Abstract
The adherence of Staphylococcus aureus to soluble proteins and extracellular-matrix components of the host is one of the key steps in the pathogenesis of staphylococcal infections. S. aureus presents a family of adhesins called MSCRAMMs (microbial surface components recognizing adhesive matrix molecules) that specifically recognize host matrix components. We examined the influence of biofilm-associated protein (Bap) expression on S. aureus adherence to host proteins, epithelial cell cultures, and mammary gland sections and on colonization of the mammary gland in an in vivo infection model. Bap-positive strain V329 showed lower adherence to immobilized fibrinogen and fibronectin than isogenic Bap-deficient strain m556. Bacterial adherence to histological sections of mammary gland and bacterial internalization into 293 cells were significantly lower in the Bap-positive strains. In addition, the Bap-negative strain showed significantly higher colonization in vivo of sheep mammary glands than the Bap-positive strain. Taken together, these results strongly suggest that the expression of the Bap protein interferes with functional properties of the MSCRAMM proteins, preventing initial bacterial attachment to host tissues and cellular internalization.
Staphylococcus aureus is a major pathogen responsible for a wide range of both acute and chronic infections. The first step in S. aureus infections is attachment to various surfaces and colonization of host tissues. For this purpose, S. aureus carries several surface adhesins (MSCRAMMs; microbial surface components recognizing adhesive matrix molecules) (13). Many MSCRAMM proteins interact with various human or animal tissues, serum proteins, and polypeptides of the extracellular matrix. For example, protein A (Spa) binds to the Fc portion of immunoglobulins (Igs) (39), a mechanism that is thought to prevent opsonophagocytosis of staphylococci after their entry into the human host (21, 31). The binding of clumping factors ClfA and ClfB to fibrinogen promotes bacterial adhesion to thrombi on the surfaces of heart valves (28). The FnbA and FnbB proteins bind to fibronectin and fibrinogen (12, 20, 45). These interactions allow staphylococci to adhere to a variety of cell lines and promote the invasion and apoptotic death of infected epithelial cells (9, 38, 46). In addition, the ability of MSCRAMMs to bind to host serum proteins that rapidly coat indwelling medical devices after implantation promotes the adherence of S. aureus to the surfaces of biopolymers (25, 29, 44).
Another step in S. aureus colonization is formation of a biofilm. Biofilm formation is a major concern in nosocomial infections because it protects microorganisms from opsonophagocytosis and antibiotics, leading to chronic infection and sepsis (5). To date, two surface components have been implicated in biofilm formation by S. aureus, (i) the product of the icaADBC operon, which encodes proteins involved in the synthesis of polysaccharide poly-N-succinyl-β-1-6-glucosamine (PIA/PNSG) (6, 26), and (ii) biofilm-associated protein (Bap), a surface protein of 2,276 amino acids, which contains 13 repeats of 86 residues (7). Bap promotes both primary attachment to inert surfaces and intercellular adhesion, whereas PIA/PNSG seems to be involved only in intercellular adhesion.
Previous studies have shown that the expression of an S. aureus extracellular polysaccharide(s) interferes with adherence by masking adhesins (14, 33). Similar phenomena occur with other pathogenic bacteria, such as Staphylococcus epidermidis (1), Streptococcus pneumoniae (42), group A streptococci (16), Klebsiella pneumoniae (11), Neisseria meningitidis (40), Escherichia coli (34), and Haemophilus influenzae type b (41). Similarly, we have observed in a mouse foreign-body infection model that a Bap-deficient mutant colonized a catheter more rapidly at the initial stages of infection than the wild-type bacteria, strongly suggesting that the presence of Bap might hinder the interaction between bacterial cell receptors and the host proteins on the catheter (7).
In this study, we have investigated the relationship between biofilm formation and the functionality of the MSCRAMM proteins in S. aureus. Our results demonstrate that Bap interfered with the activity of the MSCRAMM proteins and decreased (i) adherence to immobilized fibrinogen and fibronectin, (ii) adherence to mammary gland tissue ex vivo, (iii) internalization by epithelial cells, and (iv) infection of the mammary glands of ewes.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The strains and plasmids used in this study are listed in Table 1. Phage 85 was used to transduce the pBT2 and pBT2:Bap plasmids from RN4220 to Newman and P1 strains.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype | Relevant properties | Source or reference |
|---|---|---|---|
| S. aureus strains | |||
| RN4220 | Restriction-deficient 8325-4 mutant | 22 | |
| Newman | Expresses clumping factor | 8 | |
| DU5944 | Newman clfA2::Tn917 EmrclfB::Tcr | Deficient in clumping factors A and B | 29 |
| P1 | Expresses FnBPs | 37 | |
| P1 FnBP− | P1 fnbA::TcrfnbB::Emr | Deficient in FnBPA and -B | T. J. Foster |
| V329 | Expresses Bap | 7 | |
| m556 | V329 bap::Tn917 Emr | Deficient in Bap | 7 |
| SA113 | Expresses PIA/PNSG | 6 | |
| SA113Δica | SA113 ΔicaADBC::Tcr | Deficient in PIA/PNSG | 6 |
| Plasmids | |||
| pBT2 | Shuttle plasmid | 3 | |
| pBT2:Bap | pBT2 with bap gene | 7 |
Staphylococcal strains were cultured in Trypticase soy agar and in Trypticase soy broth (TSB) supplemented with glucose (0.25% [wt/vol]). Media were supplemented when appropriate with chloramphenicol (10 μg/ml for plasmid pBT2).
Bacterial adherence to immobilized fibrinogen and fibronectin.
The binding of cells to fibrinogen immobilized on plates was measured by the assay of Hartford et al. (17) with fibrinogen or fibronectin coating concentrations ranging from 0.1 to 10 μg/ml. Briefly, fibrinogen or fibronectin (Calbiochem) was diluted in sodium carbonate buffer (40 mM, pH 9.6) to the appropriate concentration, and 100 μl was used to coat 96-well flat-bottom enzyme-linked immunosorbent assay (ELISA) plates (Sarstedt) overnight at 4°C. Control wells contained carbonate buffer only. After being washed with phosphate-buffered saline (PBS), the plates were blocked for 2 h at 37°C in 2% bovine serum albumin (BSA) in PBS. Cells from an overnight culture (stationary phase) were washed and diluted in PBS (optical density at 600 nm [OD600] = 1.0). A volume of 100 μl of this cell suspension was added, and the plates were incubated for 2 h at 37°C. After gentle washing, adherent cells were fixed by the addition of 100 μl of 25% aqueous formaldehyde and incubation at room temperature for 30 min. The plates were then washed gently, stained with crystal violet, washed again, and read on an ELISA reader at 570 nm.
SDS-PAGE and Western immunoblotting or Western ligand affinity blotting.
For ClfA detection, S. aureus cells from a stationary-phase culture were suspended to an OD600 of 40 in 100 mM PBS containing 5 mM EDTA and 1 mM phenylmethylsulfonyl fluoride. Cells were centrifuged and resuspended in 1 ml of digestion buffer (50 mM Tris-HCl [pH 7.5], 20 mM MgCl2, 30% raffinose [Sigma]) (17). To each 1-ml sample, 60 μl of protease inhibitors (Complete cocktail; Boehringer Mannheim) and 60 μl of a 2-mg/ml solution of lysostaphin (Sigma) were then added, and the suspension was incubated in a 37°C water bath for 30 min. Protoplasts were sedimented by centrifugation at 6,000 × g, and the supernatant fraction, which contained the wall-associated proteins, was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; 10% separation gel, 4.5% stacking gel).
For Western blot analysis, protein extracts were prepared and analyzed by SDS-PAGE as described above and blotted onto an Immobilon-P membrane (Millipore). The primary anti-ClfA antibody (25) was used at a 1:1,000 dilution with Tris-buffered saline (TBS; 50 mM Tris-HCl [pH 7.5], 150 mM NaCl)-1% skim milk. Alkaline phosphatase-conjugated goat anti-rabbit IgG (Sigma) diluted 1:15,000 in TBS-1% skim milk was used, and the subsequent chemiluminescence reaction (with CSPD [Roche]) was recorded.
The Bap immunoblotting assay was performed as described previously (7). Briefly, protein extracts were prepared and analyzed by SDS-PAGE as described above and blotted onto an Immobilon-P membrane (Millipore). Anti-Bap serum was diluted 1:2,500 with TBS and immunoabsorbed with 5% skim milk. Alkaline phosphatase-conjugated goat anti-rabbit IgG (Sigma) diluted 1:15,000 in TBS-5% skim milk was used, and the subsequent chemiluminescence reaction (CSPD; Roche) was recorded.
For detection of fibronectin-binding proteins (FnBPs), the EZ-Link sulfo-N-hydroxysuccinimide-LC biotinylation kit (Pierce) was used to biotinylate human fibronectin (Calbiochem). Bacteria grown to an OD600 of 0.2 in 20 ml of TSB in a 250-ml Erlenmeyer flask shaken at 200 rpm at 37°C were harvested by centrifugation at 12,000 × g and resuspended and treated as described above. FnBPs were detected by Western ligand affinity blotting by incubation for 1 h with biotinylated human fibronectin (50 μg/ml) in PBS containing 0.1% Tween 20 (PBST). They were then given three washes in PBST and incubated for 1 h with streptavidin-peroxidase conjugate (Roche; 1:3,000 dilution). The membranes were washed as before and developed by enhanced chemiluminescence (Roche).
Quantitative assay of biofilm formation on polystyrene.
A late-adherence assay was carried out essentially as described elsewhere (19). Briefly, S. aureus strains were grown overnight in TSB at 37°C. The culture was diluted 1:40 in TSB-0.25% glucose, and 200 μl was used to inoculate sterile 96-well polystyrene microtiter plates (Iwaki). After 18 h, the wells were gently washed three times with 200 μl of sterile PBS, dried in an inverted position, and stained with 0.25% crystal violet for 1 min. Wells were rinsed again, and the absorbance at 570 nm was determined (Micro-ELISA Autoreader Elx800; Bio-Tek Instruments). Each assay was performed in triplicate and repeated five times.
Electron microscopy.
Bacteria grown in TSB-0.25% glucose were fixed and stained with alcian blue and examined by transmission electron microscopy as previously described (10).
Bacterial adherence to mammary gland tissue.
The adherence assay was performed as described by Okada et al. (30). Sections of mammary gland tissue from goats on glass slides were deparaffinized and blocked with PBS-0.2% BSA-0.05% Tween 20 for 45 min. They were incubated with 200 μl of bacterial suspensions (OD600 = 1; three slides per sample) in a humidified chamber (37°C, 2 h). After being washed in PBS (six times), the slides were dried, heat fixed, and Gram stained.
Adhesion and invasion of 293 cells.
Adherence and invasion studies were performed as described previously (9). An established epithelial cell line (293; ATCC CRL-1573) was grown in Dulbecco's modified Eagle medium (Invitrogen Life Technologies) containing 10% fetal bovine serum (Invitrogen Life Technologies), 100 U of penicillin G/ml, and 100 μg of streptomycin sulfate/ml (Invitrogen Life Technologies). Prior to use, cells were seeded at 6 × 104 cells/well in 24-well tissue culture plates (Iwaki) and grown for 3 days at 37°C with 5% CO2.
Approximately 16 h prior to invasion experiments, the 293 cell growth medium was replaced with 0.5 ml of invasion medium (growth medium without antibiotics or fetal bovine serum). The medium was removed, and the 293 cells were washed once with invasion medium and given 0.5 ml of fresh invasion medium. Wells were inoculated with 106 CFU of washed S. aureus cells from an overnight culture and incubated at 37°C with 5% CO2. After 2 h, media were removed and replaced with 1 ml of invasion medium containing 100 μg of gentamicin (Sigma) per ml. After incubation of cocultures at 37°C with 5% CO2 continued for an additional 1 h with gentamicin to kill extracellular bacteria, the supernatants were removed and discarded. Cell monolayers were washed three times with sterile PBS and lysed with 0.025% Triton X-100 (Sigma) in sterile distilled water. Cell lysates were serially diluted 10-fold and plated in triplicate on Trypticase soy agar plates. The results of representative experiments are shown. In some experiments, gentamicin was omitted so that the adherent and internalized bacteria could be quantified.
Experimental infection.
The experimental infection was carried out essentially as described elsewhere (2). Thirty-two healthy lactating Rasa Aragonesa ewes were inoculated with 1 ml of bacterial suspension (5 × 102 CFU) 20 to 25 days after parturition. Mothers were separated from their offspring 2 h before inoculation to ensure the presence of milk (as a natural lubricant) in the teat duct at the time of inoculation. After teats were disinfected with 70% ethanol, the inoculum was introduced in both glands through a 21-gauge cannula.
Since suckling favored removal of bacteria, lambs were separated from their mothers for two different time periods (120 and 240 min) in order to study the capacity of bacteria to colonize the mammary gland.
Milk samples for bacteriological analysis were obtained 1, 2, 3, 7, and 14 days after inoculation. Aliquots of milk samples were placed directly on blood agar. In addition, to exclude the possibility of contamination, bacteria recovered at the end of the experimental period were compared with the parental strains by DNA typing and by determination of erythromycin resistance, Congo red agar colony morphology phenotype, and biofilm formation (7).
Statistical analysis.
The data were analyzed by Student's t test for unpaired data to determine statistically significant differences. For analysis of the cure ratio in the experimental infection, a two-by-two contingency table was produced and Fisher's exact test was applied. Differences were considered statistically significant when P was <0.05 in all cases.
RESULTS
Bap expression blocks in vitro adherence to fibrinogen and fibronectin.
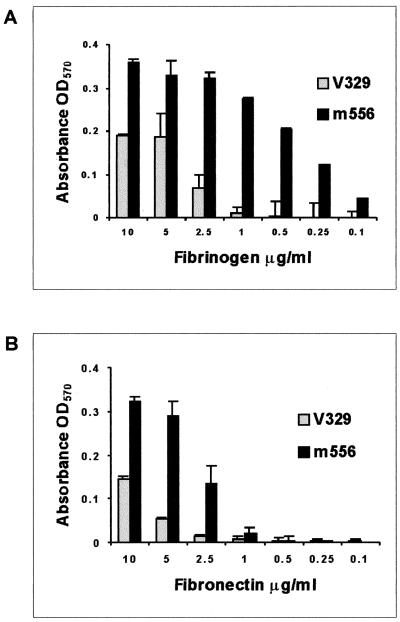
To study the influence of Bap on bacterial interactions with fibrinogen and fibronectin, wild-type S. aureus strain V329 and isogenic Bap-deficient strain m556 were tested for adherence to immobilized proteins. As shown in Fig. 1, the Bap mutant m556 cells showed a significantly higher fibrinogen and fibronectin concentration-dependent adherence than wild-type strain V329.
FIG. 1.
Adhesion of S. aureus V329 and m556 to immobilized fibrinogen (A) and fibronectin (B). Wells in ELISA plates were coated with different concentrations of human fibrinogen or fibronectin. Adherence of strain V329 and the Bap mutant m556 was indicated by staining with crystal violet and measuring the absorbance at 570 nm. Data are the average results ± standard deviations of triplicate determinations. Background values of bacteria adhering to BSA-coated wells were subtracted.
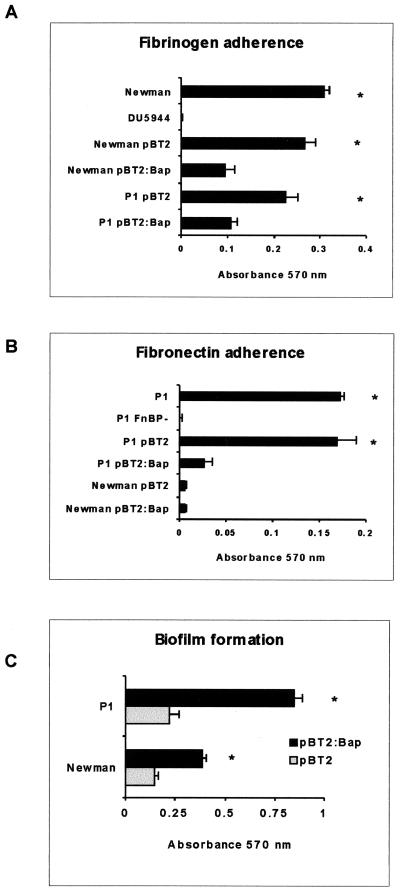
To confirm that the decreased adherence of V329 to fibrinogen and fibronectin was due to the presence of Bap and not to additional factors, naturally occurring Bap-deficient S. aureus strains Newman and P1 were converted to Bap-positive strains by introduction of plasmid pBT2:Bap. As shown in Fig. 2A, complementation of both strains with Bap produced a significant decrease in the adherence to fibrinogen compared with that for the same strains harboring the empty vector (P < 0.001). Note that fibronectin binding was tested only in P1; the testing showed the decreased adherence in the Bap-positive complemented strain (Fig. 2B; P < 0.001). Newman does not adhere to fibronectin despite expressing FnBPA and FnBPB at low levels (32). We also compared the adherence of S. aureus Newman cells and that of the isogenic ClfA− ClfB− mutant (DU5944) to fibrinogen and the adherence of S. aureus P1 strain and that of the isogenic FnBPA− FnBPB− mutant (P1 FnBP−) to fibronectin. As expected, DU5944 did not adhere to fibrinogen and P1 FnBP− did not adhere to fibronectin, clearly showing that adherence was dependent on the presence on the surfaces of these bacteria of the ClfA and ClfB proteins or FnBPA and FnBPB proteins, respectively. The production of Bap was verified by Western immunoblotting using polyclonal antibodies raised against the N-terminal region of Bap (data not shown).
FIG. 2.
Adhesion of Bap-positive S. aureus Newman and P1 strains to immobilized fibrinogen (A) and fibronectin (B). Data are the average results ± standard deviations of triplicate determinations of the increase of bacterial adherence to uncoated versus protein-coated surfaces. (C) Biofilm formation of the complemented S. aureus Newman and P1 strains. ∗, P < 0.001 (t tests).
In agreement with previous results (7), expression of Bap in Newman and P1 strains increased both intercellular adhesion, as shown by the presence of macroscopic cell aggregates at the bottom of culture tubes (data not shown), and biofilm formation on the wells of microtiter plates (Fig. 2C).
Detection of ClfA and FnBPA on adherent and nonadherent S. aureus strains.
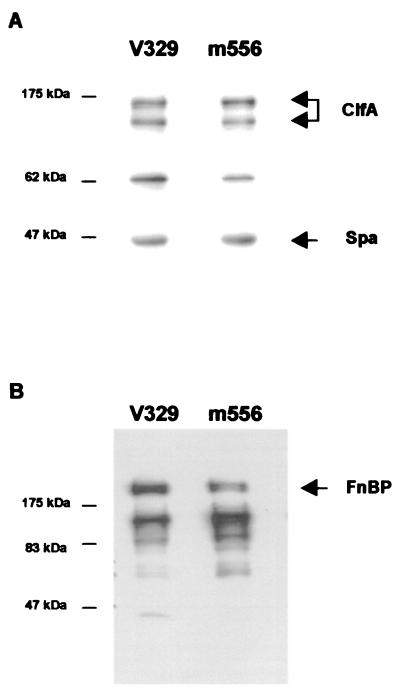
To exclude the possibility that decreased adherence to fibrinogen or fibronectin in the Bap-producing S. aureus strains was due to a decrease in the expression of the corresponding MSCRAMM protein, we determined by Western blotting or Western ligand affinity blotting the presence of ClfA and FnBPA and FnBPB proteins solubilized by lysostaphin in V329 and m556 cells. As shown in Fig. 3, Bap-positive strain V329 produced amounts of ClfA and FnBP proteins similar to those produced by the isogenic Bap-deficient m556 strain. Similar results were obtained when the levels of expression of these proteins in the Bap-positive and Bap-negative complemented Newman or P1 strains were compared (data not shown). These results indicated that the decreased adherence to fibrinogen and fibronectin in the Bap-producing strains is not due to reduced levels of expression of the corresponding MSCRAMM protein.
FIG. 3.
Visualization of ClfA protein (A) and FnBPs (B) by Western blotting and ligand affinity blots, respectively. Positions of protein size markers are shown at the left of each panel. Bands corresponding to native and truncated ClfA proteins, FnBPs, and Spa are identified. Similar amounts of ClfA, FnBPs, and protein A released into the supernatant by protoplasts stabilized in raffinose were observed in V329 and m556.
Control experiments showed that the 45-kDa band present in the Western blotting experiments is protein A. No differences in protein A expression between the Bap-producing and nonproducing strains were observed.
Implications of PIA/PNSG in the functionality of MSCRAMM.
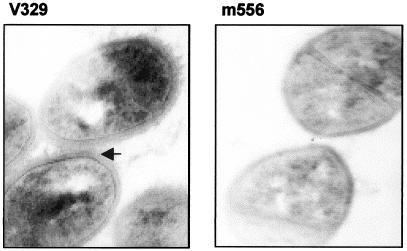
Alcian blue staining and observation of ultrathin sections by transmission electron microscopy of bacterial cells of wild-type strain V329 and Bap mutant m556 revealed the presence of a matrix surrounding the cell wall of V329. This layer was absent from m556 (Fig. 4). Because alcian blue selectively stains mucopolysaccharides, this finding suggests that an external polysaccharide-rich material is present in the V329 strain but is lacking in the Bap mutant strain. Taking into account previous results indicating that the presence of Bap increased the accumulation of PIA/PNSG (7), we hypothesized that the external polysaccharide surrounding V329 cells might be PIA/PNSG. To investigate whether the masking effect observed in the Bap-positive strains could be due to the presence of the PIA/PNSG exopolysaccharide, we compared the abilities of S. aureus SA113 and its isogenic PIA/PNSG-deficient mutant, SA113Δica, to adhere in vitro to fibrinogen and fibronectin. We were not able to detect any significant differences between the wild-type and the PIA/PNSG-deficient strains (data not shown), suggesting that PIA/PNSG does not interfere with MSCRAMM-mediated binding.
FIG. 4.
Ultrathin sections of Bap-positive and Bap-negative strains. Note the presence of extracellular polysaccharide material (arrow) stained with alcian blue in S. aureus V329, in contrast to what is seen in biofilm-negative mutant m556. Magnification, ×34,000.
Bap expression affects internalization by, but not adhesion to, 293 cells.
Several studies have implicated the FnBPA and FnBPB proteins in staphylococcal adherence to and internalization by epithelial cells (9, 38), an important mechanism related to the capacity of bacteria to evade the immune system of the host. With the aim of investigating whether the expression of Bap also affects FnBP-mediated internalization, we used the classical invasion assay based on the principle that bacteria inside host cells are protected from killing by gentamicin in the medium. Wild-type strain V329 was internalized with lower efficiency than the Bap-deficient strain (percent internalization of m556 relative to that for V329 [taken as 100%], based on the ratio of CFU of internalized bacteria to CFU of inoculated bacteria, 344% ± 1.3%; P < 0.05), suggesting decreased efficiency in the FnBPA and FnBPB function.
However, when we compared the adherence of S. aureus V329 and m556 to that of the 293 epithelial cells, we were not able to detect any differences (data not shown). This result suggests that an additional factor that is not masked by Bap might be involved in the bacterial adherence to epithelial cells.
The effect of Bap expression on the adherence of S. aureus strains to connective tissue in a mammary gland section.
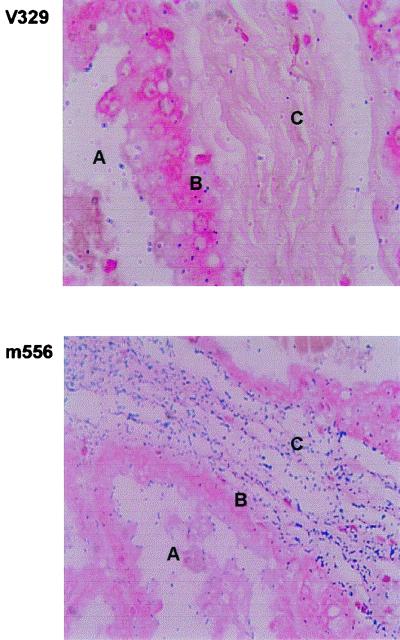
To infect the mammary gland, bacteria must invade epithelial cells and then bind to the underlying connective tissue when the epithelium is damaged. To analyze whether the Bap protein is capable of blocking adhesion to mammary gland tissue, we analyzed bacterial binding to thin sections of the caprine mammary gland. In agreement with the in vitro adhesion studies with 293 cells, no significant differences in the levels of adherence to the epithelial layer were observed (Fig. 5). However, strain m556 was able to bind significantly more efficiently to connective tissue underlying the epithelium than strain V329 (Fig. 5). Connective tissue contains in the extracellular matrix proteins such as fibronectin. The result suggests that the presence of Bap interfered with adhesion to this tissue.
FIG. 5.
Adherence of S. aureus strains to a mammary gland section. Magnification, ×200. A, lumen of mammary gland; B, epithelial layer; C, connective tissue.
The effect of Bap expression on colonization in an intramammary gland infection model.
We performed experimental mammary gland infection in ewes to determine the effect of Bap on bacterial colonization of the ovine mammary gland. The results showed that 11 out of 16 mammary glands remained infected with strain m556 14 days after inoculation when suckling was avoided for 120 min after inoculation. In contrast, only 5 out of 16 animals were infected by strain V329 (P < 0.05). Interestingly, when suckling was avoided for a longer period after inoculation (240 min), a similar number of mammary glands were infected by both strains (11 out of 15 mammary glands infected with m556 and 12 out of 15 mammary glands infected with V329).
These results suggest that early interactions between MSCRAMM proteins and host receptors are crucial for bacterial colonization. The presence of Bap significantly blocks the host-MSCRAMM interaction, reducing the infective capacity in the short term.
All ewes that were colonized after challenge had infection throughout the experimental period, and all ewes that did not become infected remained free of infection.
DISCUSSION
S. aureus is predominantly an extracellular pathogen that colonizes the host by adhering to components of the extracellular matrix. Data obtained in this study demonstrated that expression of the Bap protein blocked the activity of two MSCRAMM proteins of S. aureus and that the colonization capacity of Bap-expressing strains was reduced. Reduced adherence of Bap-positive strain V329 suggests that a nonspecific interaction between Bap and the functional domains of the MSCRAMMs occurs. This could be produced by a steric impediment caused directly by Bap or indirectly by the higher accumulation of extracellular polysaccharides that occurs in the presence of Bap. Two facts are in disagreement with the second possibility. First, Bap-positive strain V329 is a poor in vitro producer of PIA/PNSG (7), and second, the uncharacterized capsule-like material observed on the surface seems too thin and irregular to block the activity of the staphylococcal surface proteins. It is therefore more likely that Bap causes steric hindrance, as in the proposed model to explain the blocking activity of antigen 43 (Ag43) in autoaggregation by fimbria expression in E. coli (18). It has been postulated that fimbriation abolishes Ag43-mediated autoaggregation but does not affect Ag43 expression. Autoaggregation requires an intercellular Ag43-Ag43 interaction, and the presence of fimbriae on the cells seems to inhibit this. With regard to these results, Bap shows an intriguing feature, namely, the presence of dimerization domains for residues 227 to 309 and residues 528 to 548, according to the Coiled-Coil prediction program of Lupas et al. (23). Thus, two Bap molecules of the same bacterium could undergo dimerization, which could directly affect the interaction of Bap with the exopolysaccharides of the biofilm, or two Bap molecules of different bacteria could undergo dimerization, which would induce intercellular adhesion.
In previous studies with specific mutants lacking MSCRAMM proteins, the role of these proteins in the pathogenesis of S. aureus infections was sometimes not clear. The presence of other adhesins on the S. aureus surface that can complement the activity in the deficient mutant could explain these results. However, as in the S. aureus sortase mutants defective in the display of many surface proteins (24), inhibition of MSCRAMM proteins by Bap was correlated with a decreased capacity to infect the mammary gland (suckling was delayed for 2 h after infection), although adherence to epithelial cells in the wild type was the same as that in the Bap mutant. This attachment implies that structures other than FnBPs could be involved in this process. An extracellular module, HYR, that is involved in cellular adhesion in several eukaryotic and bacterial proteins has been described (4). An HYR domain is present in the C repeats of Bap. However, the different capacities of Bap-positive and Bap-negative strains to invade indicate that adherence to epithelial cells is not the most important step in the colonization process for these strains. Possibly, direct adherence to connective tissue after epithelial damage caused by milking or by action of bacterial toxins is more relevant in the initial phase of the infection.
In contrast with the limited value that Bap appears to have in promoting early (at 2 h) adherence, this protein has been shown to play a significant role in establishing long-lasting infections in a mouse model and in making biofilms in studies of late in vitro adherence (7). Interestingly, animals infected with Bap-positive strains show a decreased number of somatic (phagocytic) cells in milk compared to animals naturally infected with Bap-negative strains (C. Cucarella et al., unpublished data). This suggests that the natural increase in milk somatic cell counts occurring in mammary gland bacterial infections is impaired in the presence of Bap, further favoring the establishment of chronic infections. Miyamoto and coworkers demonstrated the implications of FnBPA in T-lymphocyte coactivation (27). The blockade of this protein by Bap might produce a lower stimulation of the immune system and, as a consequence, a higher persistence of Bap-positive strains in the mammary gland.
In this study, the blocking capacity of the Bap protein was observed by using stationary-phase cells. If the Bap protein was expressed in stationary phase the MSCRAMM-blocking activity would be irrelevant, because the staphylococci could colonize host tissue using their adhesins expressed earlier in the growth cycle. However, our preliminary results show that Bap is expressed in the exponential phase of growth, as are the FnBPs (35), the collagen-binding protein (15), and clumping factor B (29). Bap expressed during this phase of growth would block MSCRAMM activity, suggesting an important role for this protein in staphylococcal pathogenesis.
Recently, surface protein of methicillin-resistant S. aureus Pls has been shown to prevent the interaction of surface adhesins with its ligands in vitro (36). Pls contains three different types of repeat sequences and presents high homology with Aap, an S. epidermidis surface protein involved in biofilm formation on glass or polystyrene surfaces (GenBank accession no. CAB77251). Interestingly, we have recently shown that a surface protein of Enterococcus faecalis named Esp, which has high global structural similarity to Bap, is also involved in biofilm formation (43). Therefore, four surface proteins, Bap, Esp, Pls, and Aap, of three gram-positive bacteria have two common features: (i) presence of extensive repeat regions and (ii) absence of a known adhesin ligand. In addition, Bap, Esp, and Aap are required for biofilm formation and Bap and Pls (the only two that have been studied in this respect) have an antiadhesin function. The key question to address now is how the interactions between MSCRAMMs, antiadhesins, and exopolysaccharides are spatially (at the surface of the bacteria) and temporally (during the infection process) coordinated to help bacteria adhere to tissues and structures of the host or allow spreading of the cells during the infection process.
Acknowledgments
This work was supported by grant BIO99-0285 from the Comisión Interministerial de Ciencia y Tecnología (C.I.C.Y.T.) and grants from the Cardenal Herrera-CEU University to J.R.P. and from the Wellcome Trust to T.J.F. Fellowship support for C. Cucarella and M. Á. Tormo from the Cardenal Herrera-CEU University is gratefully acknowledged. J. R. Penadés was partially supported by the “Conselleria de Cultura, Educació i Ciència” of the Generalitat Valenciana (Spain).
Editor: E. I. Tuomanen
REFERENCES
- 1.Baldassarri, L., G. Donelli, A. Gelosia, A. W. Simpson, and G. D. Christensen. 1997. Expression of slime interferes with in vitro detection of host protein receptors of Staphylococcus epidermidis. Infect. Immun. 65:1522-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baselga, R., I. Albizu, M. de la Cruz, E. del Cacho, M. Barberan, and B. Amorena. 1993. Phase variation of slime production in Staphylococcus aureus: implication in colonization and virulence. Infect. Immun. 61:4857-4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brückner, R. 1997. Gene replacement in Staphylococcus carnosus and Staphylococcus xylosus. FEMS Microbiol. Lett. 151:1-8. [DOI] [PubMed] [Google Scholar]
- 4.Callebaut, I., D. Gilgès, I. Vigon, and J. P. Mornon. 2000. HYR, an extracellular module involved in cellular adhesion and related to the immunoglobulin-like fold. Protein Sci. 9:1382-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 6.Cramton, S. E., C. Gerke, N. F. Schnell, W. W. Nichols, and F. Götz. 1999. The intercellular adhesion (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infect. Immun. 67:5427-5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cucarella, C., C. Solano, J. Valle, B. Amorena, I. Lasa, and J. R. Penadés. 2001. Bap, a Staphylococcus aureus surface protein involved in biofilm formation. J. Bacteriol. 183:2888-2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duthie, E. S., and L. L. Lorenz. 1952. Staphylococcal coagulase: mode of action and antigenicity. J. Gen. Microbiol. 6:95-107. [DOI] [PubMed] [Google Scholar]
- 9.Dziewanowska, K., J. M. Patti, C. F. Deobald, K. W. Bayles, W. R. Trumble, and G. A. Bohach. 1999. Fibronectin binding protein and host cell tyrosine kinase are required for internalization of Staphylococcus aureus by epithelial cells. Infect. Immun. 67:4673-4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fassel, T. A., and C. E. Edmiston. 1999. Bacterial biofilms: strategies for preparing glycocalix for electron microscopy. Methods Enzymol. 310:194-203. [DOI] [PubMed] [Google Scholar]
- 11.Favre-Bonte, S., B. Joly, and C. Forestier. 1999. Consequences of reduction of Klebsiella pneumoniae capsule expression on interactions of this bacterium with epithelial cells. Infect. Immun. 67:554-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flock, J. I., G. Froman, K. Jonsson, B. Guss, C. Signas, B. Nilsson, G. Raucci, M. Hook, T. Wadstrom, and M. Lindberg. 1987. Cloning and expression of the gene for a fibronectin-binding protein from Staphylococcus aureus. EMBO J. 6:2351-2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foster, T. J., and M. Höök. 1998. Surface protein adhesins of Staphylococcus aureus. Trends Microbiol. 6:484-488. [DOI] [PubMed] [Google Scholar]
- 14.Gillaspy, A. F., C. Y. Lee, S. Sau, A. L. Cheung, and M. S. Smeltzer. 1998. Factors affecting the collagen binding capacity of Staphylococcus aureus. Infect. Immun. 66:3170-3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gillaspy, A. F., J. M. Patti, and M. S. Smeltzer. 1997. Transcriptional regulation of the Staphylococcus aureus collagen adhesin gene, cna. Infect. Immun. 65:1536-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hagman, M. M., J. B. Dale, and D. L. Stevens. 1999. Comparison of adherence to and penetration of a human laryngeal epithelial cell line by group A streptococci of various M protein types. FEMS Immunol. Med. Microbiol. 23:195-204. [DOI] [PubMed] [Google Scholar]
- 17.Hartford, O., P. Francois, P. Vaudaux, and T. J. Foster. 1997. The dipeptide repeat region of the fibrinogen-binding protein (clumping factor) is required for functional expression of the fibrinogen-binding domain on the Staphylococcus aureus cell surface. Mol. Microbiol. 25:1065-1076. [DOI] [PubMed] [Google Scholar]
- 18.Hasman, H., T. Chakraborty, and P. Klemm. 1999. Antigen-43-mediated autoaggregation of Escherichia coli is blocked by fimbriation. J. Bacteriol. 181:4834-4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heilmann, C., O. Schweitzer, C. Gerke, N. Vanittanakom, D. Mack, and F. Götz. 1996. Molecular basis of intercellular adhesion in the biofilm-forming Staphylococcus epidermidis. Mol. Microbiol. 20:1083-1091. [DOI] [PubMed] [Google Scholar]
- 20.Jonsson, K., C. Signas, H. P. Muller, and M. Lindberg. 1991. Two different genes encode fibronectin binding proteins in Staphylococcus aureus. The complete nucleotide sequence and characterization of the second gene. Eur. J. Biochem. 202:1041-1048. [DOI] [PubMed] [Google Scholar]
- 21.Jonsson, P., M. Lindberg, I. Haraldsson, and T. Wadstrom. 1985. Virulence of Staphylococcus aureus in a mouse mastitis model: studies of alpha hemolysin, coagulase, and protein A as possible virulence determinants with protoplast fusion and gene cloning. Infect. Immun. 49:765-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kreiswirth, B. N., S. Lofdahl, M. J. Betley, M. O'Reilly, P. M. Schlievert, M. S. Bergdoll, and R. P. Novick. 1983. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature 305:709-712. [DOI] [PubMed] [Google Scholar]
- 23.Lupas, A., M. Van Dyke, and J. Stock. 1991. Predicting coiled coils from protein sequences. Science 252:1162-1164. [DOI] [PubMed] [Google Scholar]
- 24.Mazmanian, S. K., G. Liu, E. R. Jensen, E. Lenoy, and O. Schneewind. 2000. Staphylococcus aureus sortase mutants defective in the display of surface proteins and in the pathogenesis of animal infections. Proc. Natl. Acad. Sci. USA 97:5510-5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McDevitt, D., P. Francois, P. Vaudaux, and T. J. Foster. 1994. Molecular characterization of the clumping factor (fibrinogen receptor) of Staphylococcus aureus. Mol. Microbiol. 11:237-248. [DOI] [PubMed] [Google Scholar]
- 26.McKenney, D., K. L. Pouliot, Y. Wang, V. Murthy, M. Ulrich, G. Döring, J. C. Lee, D. A. Goldmann, and G. B. Pier. 1999. Broadly protective vaccine for Staphylococcus aureus based on an in vivo-expressed antigen. Science 284:1523-1527. [DOI] [PubMed] [Google Scholar]
- 27.Miyamoto, Y. J., E. R. Wann, T. Fowler, E. Duffield, M. Höök, and B. W. McIntyre. 2001. Fibronectin binding protein A of Staphylococcus aureus can mediate human T lymphocyte adhesion and coactivation. J. Immunol. 166:5129-5138. [DOI] [PubMed] [Google Scholar]
- 28.Moreillon, P., J. M. Entenza, P. Francioli, D. McDevitt, T. J. Foster, P. Francois, and P. Vaudaux. 1995. Role of Staphylococcus aureus coagulase and clumping factor in pathogenesis of experimental endocarditis. Infect. Immun. 63:4738-4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ni Eidhin, D., S. Perkins, P. Francois, P. Vaudaux, M. Höök, and T. J. Foster. 1998. Clumping factor B (ClfB), a new surface-located fibrinogen-binding adhesin of Staphylococcus aureus. Mol. Microbiol. 30:245-257. [DOI] [PubMed] [Google Scholar]
- 30.Okada, N., A. P. Pentland, P. Falk, and M. G. Caparon. 1994. M protein and protein F act as important determinants of cell-specific tropism of Streptococcus pyogenes in skin tissue. J. Clin. Investig. 94:965-977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel, A. H., P. Nowlan, E. D. Weavers, and T. J. Foster. 1987. Virulence of protein A-deficient and alpha-toxin-deficient mutants of Staphylococcus aureus isolated by allele replacement. Infect. Immun. 55:3103-3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peacock, S. J., N. P. Day, M. G. Thomas, A. R. Berendt, and T. J. Foster. 2000. Clinical isolates of Staphylococcus aureus exhibit diversity in fnb genes and adhesion to human fibronectin. J. Infect. 41:23-31. [DOI] [PubMed] [Google Scholar]
- 33.Pöhlmann-Dietze, P., M. Ulrich, K. B. Kiser, G. Döring, J. C. Lee, J. M. Fournier, K. Botzenhart, and C. Wolz. 2000. Adherence of Staphylococcus aureus to endothelial cells: influence of capsular polysaccharide, global regulator agr, and bacterial growth phase. Infect. Immun. 68:4865-4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Runnels, P. L., and H. W. Moon. 1984. Capsule reduces adherence of enterotoxigenic Escherichia coli to isolated intestinal epithelial cells of pigs. Infect. Immun. 45:737-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saravia-Otten, P., H. P. Müller, and S. Arvidson. 1997. Transcription of Staphylococcus aureus fibronectin binding protein genes is negatively regulated by agr and an agr-independent mechanism. J. Bacteriol. 179:5259-5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Savolainen, K., L. Paulin, B. Westerlund-Wikström, T. J. Foster, T. K. Korhonen, and P. Kuusela. 2001. Expression of pls, a gene closely associated with the mecA gene of methicillin-resistant Staphylococcus aureus, prevents bacterial adhesion in vitro. Infect. Immun. 69:3013-3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sherertz, R. J., W. A. Carruth, A. A. Hampton, M. P. Byron, and D. D. Solomon. 1993. Efficacy of antibiotic-coated catheters in preventing subcutaneous Staphylococcus aureus infection in rabbits. J. Infect. Dis. 167:98-106. [DOI] [PubMed] [Google Scholar]
- 38.Sinha, B., P. P. François, O. Nübe, M. Foti, O. M. Hartford, P. Vaudaux, T. J. Foster, D. P. Lew, M. Herrmann, and K. H. Krause. 1999. Fibronectin-binding protein acts as Staphylococcus aureus invasin via fibronectin bridging to integrin α5β1. Cell. Microbiol. 1:101-117. [DOI] [PubMed] [Google Scholar]
- 39.Sjodahl, J. 1977. Repetitive sequences in protein A from Staphylococcus aureus. Arrangement of five regions within the protein, four being highly homologous and Fc-binding. Eur. J. Biochem. 73:343-351. [DOI] [PubMed] [Google Scholar]
- 40.Stephens, D. S., P. A. Spellman, and J. S. Swartley. 1993. Effect of the (alpha 2→8)-linked polysialic acid capsule on adherence of Neisseria meningitidis to human mucosal cells. J. Infect. Dis. 167:475-479. [DOI] [PubMed] [Google Scholar]
- 41.St. Geme, J. W., III, and S. Falkow. 1991. Loss of capsule expression by Haemophilus influenzae type b results in enhanced adherence to and invasion of human cells. Infect. Immun. 59:1325-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Talbot, U. M., A. W. Paton, and J. C. Paton. 1996. Uptake of Streptococcus pneumoniae by respiratory epithelial cells. Infect. Immun. 64:3772-3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toledo-Arana, A., J. Valle, C. Solano, M. J. Arrizubieta, C. Cucarella, M. Lamata, B. Amorena, J. Leiva, J. R. Penadés, and I. Lasa. 2001. The enterococcal surface protein, Esp, is involved in Enterococcus faecalis biofilm formation. Appl. Environ. Microbiol. 67:4538-4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vaudaux, P. E., P. Francois, R. A. Proctor, D. McDevitt, T. J. Foster, R. M. Albrecht, D. P. Lew, H. Wabers, and S. L. Cooper. 1995. Use of adhesion-defective mutants of Staphylococcus aureus to define the role of specific plasma proteins in promoting bacterial adhesion to canine arteriovenous shunts. Infect. Immun. 63:585-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wann, E. R., S. Gurusiddappa, and M. Höök. 2000. The fibronectin-binding MSCRAMM FnbpA of Staphylococcus aureus is a bifunctional protein that also binds to fibrinogen. J. Biol. Chem. 275:13863-13871. [DOI] [PubMed] [Google Scholar]
- 46.Wesson, C. A., L. E. Liou, K. M. Todd, G. A. Bohach, W. R. Trumble, and K. W. Bayles. 1998. Staphylococcus aureus Agr and Sar global regulators influence internalization and induction of apoptosis. Infect. Immun. 66:5238-5243. [DOI] [PMC free article] [PubMed] [Google Scholar]