkrumah. 1995. Malaria infection, morbidity and transmission in two ecological zones in southern Ghana. Afr. J. Health Sci. 2:312-316. [PubMed]
- 2.Afari, E. A., K. A. Koram, S. K. Dunyo, and F. K. Nkrumah. 1992. The epidemiology of malaria with special emphasis on transmission, morbidity, mortality and disease control in Ghana. Internal report. Noguchi Memorial Institute for Medical Research, Legon, Ghana.
- 3.Barnwell, J. W., A. S. Asch, R. L. Nachman, M. Yamaya, M. Aikawa, and P. Ingravallo. 1989. A human 88-kD membrane glycoprotein (CD36) functions in vitro as a receptor for a cytoadherence ligand on Plasmodium falciparum-infected erythrocytes. J. Clin. Investig. 84:765-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnwell, J. W., C. Ockenhouse, and D. M. Knowles II. 1985. Monoclonal antibody OKM5 inhibits the in vitro binding of Plasmodium falciparum infected erythrocytes to monocytes, endothelial, and C32 melanoma cells. J. Immunol. 135:3494-3497. [PubMed] [Google Scholar]
- 5.Baruch, D. I., B. L. Pasloske, H. B. Singh, et al. 1995. Cloning the P. falciparum gene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell 82:77-87. [DOI] [PubMed] [Google Scholar]
- 6.Beeson, J. G., G. V. Brown, M. E. Molyneux, C. Mhango, F. Dzinjalamala, and S. J. Rogerson. 1999. Plasmodium falciparum isolates from infected pregnant women and children are associated with distinct adhesive and antigenic properties. J. Infect. Dis. 180:464-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berendt, A. R., D. L. Simmons, J. Tansey, C. I. Newbold, and K. Marsh. 1989. Intercellular adhesion molecule-1 is an endothelial cell adhesion receptor for Plasmodium falciparum. Nature 341:57-59. [DOI] [PubMed] [Google Scholar]
- 8.Bull, P. C., M. Kortok, O. Kai, et al. 2000. Plasmodium falciparum-infected erythrocytes: agglutination by diverse Kenyan plasma is associated with severe disease and young host age. J. Infect. Dis. 182:252-259. [DOI] [PubMed] [Google Scholar]
- 9.Bull, P. C., B. S. Lowe, M. Kortok, and K. Marsh. 1999. Antibody recognition of Plasmodium falciparum erythrocyte surface antigens in Kenya: evidence for rare and prevalent variants. Infect. Immun. 67:733-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bull, P. C., B. S. Lowe, M. Kortok, C. S. Molyneux, C. I. Newbold, and K. Marsh. 1998. Parasite antigens on the infected red cell are targets for naturally acquired immunity to malaria. Nat. Med. 4:358-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dodoo, D., T. Staalsoe, H. Giha, et al. 2001. Antibodies to variant antigens on the surfaces of infected erythrocytes are associated with protection from malaria in Ghanaian children. Infect. Immun. 69:3713-3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dodoo, D., T. G. Theander, J. A. L. Kurtzhals, et al. 1999. Levels of antibody to conserved parts of Plasmodium falciparum merozoite surface protein 1 in Ghanaian children are not associated with clinical protection from malaria. Infect. Immun. 67:2131-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forsyth, K. P., G. Philip, T. Smith, E. Kum, B. Southwell, and G. V. Brown. 1989. Diversity of antigens expressed on the surface of erythrocytes infected with mature Plasmodium falciparum parasites in Papua New Guinea. Am. J. Trop. Med. Hyg. 41:259-265. [PubMed] [Google Scholar]
- 14.Fried, M., F. Nosten, A. Brockman, B. T. Brabin, and P. E. Duffy. 1998. Maternal antibodies block malaria. Nature 395:851-852. [DOI] [PubMed] [Google Scholar]
- 15.Giha, H. A., T. Staalsoe, D. Dodoo, et al. 1999. Nine-year longitudinal study of antibodies to variant antigens on the surface of Plasmodium falciparum-infected erythrocytes. Infect. Immun. 67:4092-4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giha, H. A., T. Staalsoe, D. Dodoo, et al. 1999. Overlapping antigenic repertoires of variant antigens expressed on the surface of erythrocytes infected by Plasmodium falciparum. Parasitology 119:7-17. [DOI] [PubMed] [Google Scholar]
- 17.Gupta, S., R. W. Snow, C. A. Donnelly, K. Marsh, and C. Newbold. 1999. Immunity to non-cerebral severe malaria is acquired after one or two infections. Nat. Med. 5:340-343. [DOI] [PubMed] [Google Scholar]
- 18.Howard, R. J., and J. W. Barnwell. 1984. Roles of surface antigens on malaria-infected red blood cells in evasion of immunity. Contemp. Top. Immunobiol. 12:127-191. [DOI] [PubMed] [Google Scholar]
- 19.Leech, J. H., J. W. Barnwell, L. H. Miller, and R. J. Howard. 1984. Identification of a strain-specific malarial antigen exposed on the surface of Plasmodium falciparum-infected erythrocytes. J. Exp. Med. 159:1567-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marsh, K., and R. J. Howard. 1986. Antigens induced on erythrocytes by P. falciparum: expression of diverse and conserved determinants. Science 231:150-153. [DOI] [PubMed] [Google Scholar]
- 21.Marsh, K., L. Otoo, R. J. Hayes, D. C. Carson, and B. M. Greenwood. 1989. Antibodies to blood stage antigens of Plasmodium falciparum in rural Gambians and their relation to protection against infection. Trans. R. Soc. Trop. Med. Hyg. 83:293-303. [DOI] [PubMed] [Google Scholar]
- 22.Newbold, C. I., R. Pinches, D. J. Roberts, and K. Marsh. 1992. Plasmodium falciparum: the human agglutinating antibody response to the infected red cell surface is predominantly variant specific. Exp. Parasitol. 75:281-292. [DOI] [PubMed] [Google Scholar]
- 23.Ockenhouse, C. F., N. N. Tandon, C. Magowan, G. A. Jamieson, and J. D. Chulay. 1989. Identification of a platelet membrane glycoprotein as a falciparum malaria sequestration receptor. Science 243:1469-1471. [DOI] [PubMed] [Google Scholar]
- 24.Ockenhouse, C. F., T. Tegoshi, Y. Maeno, et al. 1992. Human vascular endothelial cell adhesion receptors for Plasmodium falciparum-infected erythrocytes: roles for endothelial leukocyte adhesion molecule 1 and vascular adhesion molecule 1. J. Exp. Med. 176:1183-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oquendo, P., E. Hundt, J. Lawler, and B. Seed. 1989. CD36 directly mediates cytoadherence of Plasmodium falciparum parasitized erythrocytes. Cell 58:95-101. [DOI] [PubMed] [Google Scholar]
- 26.Ricke, C. H., T. Staalsoe, K. Koram, et al. 2000. Plasma antibodies from malaria-exposed pregnant women recognize variant surface antigens on Plasmodium falciparum-infected erythrocytes in a parity-dependent manner and block parasite adhesion to chondroitin sulphate A. J. Immunol. 165:3309-3316. [DOI] [PubMed] [Google Scholar]
- 27.Riley, E. M., L. Hviid, and T. G. Theander.1994. Malaria, p. 119-143. In F. Kierszenbaum (ed.), Parasitic Infections and the Immune System. Academic Press, New York, N.Y.
- 28.Robert, C., B. Pouvelle, P. Meyer, et al. 1995. Chondroitin-4-sulphate (proteoglycan), a receptor for Plasmodium falciparum-infected erythrocyte adherence on brain microvascular endothelial cells. Res. Immunol. 146:383-393. [DOI] [PubMed] [Google Scholar]
- 29.Roberts, D. D., J. A. Sherwood, S. L. Spitalnik, et al. 1985. Thrombospondin binds falciparum malaria parasitized erythrocytes and may mediate cytoadherence. Nature 318:64-66. [DOI] [PubMed] [Google Scholar]
- 30.Rogerson, S. J., S. C. Chaiyaroj, K. Ng, J. C. Reeder, and G. V. Brown. 1995. Chondroitin sulfate A is a cell surface receptor for Plasmodium falciparum-infected erythrocytes. J. Exp. Med. 182:15-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sherwood, J. A., K. Marsh, R. J. Howard, and J. W. Barnwell. 1985. Antibody mediated strain-specific agglutination of Plasmodium falciparum-parasitized erythrocytes visualized by ethidium bromide staining. Parasite Immunol. 7:659-663. [DOI] [PubMed] [Google Scholar]
- 32.Snounou, G., X. P. Zhu, N. Siripoon, et al. 1999. Biased distribution of msp1 and msp2 allelic variants in Plasmodium falciparum populations in Thailand. Trans. R. Soc. Trop. Med. Hyg. 93:369-374. [DOI] [PubMed] [Google Scholar]
- 33.Staalsoe, T., H. A. Giha, D. Dodoo, T. G. Theander, and L. Hviid. 1999. Detection of antibodies to variant antigens on Plasmodium falciparum infected erythrocytes by flow cytometry. Cytometry 35:329-336. [DOI] [PubMed] [Google Scholar]
- 34.Staalsoe, T., and L. Hviid. 1998. The role of variant-specific immunity in asymptomatic infections: maintaining a fine balance. Parasitol. Today 14:177-178. [DOI] [PubMed] [Google Scholar]
- 35.Staalsoe, T., R. Megnekou, N. Fievet, et al. 2001. Acquisition and decay of antibodies to pregnancy-associated variant antigens on the surface of Plasmodium falciparum infected erythrocytes that are associated with protection against placental parasitemia. J. Infect. Dis. 184:618-626. [DOI] [PubMed] [Google Scholar]
- 36.Treutiger, C. J., A. Heddini, V. Fernandez, W. A. Muller, and M. Wahlgren. 1997. PECAM-1/CD31, an endothelial receptor for binding Plasmodium falciparum-infected erythrocytes. Nature Med. 3:1405-1408. [DOI] [PubMed] [Google Scholar]
- 37.Udeinya, I. J., L. H. Miller, I. A. McGregor, and J. B. Jensen. 1983. Plasmodium falciparum strain-specific antibody blocks binding of infected erythrocytes to amelanotic melanoma cells. Nature 303:429-431. [DOI] [PubMed] [Google Scholar]