Abstract
The role lysogenic bacteriophage play in the pathogenesis of the host bacterium is poorly understood. In a previous study, we found that streptococcal coculture with human pharyngeal cells resulted in the induction of lysogenic bacteriophage as well as the phage-associated streptococcal pyrogenic exotoxin C (SpeC). In this study, we have determined that in addition to SpeC induction, a number of other streptococcal proteins are also released by the bacteria during coculture with pharyngeal cells. Among these, we identified and characterized a novel 27-kDa secreted protein. Sequence analysis of this novel protein demonstrated it to be encoded by the same lysogenic bacteriophage which harbors speC. Protein sequence analysis revealed varied homologies with several streptococcal DNases. Further biochemical characterization of the recombinantly expressed protein verified it to be a divalent cation-dependent streptococcal phage-encoded DNase (Spd1). Although functionally distinct, SpeC and Spd1 are associated by a number of parameters, including genetic proximity and transcriptional regulation. Finally, we speculate on the induction of phage-encoded DNase (Spd1) enhancing the fitness of both bacteria and phage.
The pathogenic group A streptococcus Streptococcus pyogenes is a bacterium responsible for a wide spectrum of diseases. In the United States alone, there are between 20 and 30 million cases of S. pyogenes-associated infections every year (9). These diseases range from streptococcal pharyngitis and associated rheumatic fever to the more invasive necrotizing fasciitis and streptococcal toxic shock syndrome. Many of these diseases are mediated by streptococcus-specific soluble proteins which act on the host cells; these include toxins (3), mitogens (31), and hydrolases (28, 29).
Of the extracellular products produced by group A streptococci, the streptococcal pyrogenic exotoxins (Spes) have been most studied. The Spes are superantigens, and as such, directly cross-link the major histocompatibility complex class II on antigen-presenting cells with T-cell receptors, stimulating between 5 and 25% of the total T-cell population (14). This random stimulation induces a host immunological response, often leading to tissue damage and organ failure. While less characterized, the streptococcal DNases represent another class of extracellular proteins. These enzymes possess a lengthy history in streptococcal research, as McCarty (18) identified the first extracellular DNase activity over half a century ago. Since then, the four exoenzymes, DNases A, B, C, and D, have been identified (28, 29). Interestingly, DNase B has been shown to be the same molecule as the streptococcal mitogenic factor (MF), a putative virulence protein (24). We refer to this molecule as MF/DNase B. While antibodies to DNases such as MF/DNase B have been found in the sera of S. pyogenes-infected individuals (10, 20), the role of these DNases in streptococcal pathogenesis has never been fully elucidated. Yet the potent DNA-degrading activity that these enzymes possess cannot be easily dismissed.
Many bacterial toxins, such as SpeA and SpeC, are often harbored and disseminated by genetic mobile elements, such as lysogenic bacteriophage. Lysogeny affords the bacteria the ability to acquire massive amounts of new genetic information in a single event (22), resulting in what has been called “quantum evolutionary leaps.” Additionally, phage induction has long been observed to result in the up-regulation of extracellular toxins in bacteria such as S. pyogenes (32) and Corynebacterium diphtheriae (17). This phenomenon has recently been identified at the molecular level in Escherichia coli (27). In the group A streptococci, we have further demonstrated that the pharyngeal cell itself (i.e., the cell at the most common site of streptococcal infection) generates the signal for the induction of both phage and the associated SpeC toxin (6). The importance of lysogenic bacteriophage as an agent of horizontal gene transfer as well as a means for the regulation of those genes makes them a critical component in studying bacterial pathogenesis.
In this study, we further our hypothesis that S. pyogenes is more than a static particle during the earliest stages of infection. Once it is exposed to the host cell environment, we propose that other extracellular proteins are likely to be induced. Such regulation has been observed with both intracellular (1, 2) and nonintracellular bacteria (6, 19), as well as other microorganisms, including Candida albicans (4). Our present work identifies a novel streptococcal DNase (Spd1) that is closely associated with SpeC and is induced along with the exotoxin during coculture with human pharyngeal cells. This study also suggests that nearly all the group A streptococcal DNases described may be phage encoded.
MATERIALS AND METHODS
Growth conditions for pharyngeal cells and S. pyogenes.
The growth conditions for S. pyogenes strain D471 (from the Rockefeller University collection) and the human pharyngeal cell line Detroit 562 (ATCC CCL 138) were identical to those outlined in our previous experiments (6).
S. pyogenes-human pharyngeal cell coculture system.
Pathogenic S. pyogenes (strain D471) was grown overnight and suspended in phosphate-buffered saline. After the optical density at 650 nm was adjusted to 1.0 (∼5 × 108 CFU/ml), the bacteria were centrifuged and resuspended in serum-free minimal essential medium (methionine deficient). The Detroit 562 pharyngeal cells were grown to confluence, washed three times with serum-free minimal essential medium (Met−), and then treated with cycloheximide (150 μg/ml) for 45 min. Following the incubation, bacteria and [35S]methionine were added to the coculture medium to concentrations of ∼108 CFU/ml and 80 μCi/ml, respectively. The coculture was allowed to incubate for 3 h at 37°C under 5% CO2. The medium was then centrifuged (10,000 × g; 15 min) and sterile filtered through a 0.45-μm-pore-size membrane. As controls, bacteria were incubated alone and pharyngeal cells were incubated alone. The control medium was processed in parallel with the coculture sample.
Detection of secreted proteins in coculture media.
Both bacterial and pharyngeal-cell medium supernatants, along with the coculture medium supernatant, were concentrated 100-fold by trichloroacetic acid precipitation. Labeled proteins in the concentrate were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to a polyvinylidene difluoride membrane, and visualized by exposure to X-ray film. Additionally, the pharyngeal-cell control was treated with 0.1% SDS, and the whole-cell extract was examined by SDS-PAGE for the presence of radioactive proteins.
Sequencing of Spd1.
The concentrated coculture medium was prepared and blotted as described above. The polyvinylidene difluoride membrane was stained with Coomasie brilliant blue-R, and the portion of the membrane containing the 27-kDa band was cut out and subjected to direct N-terminal sequencing via automated Edman degradation in the Rockefeller University Biotechnology Center.
DNA manipulations.
Two spd1 internal primers (5′-CTAGGCTACGAACTTATCCG-3′ and 5′-TTTAGTTTTTAGGAGTGGCA-3′) were used to PCR amplify a spd1 fragment from a D471 genomic DNA template.
A Stratagene Lambda Zap D471 genomic DNA phage library was screened for spd1 with the spd1 gene probe according to the recommended procedure. The selected phagemid, carrying the D471 genomic DNA insert, was used as a template for the PCR-based spd1 sequencing reaction (performed at the Rockefeller University Protein/DNA Technology Center). Southern blot analysis was performed according to the standard protocol.
Recombinant expression of Spd1.
The portion of spd1 encoding the mature protein was cloned into the Streptococcus gordonii expression vector pSMB104/pSTOP (kindly provided by Siga Technologies, Corvalis, Oreg.). The protocol outlined by Dutton et al. was then followed for transformation and expression of the molecule in S. gordonii strain GP251 (7).
Functional characterization of Spd1.
S. gordonii clones which recombinantly secrete either staphylococcal nuclease or streptococcal M protein were obtained from Siga Technologies to be used as controls in nuclease assays. All S. gordonii strains, including the parent strain (GP251) and the recombinant Spd1-secreting strain, were grown overnight in Todd Hewitt broth-1% yeast extract at 37°C. The overnight cultures were centrifuged at 1,500 × g for 30 min, and the supernatants were filtered (0.45-μm pore size). The supernatants were then mixed with ∼500 ng of pBluescript in a 50 mM Tris (pH 8.0)-500 μM CaCl2-500 μM MgCl2 buffer with or without EDTA (20 mM). The mixture was incubated at 37°C for 45 min. The completed reaction was analyzed by agarose gel electrophoresis and visualized by ethidium bromide staining. Additionally, the same nuclease assay was performed using 8 μg of total RNA (from S. pyogenes strain D471) in place of the DNA substrate. Total RNA was digested with ∼2,500 U of RNase T1 (Gibco-BRL, Gaithersburg, Md.) to serve as a positive experimental control in the RNA digestion assay.
PBMC proliferation assay.
The protocol employed by Visvanathan et al. (26) was used to isolate human peripheral blood mononuclear cells (PBMCs) and to test whether recombinant Spd1 was able to stimulate PBMC proliferation.
RT-PCR for spd1/speC polycistronic transcript.
The Detroit 562-D471 coculture was trypsinized, and the mixed cellular suspension was subjected to differential centrifugation to separate the bacteria from the eukaryotic cells (150 × g for 10 min to pellet eukaryotic cells; 1,500 × g for 30 min to pellet the bacteria). Total RNA was extracted from the cocultured D471 sample, as well as the pharyngeal-cell and S. pyogenes controls. Reverse transcription was carried out on total RNA extracted from the cocultured D471 sample using the standard Gibco-BRL Superscript Reverse Transcriptase protocol with a reverse primer for speC (5′-GGCGTAATTCCTCCATAGAT-3′). The subsequent PCR used an spd1 sense primer and the same speC antisense primer used in the reverse transcription reaction. A separate control reaction containing no reverse transcriptase (RT) enzyme was also performed.
Northern blot analysis for spd1 and speC transcripts.
Total RNA from the cocultured S. pyogenes, as well as the pharyngeal-cell and S. pyogenes controls, was analyzed by Northern blotting and probed for the spd1 or speC transcript with [32P]CTP-labeled gene probes (Boehringer Mannheim High Prime DNA-labeling reaction). The blot was then exposed to X-ray film, and intensities were quantified using AlphaEase imaging software.
RESULTS AND DISCUSSION
Induction of secreted proteins after coculture with human pharyngeal cells.
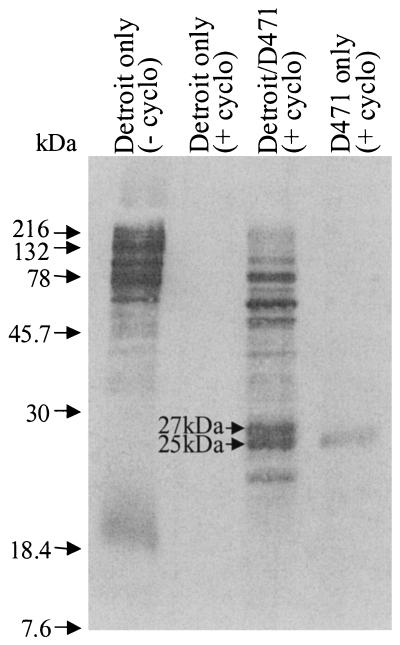
By modifying our Detroit pharyngeal-cell in vitro model of streptococcal infection (6) to specifically radiolabel bacterial proteins produced during the coculture, we demonstrate that S. pyogenes M6 strain D471 releases a number of proteins ranging in size from ∼20 to ∼200 kDa (Fig. 1). We found no apparent down-regulation of constitutively expressed bacterial proteins. In fact, the amount of protein secreted by strain D471 incubated in minimal medium, in the absence of Detroit pharyngeal cells, is extremely low (Fig. 1). Thus, the induction of these secreted bacterial proteins is dependent upon the interaction of streptococcus with its pharyngeal-cell host, suggesting that these secreted proteins are produced to enhance early pathogenic processes and are not produced merely for some basal metabolic function.
FIG. 1.
[35S]Met-labeled proteins in the culture media of the non-cycloheximide-treated pharyngeal-cell control [Detroit only (− cyclo)] and the cycloheximide-treated samples, including the pharyngeal-cell control [Detroit only (+ cyclo)], pharyngeal-cell-S. pyogenes coculture (Detroit/D471), and S. pyogenes control (D471 only).
Identification and sequence analysis of a 27-kDa protein.
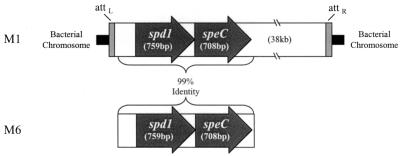
In our previous studies, we showed that the 25-kDa protein (Fig. 1) is SpeC (6). When compared to the S. pyogenes M1 genome (http://www.genome.ou.edu/strep), the N-terminal sequence of the adjacent 27-kDa protein revealed 90% identity with an open reading frame encoding a protein of similar size. Remarkably, in the M1 genome, these two genes were located adjacent to each other and positioned precisely at the distal end of the prophage which harbors speC (Fig. 2), proving that the novel 27-kDa protein is also of bacteriophage origin. Furthermore, the 27-kDa protein demonstrated homology with a set of streptococcal DNases, including the S. pyogenes MF/DNase B (21% amino acid identity), prompting speculation that it was a streptococcal phage DNase (Spd1).
FIG. 2.
Schematic alignment of spd1 and speC sequences from the integrated prophage in the M1 genome and the region of the M6 strain D471 which was sequenced. attL and attR, left and right attachment sites, respectively, of the integrated prophage.
The 759-bp spd1 gene encodes a preprotein with a predicted mass of 28.4 kDa. Based on N-terminal amino acid sequence analysis of the secreted Spd1 molecule, it is apparent that Spd1 has a hydrophobic 29-amino-acid leader sequence which is removed when the protein is secreted. The resulting mature protein is predicted to have a molecular mass of ∼26.2 kDa, which is consistent with the 27-kDa mass observed by SDS-PAGE.
The sequences of both spd1 and speC from the M6 serotype (strain D471) of S. pyogenes (which was used in our experiments) demonstrated nearly complete identity with those of the spd1 and speC homologues of the M1 genome. The physical locations of spd1 and speC were also preserved in the M6 strain (Fig. 2). While Spd1 is phage encoded, its origin may have been chromosomal as well. As Spd1 is located at the very end of its prophage, it is likely that the bacteriophage recently acquired the gene from a host chromosome through an improper excision event.
Distribution and regulation of spd1 and speC.
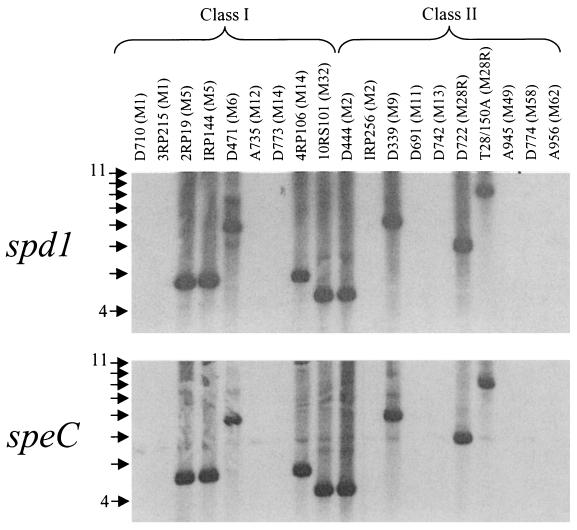
When 19 randomly selected strains of S. pyogenes were screened, the genetic proximity of speC and spd1 was always preserved. While not ubiquitous, the two genes were consistently found on the same restriction fragment (Fig. 3), suggesting that the bacteriophage acquired these two genes in a single event. As the two genes are phage encoded, one interpretation for the variation in restriction fragment size is the locations of bacteriophage integration sites in the different streptococcal genomes. While 9 of 19 (47%) strains contained both genes, no streptococcal antigenic class association (5) was observed (Fig. 3). The fact that, when present, spd1 is always phage associated suggests that the presence of the nuclease activity may have a benefit for the bacteriophage.
FIG. 3.
Southern blot of HindIII-digested genomic DNAs from 19 strains of S. pyogenes probed with either spd1 or speC. The M serotype is designated in parentheses next to the strain number, and the strains are grouped by their surface-exposed antigenic properties as either class I or class II. The DNA ladder is shown to the left, with arrows indicating 1-kb increments.
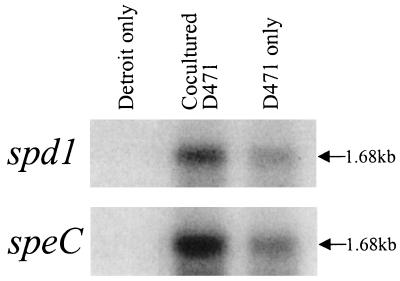
The intimate physical association between the two genes prompted us to speculate that spd1 and speC may be linked at a level beyond that of genetic neighbors. RT-PCR analysis confirmed that both spd1 and speC are encoded by a common transcript (data not shown). Northern blot analysis supported this finding, as both genes were found to lie on a 1.68-kb transcript (Fig. 4). Additionally, the observed up-regulation of the Spd1 and SpeC proteins during coculture is supported by the ∼3-fold induction of each transcript over that of the bacterial control (Fig. 4).
FIG. 4.
RNAs extracted from Detroit 562 cells, D471 cocultured with Detroit 562 (Cocultured D471), and D471 alone were blotted and probed for either spd1 or speC.
It is tempting to postulate that the pharyngeal-cell-mediated induction of spd1 and speC results from the previously observed induction of their associated bacteriophage (6). When the bacteriophage is induced and its genome is replicated, the copy numbers of spd1 and speC are largely increased, creating more DNA templates for transcription. However, this method of induction is likely not to be the primary source of regulation, as the increase in gene copy number (i.e., replicated phage genomes) far outweighs the threefold induction observed at the transcriptional level. Translational control may offer an additional level of regulation and may very well explain the increased production of SpeC over Spd1 observed in the S. pyogenes control (Fig. 1, lane 4).
Our finding that the bacteriophage genetic block encoding speC and spd1 is induced in response to coculture with pharyngeal cells has pathogenic implications. Numerous accounts describing blocks of virulence factors, originating from extrachromosomal sources (e.g., plasmids, transposons, or bacteriophage), have been reported for several pathogenic bacteria (13). The close proximity of spd1 to speC, coordinate transcription, and common induction following interaction with pharyngeal cells strongly indicate that these two genes may be part of a bacteriophage-encoded pathogenicity locus. Since expression of virulence factors is likely to be under tight regulatory control, the presence of multiple factors on a common transcript affords the bacteria the ability to regulate two genes with one signal. The human pharyngeal cell itself or, more precisely, a currently unidentified pharyngeal-cell soluble phage-inducing factor (SPIF) (6), provides the bacterial signal necessary for this gene expression (unpublished data).
Biochemical characterization of Spd1 function.
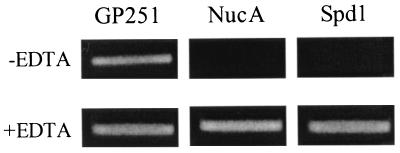
The homologies found between Spd1 and MF/DNase B prompted us to investigate whether Spd1 actually possesses DNase activity. In our DNA hydrolysis assay, we found that Spd1 possesses DNase activity which is inhibited by EDTA, demonstrating the requirement for divalent cations common for DNases (Fig. 5). As expected, staphylococcal nuclease (NucA) hydrolyzed DNA and was equally inhibited by EDTA. Furthermore, we identified the nuclease activity of Spd1 to be substrate specific, as the enzyme failed to digest RNA (data not shown). These results clearly demonstrate that Spd1 is an extracellular streptococcal DNase.
FIG. 5.
Culture supernatants from S. gordonii (GP251) parent strain and supernatants from recombinant S. gordonii (secreting either NucA or Spd1) were collected. Each supernatant was incubated with pBlueScript DNA in Tris-Ca2+-Mg2+ buffer, either with (+) or without (−) EDTA present. The completed reaction was analyzed by agarose gel electrophoresis and visualized by ethidium bromide staining.
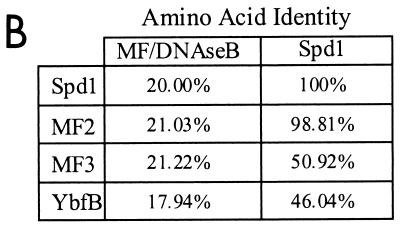
While recently debated in the literature (11), the S. pyogenes MF/DNase B protein has been thought to possess mitogenic activity. In fact, the 21% amino acid identity found between the streptococcal MF/DNase B and the product of the gene which we have now labeled spd1 (Fig. 6B) prompted Ferretti et al. (8) to name this gene mf2 (for mitogenic factor 2) in the recently completed S. pyogenes M1 genome. However, using various concentrations of recombinant Spd1 in cellular proliferation assays, we were unable to detect any proliferative response from human PBMCs; 2.0 μg of the staphylococcal enterotoxin B (a known mitogen) served as a positive control and stimulated PBMC proliferation (data not shown). Our characterization of Spd1, whose sequence is virtually identical to that of MF2 (99%) (Fig. 6B), clearly demonstrates that this molecule has DNase activity but lacks mitogenic capacity. Thus, we propose that Spd1 is a more appropriate name for MF2.
FIG. 6.
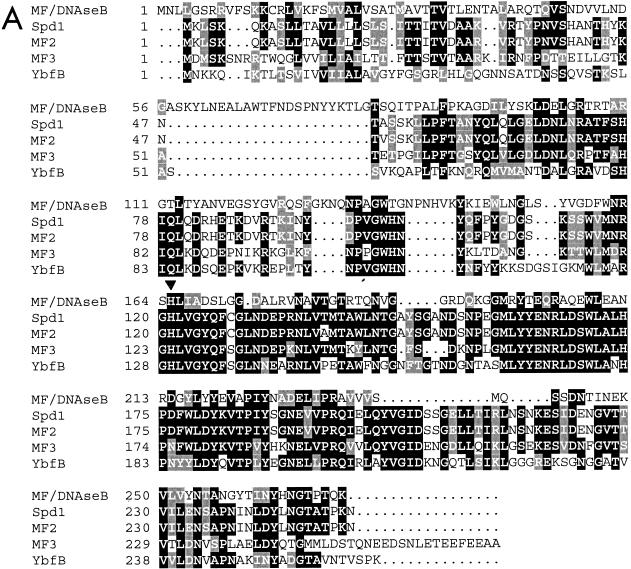
(A) Amino acid sequence alignment of the Spd1 nuclease with the known nuclease MF/DNase B and three other putative DNases, including two from S. pyogenes (MF2 and MF3) and one from L. lactis (YbfB). The conserved histidine is indicated with an arrowhead. The dots represent spaces in the sequence alignment. Solid boxes represent amino acid identity, and shaded boxes represent amino acid similarity based on Clustal W sequence analysis. (B) Amino acid sequence identities between MF/DNase B or Spd1 and the Spd1-related putative DNases.
Spd1-related DNases.
Having firmly established Spd1 to be a DNase, we are now able to more clearly identify a number of putative molecules whose functions were thought to be DNases based on sequence homology. The putative S. pyogenes proteins MF2 (GenBank accession no. AAK33665) and MF3 (accession no. AAK34241), as well as the Lactococcus lactis putative protein YbfB (accession no. AAK04254), have all been labeled DNases based solely on their homologies to other nucleases, principally the streptococcal MF/DNase B (Fig. 6A). Indeed, while all three of these molecules have >17% sequence identity with MF/DNase B, they have two to three times as much identity with Spd1 (Fig. 6B). Thus, sequence analysis of the Spd1 sequence determined from our M6 S. pyogenes strain (D471) and the completed genomes of both the M1 S. pyogenes strain (SF370) and L. lactis indicates the existence of a class of highly conserved DNases, all similar to Spd1.
Likely to be of critical importance to this class of DNases is a thoroughly conserved histidine residue (His121 of Spd1). Histidines are often critical to DNase catalytic function (30), and this specific amino acid has been demonstrated by Iwasaki et al. (15) to be required for the nuclease activity of the related MF/DNase B.
While MF/DNase B and the putative L. lactis DNase, YbfB, appear to be encoded by chromosomal genes, MF2 (i.e., Spd1) and MF3 are encoded by integrated streptococcal bacteriophages (8). The role of bacteriophages in relation to these DNases is not well understood. However, evidence presented here and elsewhere leads us to believe that phage-encoded nuclease expression is directly related to the phage life cycle and survival. Sargent et al. (23) propose that the B. subtilis SPβ prophage encodes a nuclease and that the nucleolytic activity is likely related to the repression state of the prophage. Indeed, phage-encoded virulence factors have long been observed to be expressed following the derepression of lysogenic bacteriophage (17, 32). While the molecular link of toxin up-regulation with bacteriophage induction is unknown for a number of organisms, Wagner et al. (27) have provided detailed evidence, in the E. coli system, that Shiga toxin induction and lytic activation are synonymous events, as both are driven by the same late phage promoter. Thus, it is becoming more and more apparent that bacteriophage induction is more than a means for horizontal gene transfer. It also serves as a method to induce the expression of many phage-encoded virulence determinants.
Identification of the four classical DNases of S. pyogenes.
The four known extracellular DNases of S. pyogenes (A, B, C, and D) are all ∼25 to 30 kDa (12). Substrate specificities have previously been examined for these enzymes; DNases A and C are specific for DNA, while DNases B and D are less specific and will act on either DNA or RNA substrates. Thus far, only DNase B and DNase D have been identified at the molecular level (20, 24); however, the distribution of all four DNases across a wide range of streptococcal strains has been thoroughly investigated. Tiesler and Beck (25) determined that DNases A, B, C, and D are secreted by a respective 33, 100, 50, and 20% of all streptococcal strains screened. This simple distribution analysis validates the fact that DNase B is chromosomally encoded (8) and therefore present in all strains. The remaining three DNases are present in only a fraction of streptococcal strains, leading us to believe that these genes may be encoded by mobile elements, such as bacteriophage. The recently completed S. pyogenes genome does not appear to contain the gene for DNase D, and other than DNase B, the genome sequence identified only two putative extracellular DNases, MF2 and MF3. We have characterized mf2 to be spd1, a bacteriophage gene which, like DNase C, is present in ∼50% of the strains examined. Additionally, similar to DNase C, we have characterized Spd1 to be DNA substrate specific, as the enzyme does not digest RNA. Thus, the common size, extracellular location, possible mobile-element origin, equal distribution, and nucleic acid substrate specificity suggest that Spd1 may in fact be DNase C. Furthermore, Ferretti et al.'s identification of MF3 as a streptococcal phage product (8) predicts that the gene will not be found in all strains. As the only remaining known extracellular DNase of S. pyogenes, DNase A (secreted by 33% of strains and possibly of bacteriophage origin) may very well be the equivalent of MF3, a putative molecule of similar size and extracellular action.
Streptococcal DNase function in vivo.
Antibodies to DNase B (10) and DNase D (20) have been detected in patients recovering from streptococcal sepsis, indicating that these proteins are produced by the bacteria during infection, yet there has been little more than speculation as to what such DNases may be doing in vivo. To elucidate one possible Spd1 DNase function, we constructed an Spd1− mutant (via allelic exchange) and tested it in an in vitro pharyngeal-cell adherence and invasion assay (21); however, no difference was detected between the parent Spd1+ strain and the Spd1− mutant. Thus, it seems plausible that the Spd1 DNase has a function independent of adherence. While there is little direct evidence, it has long been speculated that streptococcal DNases act during bacterial infection to liquefy pus and allow the bacteria to further invade the host. Additionally, there are several examples of extracellular bacterial DNases acting as pathogenic determinants which cause eukaryotic chromatin degradation and eventual cell death (16). Since Spd1 is induced by bacteria upon encountering the pharyngeal cell, it is tempting to postulate that it too may act as a pathogenic determinant, perhaps in a fashion similar to the other toxin DNases, thus increasing the bacterial pathogenic fitness with respect to its mammalian host.
It must also be considered that phage-encoded DNases may function to increase the pathogenic fitness of the bacteriophage with respect to its host. Such DNases have been suggested to digest the bacterial chromosome into bacteriophage genome building blocks; however, since the Spd1 DNase has a leader sequence and is thus professionally secreted, it is unlikely that it acts cytoplasmically within the bacteria. It is important to remember that the Spd1 enzyme is induced when the bacteriophage themselves are induced. We suggest that in vivo, phage induction occurs in the pharynx, where other strains of S. pyogenes are likely to be present (up to 33% of humans are colonized with group A streptococci) and could then be lysogenized by the newly released bacteriophage. However, as a result of lysis, bacterial DNA is spilled out, likely contributing to the viscous environment at the site of streptococcal infection, making it difficult for the newly released bacteriophage to randomly encounter the colonizing strain. Selective pressure during the evolution of some bacteriophage (such as the S. pyogenes spd1 phage) may very well have resulted in the incorporation of extracellular DNases, which are upregulated just prior to phage induction in order to digest the bacterial chromosomal DNA released during phage lysis. Thus, the bacteriophage would encode the very molecule which would allow it to more freely diffuse and ultimately disseminate its genetic material into any other phage-naïve strains it may encounter.
Acknowledgments
We thank Joshua Lederberg and Alexander Tomasz for their insightful discussion of the subject matter. Additionally, we thank Patricia Ryan, Daniel Nelson, Corrie Broudy, and Raymond Schuch for critical comments throughout the execution of this work and preparation of the manuscript. We are also greatly indebted to Clara Eastby for her cell culture expertise and to Alain Charles and John Zabriskie for their assistance with mitogenic assays.
This work was supported by U.S. Public Health Service grant AI11822 to V.A.F.
Editor: A. D. O'Brien
REFERENCES
- 1.Abshire, K. Z., and F. C. Neidhardt. 1993. Analysis of proteins synthesized by Salmonella typhimurium during growth within host macrophage. J. Bacteriol. 175:3734-3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abu Kwaik, Y. 1998. Induced expression of the Legionella pneumophila gene encoding a 20-kilodalton protein during intracellular infection. Infect. Immun. 66:203-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alouf, J. E. 1986. Streptococcal toxins (streptolysin O, streptolysin S, erythrogenic toxin), p. 635-691. In F. Dorner and J. Drews (ed.), Pharmacology of bacterial toxins. Pergamon Press, New York, N.Y. [DOI] [PubMed]
- 4.Bailey, A., E. Wadsworth, and R. Calderone. 1995. Adherence of Candida albicans to human buccal epithelial cells: host-induced protein synthesis and signaling events. Infect. Immun. 63:569-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bessen, D., K. F. Jones, and V. A. Fischetti. 1989. Evidence for two distinct classes of streptococcal M protein and their relationship to rheumatic fever. J. Exp. Med. 169:269-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Broudy, T. B., V. Pancholi, and V. A. Fischetti. 2001. Induction of lysogenic bacteriophage and phage-associated toxin from group A streptococci during coculture with human pharyngeal cells. Infect. Immun. 69:1440-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dutton, E. K., S. A. Ottum, T. C. Bolken, C. A. Franke, and D. E. Hruby. 2000. Expression of active monomeric and dimeric nuclease A from the gram-positive Streptococcus gordonii surface protein expression system. Protein Expr. Purif. 19:158-172. [DOI] [PubMed] [Google Scholar]
- 8.Ferretti, J. J., W. M. McShan, D. Ajdic, D. J. Savic, G. Savic, K. Lyon, C. Primeaux, S. Sezate, A. N. Suvorov, S. Kenton, H. S. Lai, S. P. Lin, Y. Qian, H. G. Jia, F. Z. Najar, Q. Ren, H. Zhu, L. Song, J. White, X. Yuan, S. W. Clifton, B. A. Roe, and R. McLaughlin. 2001. Complete genome sequence of an M1 strain of Streptococcus pyogenes. Proc. Natl. Acad. Sci. USA 98:4658-4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fischetti, V. A. 1991. Streptococcal M protein. Sci. Am. 264:58-65. [DOI] [PubMed] [Google Scholar]
- 10.Gerber, M. A., E. D. Gray, P. Ferrieri, and E. L. Kaplan. 1980. Enzyme-linked immunosorbent assay of antibodies in human sera to streptococcal DNase B. J. Lab. Clin. Med. 95:258-265. [PubMed] [Google Scholar]
- 11.Gerlach, D., K. Schmidt, and B. Fleischer. 2001. Basic streptococcal superantigens (SPEX/SMEZ or SPEC) are responsible for the mitogenic activity of the so-called mitogenic factor (MF). FEMS Immunol. Med. Microbiol. 30:209-216. [DOI] [PubMed] [Google Scholar]
- 12.Gray, E. D. 1972. Nucleases of group A streptococci, p. 143-155. In L. W. Wannamaker and J. M. Matsen (ed.), Streptococci and streptococcal diseases. Academic Press, New York, N.Y.
- 13.Hacker, J., G. Blum-Oehler, I. Muhldorfer, and H. Tschape. 1997. Pathogenicity islands of virulent bacteria: structure, function, and impact on microbial evolution. Mol. Microbiol. 23:1089-1097. [DOI] [PubMed] [Google Scholar]
- 14.Herman, A., J. W. Kappler, P. Marrack, and A. M. Pullen. 1991. Superantigens: mechanisms of T cell stimulation and role in immune responses. Annu. Rev. Immunol. 9:745-772. [DOI] [PubMed] [Google Scholar]
- 15.Iwasaki, M., H. Igarashi, and T. Yutsudo. 1997. Mitogenic factor secreted by Streptococcus pyogenes is a heat-stable nuclease requiring His122 for activity. Microbiology 143:2449-2455. [DOI] [PubMed] [Google Scholar]
- 16.Lara-Tejero, M., and J. E. Galán. 2000. A bacterial toxin that controls cell cycle progression as a deoxyribonuclease I-like protein. Science 290:354-357. [DOI] [PubMed] [Google Scholar]
- 17.Matsuda, M., and L. Barksdale. 1967. System for the investigation of the bacteriophage-directed synthesis of diphtherial toxin. J. Bacteriol. 93:722-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCarty, M. 1948. The occurrence of nucleases in culture filtrates of group A hemolytic streptococci. J. Exp. Med. 88:181-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pettersson, J., R. Nordfelth, E. Dubinina, T. Bergman, M. Gustafsson, K. E. Magnusson, and H. Wolf-Watz. 1996. Modulation of virulence factor expression by pathogen target cell contact. Science 273:1231-1233. [DOI] [PubMed] [Google Scholar]
- 20.Podbielski, A., I. Zarges, A. Flosdorff, and J. Weber-Heynemann. 1996. Molecular characterization of a major serotype M49 group A streptococcal DNase gene (sdaD). Infect. Immun. 64:5349-5356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryan, P. A., V. Pancholi, and V. A. Fischetti. 2001. Group A streptococci bind to mucin and human pharyngeal cells through sialic acid-containing receptors. Infect. Immun. 69:7402-7412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sapp, J. 1994. Organisms and the edge of disciplines. In Evolution by association: a history of symbiosis. Oxford University Press, New York, N.Y.
- 23.Sargent, M. G., S. Davies, and M. F. Bennett. 1985. Potentiation of a nucleolytic activity in Bacillus subtilis. J. Gen. Microbiol. 131:2795-2804. [DOI] [PubMed] [Google Scholar]
- 24.Sriskandan, S., M. Unnikrishnan, T. Krausz, and J. Cohen. 2000. Mitogenic factor (MF) is the major DNase of serotype M89 Streptococcus pyogenes. Microbiology 146:2785-2792. [DOI] [PubMed] [Google Scholar]
- 25.Tiesler, E., and U. Beck. 1976. Distribution of the isoenzymes of nucleases in group A streptococci. Zentbl. Bakteriol. 234:462-472. [PubMed] [Google Scholar]
- 26.Visvanathan, K., A. Charles, J. Bannan, P. Pugach, K. Kashfi, and J. Zabriskie. 2001. Inhibition of bacterial superantigens by peptides and antibodies. Infect. Immun. 69:875-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wagner, P. L., M. N. Neely, X. Zhang, D. W. Acheson, M. K. Waldor, and D. I. Friedman. 2001. Role for a phage promoter in Shiga toxin 2 expression from a pathogenic Escherichia coli strain. J. Bacteriol. 183:2081-2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wannamaker, L. W. 1958. The differentiation of three distinct desoxyribonucleases of group A streptococci. J. Exp. Med. 107:797-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wannamaker, L. W., B. Hayes, and W. G. Yasmineh. 1967. Streptococcal nucleases. II. Characterization of DNase D. J. Exp. Med. 126:497.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weston, S., and D. Suck. 1993. X-ray structures of two single-residue mutants on DNase I: H134Q and Y76A. Protein Eng. 6:349-357. [DOI] [PubMed] [Google Scholar]
- 31.Yutsudo, T., H. Murai, J. Gonzalez, T. Takao, Y. Shimonishi, Y. Takeda, H. Igarashi, and Y. Hinuma. 1992. A new type of mitogenic factor produced by Streptococcus pyogenes. FEBS Lett. 308:30-34. [DOI] [PubMed] [Google Scholar]
- 32.Zabriskie, J. B. 1964. The role of temperate bacteriophage in the production of erythrogenic toxin by group A streptococci. J. Exp. Med. 119:761-780. [DOI] [PMC free article] [PubMed] [Google Scholar]