Abstract
The invasive stages of apicomplexan parasites enter their host cells through mechanisms which are largely conserved throughout the phylum. Host cell invasion is divided into two distinct events, namely, adhesion onto the host cell surface and the actual host cell entry process. The former is mediated largely through microneme proteins which are secreted at the onset of establishing contact with the host cell surface. Many of the microneme proteins identified so far contain adhesive domains. We here present the genomic and corresponding cDNA sequences coding for a 460-amino-acid (aa) microneme protein in Neospora caninum tachyzoites which, due to its homology to MIC1 in Toxoplasma gondii (TgMIC1), was named NcMIC1. The deduced NcMIC1 polypeptide sequence contains an N-terminal signal peptide of 20 aa followed by two tandemly internal repeats of 48 and 44 aa, respectively. Integrated into each repeat is a CXXXCG sequence motif reminiscent of the thrombospondin-related family of adhesive proteins. The positioning of this motif is strictly conserved in TgMIC1 and NcMIC1. The C-terminal part, comprised of 278 aa, was expressed in Escherichia coli, and antibodies affinity purified on recombinant NcMIC1 were used to confirm the localization within the micronemes by immunofluorescence and immunogold transmission electron microscopy of tachyzoites. Immunohistochemistry of mouse brains infected with tissue cysts showed that expression of this protein is reduced in the bradyzoite stage. Upon initiation of secretion by elevating the temperature to 37°C, NcMIC1 is released into the medium supernatant. NcMIC1 binds to trypsinized, rounded Vero cells, as well as to Vero cell monolayers. Removal of glycosaminoglycans from the host cell surface and modulation of host cell surface glycosaminoglycan sulfation significantly reduces the binding of NcMIC1 to the host cell surface. Solid-phase binding assays employing defined glycosaminoglycans confirmed that NcMIC1 binds to sulfated glycosaminoglycans.
Neospora caninum (10), is an apicomplexan parasite which has attracted considerable attention as an important cause of bovine abortion and neuromuscular disease in cattle and dogs (for reviews, see references 11, 20, and 21). Although it shares many biological features with the closely related species Toxoplasma gondii, N. caninum is antigenically quite distinct, and N. caninum tachyzoites can be distinguished from T. gondii through unique ultrastructural criteria, antibodies directed against immunodominant proteins and specific PCR assays (20). The life cycle of N. caninum is comprised of three distinct stages, namely, (i) the rapidly proliferating tachyzoite, (ii) the very slowly proliferating bradyzoite, and (iii) the product of a sexual process which takes place within the intestine of the dog (or possibly another definitive host), which, upon sporulation following oocyst formation, results in sporozoite formation (26). Oral infection of an immunocompetent host through either sporozoite-containing oocysts or bradyzoite-containing tissue cysts is normally not accompanied by clinical signs. It is likely that during pregnancy tachyzoites are transmitted congenitally from the mother to the fetus.
During the course of its life cycle, N. caninum, like all apicomplexan parasites, must enter host cells in order to survive and proliferate. The parasite exhibits a very low host cell specificity, as it is capable of invading a wide range of tissues and cell types (20, 23). This process is dependent on the initial recognition of, and adhesion onto, the host cell surface, followed by the actual host cell entry process (16). Secretory proteins which are sequentially released during host cell adhesion and invasion originate from micronemes, rhoptries, and dense granules, respectively (3, 5, 7, 8, 12, 32). Among the secretory proteins, microneme proteins have been shown to be released early at the onset of adhesion onto the host cell surface (34). These molecules contribute largely in consolidating the physical interaction between parasite and host cell, through the functional involvement of potentially adhesive domains, which include mucin-like domains of Cryptosporidium parvum, DBL domains in Plasmodium merozoites, Cys-rich regions on microneme proteins of Theileria parva, thrombospondin (TSP) type I regions, integrin insertion domains, epidermal growth factor-like domains, and Apple domains (for reviews, see references 33 and 35). Microneme secretion has been extensively studied in T. gondii, where these organelles discharge by fusing with the apical tip of the parasite, thus delivering their content to the apical surface. The discharge of Toxoplasma micronemes was shown to be regulated by cytoplasmic Ca2+ (4). In T. gondii, up to 10 microneme proteins have been identified and at least partially characterized to date, and most but not all of them have potential adhesive functions (33, 35).
In N. caninum, three distinct microneme proteins have been characterized at the molecular level so far. The first was NcMIC2 (25), which is homologous to TgMIC2 from T. gondii and contains integrin- and TSP-like domains and thus represents a member of the TSP family of adhesive proteins (28). The secretion of this protein is dependent on the mobilization of intracellular Ca2+ stores. The second microneme protein is NcMIC3 (34). Secretion of NcMIC3 onto the parasite surface is rapidly induced upon liberation of tachyzoites from their host cells, and the protein remains bound to the parasite surface for extended periods of time. Binding of NcMIC3 onto the host cell surface was shown to be mediated through its four consecutive epidermal growth factor-like domains (27). Third, NcMIC10 was identified by Hoff et al. (22) according to its sequence similarity to TgMIC10. These two proteins do not possess any adhesive domains, and their putative function is largely unknown.
In this paper, we report on the identification, genomic sequence, cDNA cloning, and deduced polypeptide sequence of a microneme protein (NcMIC1) in N. caninum which represents a homologue of T. gondii MIC1 (14). Secreted NcMIC1 is released by the parasite as a soluble protein, and the interaction between NcMIC1 and the Vero host cell surface was assessed.
MATERIALS AND METHODS
Unless otherwise stated, all reagents and tissue culture media were purchased from Sigma (St. Louis, Mo.).
Tissue culture, parasite purification, and parasite-infected brain tissue.
Cultures of Vero cells were maintained in RPMI 1640 medium (Gibco-BRL, Basel, Switzerland) supplemented with 7% fetal calf serum, 2 mM glutamine, 50 U of penicillin ml−1, and 50 μg of streptomycin ml−1 at 37°C with 5% CO2 in T-25 tissue culture flasks. Cultures were trypsinized at least once a week. N. caninum tachyzoites of the Nc-1 isolate were used (10) and cultured as described previously (16-19, 34). Paraffin blocks of tissue harboring N. caninum bradyzoites had been used in previous studies (34) and were kindly provided by Milton McAllister, University of Illinois, Urbana.
Detergent extraction of tachyzoites, SDS-PAGE, immunoblotting, and affinity purification of antibodies.
Purified N. caninum tachyzoites were subjected to fractionation with the nonionic detergent Triton X-114 or Triton X-100 as previously described (17). Samples were precipitated in methanol-chloroform (37), and equal amounts, corresponding to the same number of tachyzoites, were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing and nonreducing conditions, respectively. Transfer onto nitrocellulose filters was carried out as previously described (17). After blocking of nonspecific binding sites in Tris-buffered saline-3% bovine serum albumin (BSA)-0.3% Tween 20, blots were labeled either with anti-N. caninum antiserum diluted 1:2,000 in Tris-buffered saline-0.3% BSA-0.3% Tween (antibody dilution buffer) or with affinity-purified antibodies at a dilution of 1:30 to 1:50. Bound antibodies were visualized using goat anti-rabbit-alkaline phosphatase conjugates (Promega) according to the instructions provided by the manufacturer. Affinity purification of antibodies directed against a 60-kDa band in detergent extracts (NcMIC1) and recombinant protein expressed in Escherichia coli (recNcMIC1) (see below) was performed as previously described (17, 34).
Immunoscreening of a λgt22 N. caninum tachyzoite cDNA library, PCR, and cDNA sequencing.
The production of an N. caninum tachyzoite cDNA expression library in the phage λgt22A has been reported previously (17, 34). Immunoscreening was performed using affinity-purified antibodies directed against a 60-kDa band in Triton X-114 membrane protein fractions which reacted with the lectins concanavalin A, wheat germ agglutinin, and Jacalin, at a dilution of 1:50 in antibody dilution buffer. Reactive clones were purified through repeated screening. Following isolation of pure plaque populations, the length of the cDNA inserts was determined by PCR amplification in a thermal cycler (Perkin-Elmer Cetus, Rotkreuz, Switzerland). The Expand High Fidelity PCR System (Roche) was used for carrying out all PCRs. Primers were derived from the 5′ flanking sequence on the sense strand (primer 1218B, 5′-GCG GAT CCG GTG GCG ACG ACT CCT GGA GCC CG-3′) and the 3′ flanking sequence on the antisense strand (primer 1222H, 5′-GCA AGC TTT TGA CAC CAG ACC AAC TGG TAA TG-3′) of the λgt22A vector (34). The primers were purchased from Gibco-BRL. PCR products were electrophoretically analyzed on 1% agarose gels and were purified using the Wizard PCR purification kit (Promega). The PCR fragments were subcloned into the pGEM-T Easy vector (Promega), and the cDNA sequences of the inserts were obtained by direct sequencing of purified PCR products by an automated sequencing service provided by Microsynth, Balgach, Switzerland. One of these fragments (1,500 bp) contained a cDNA sequence coding for the C-terminal portion of a protein which was closely related to the microneme protein TgMIC1 (14)
A cDNA fragment which contained the 5′ end of the corresponding cDNA was obtained by performing PCR using the cDNA expression library as a template, with the forward primer 1218B (5′-GCG GAT CCG GTG GCG ACG ACT CCT GGA GCC CG-3′) and the reverse primers p60revH1 (5′-GCA AGC TTC TGA TCC CTC GGG AGG CAA ACA AG-3′), located about 200 bp downstream. This resulted in a PCR product consisting of 900 bp. For amplification of the full-length NcMIC1 cDNA from the library, the forward primer NcMIC1SigPepB (5′-GCG GAT CCA TGG GCC AGT CGG TGG TTT TCG TC-3′) and reverse primer NcMIC1revH (5′-GCA AGC TTT TAC AAT TCA GAT TCA CCC GGA GA-3′) were used. The resulting PCR product had a size of 1,380 bp. The PCR fragments were subcloned into the pGEM-T Easy vector and were sequenced as described above. Each fragment was sequenced two or three times in both the sense and antisense directions in order to verify the sequence information. Sequencing revealed the presence of an open reading frame coding for a protein of 460 amino acids (aa).
Isolation of N. caninum DNA and sequencing of the NCMIC1 gene.
In order to verify the NcMIC1 sequence at the genomic level, Neospora DNA isolation was performed (34). The entire NCMIC1 gene was amplified by PCR with the forward primer NcMIC1SigPepB and reverse primer NcMIC1revH. This resulted in a 2,300-bp fragment, which was cloned into the pGEM Easy vector and sequenced as described above.
NcMIC1 cDNA and deduced polypeptide sequence analysis.
Sequences were processed and aligned by performing BLAST searches on nonredundant SWISS-PROT database sequences. For both TgMIC1 (accession no. Z71786) and NcMIC1, the presence of potential transmembrane domains was searched for using TopPred 2 (http:/www.biokemi.su.se/server/toppred2 [Stockholm University]). N-terminal signal peptides and internal repeats were identified through SMART (Simple Modular Architecture Research Tool) at the EMBL (Heidelberg, Germany) (http://www.smart.embl-heidelberg.de/smart/), potential N-glycosylation sites were identified through the Scan Prosite Tool (http://www.expasy.ch/tools/scnpsite.html), and the protein motifs or fingerprint searches were provided by the EMBL Outstation European Bioinformatics Institute (http://www2.ebi.ac.uk/ppsearch/ and http://www2.ebi.ac.uk/servicestmp/20688.html).
Expression of the C-terminal domain of NcMIC1 (recNcMIC1) in E. coli.
By using a 5′ SalI site (provided by the phage vector λgt22A) and a 3′ HindIII site (introduced via the primer 1222H), the PCR-amplified partial C-terminal cDNA fragment was inserted in frame into the XhoI- and HindIII-digested expression vector pTRC-HisA (Promega). The cloned sequence was expressed in E. coli as polyhistidine (His6) fusion proteins (34). Bacteria were harvested by centrifugation, and the pellet was solubilized in sample buffer and processed for SDS-PAGE and immunoblotting.
Immunofluorescence.
Immunolabeling of NcMIC1 in cell culture-derived N. caninum tachyzoites was carried out using affinity-purified antibodies directed against recNcMIC1 at a dilution of 1:2 in phosphate-buffered saline (PBS)-0.5% BSA. Cell culture, fixation, and processing have been described previously (17, 27, 34). Immunofluorescence labeling on paraffin-embedded mouse brain tissue infected with N. caninum bradyzoites was carried out as described by Sonda et al. (34). For double staining, antibodies directed against recNcMIC were applied as described above, and monoclonal antibody (MAb) CC2 (15) was used at a dilution of 1:250. Specimens were inspected on a Nikon Eclipse E800 digital confocal fluorescence microscope. Processing of images was performed using the Openlab 2.0.7 software (Improvision, Heidelberg, Germany).
Immunogold TEM.
LR-White embedding and on-section labeling of N. caninum-infected Vero cell cultures were performed essentially as previously described (17, 19, 34). Sections were incubated in affinity-purified antibodies directed against recNcMIC1 diluted 1:2 in electron microscopy (EM) blocking buffer (PBS-1% BSA) for 1 h. As a negative control, incubations with an affinity-purified rabbit anti-β-galactosidase antibody and with the preimmune serum of the anti-N. caninum antiserum were performed. After washing in five changes of PBS (2 min each), the goat anti-rabbit antibody conjugated to 10-nm-diameter gold particles (purchased from Amersham, Zurich, Switzerland) was applied. Finally, grids were stained with lead citrate and uranyl acetate (18) and were subsequently viewed on a Hitachi 600 transmission EM (TEM) operating at 100 kV.
In vitro secretion assay.
Secretion assays were carried out as described by Naguleswaran et al. (27), by subjecting purified tachyzoites (5 × 107/ml) in Earle's balanced salt solution (EBSS) to a temperature of 37°C for 10 min. After the incubation, parasites were centrifuged at 2,000 × g, and the supernatant was collected and centrifuged again at 10,000 × g for 30 min at 4°C. The parasite pellet and the supernatant resulting from the second centrifugation were processed for SDS-PAGE, and equal amounts were analyzed by immunoblotting with affinity-purified antibodies directed against recNcMIC1 (see above). Prior to binding experiments (see below), supernatants were stored at −80°C.
In vitro binding of NcMIC1 to prefixed host cells.
The interaction between NcMIC1 and Vero cells was studied either by coprecipitation assays or by incubation of either Triton X-100 or secreted supernatants with host cell monolayers. Coprecipitation assays were performed as described by Naguleswaran et al. (27). Freshly trypsinized nonadherent Vero cells were fixed in 2.5% glutaraldehyde in EBSS for 30 min, followed by postfixation in 0.5% OsO4 in 100 mM sodium phosphate buffer, pH 7.2. Subsequently, free aldehyde groups were blocked by incubation in 100 mM ethanolamine (pH 8) at 4°C overnight, and nonspecific binding sites were blocked by incubating the cells in PBS-1.5% BSA for 2 h at room temperature. Triton X-100 extracts containing N. caninum proteins were prepared by incubating 5 × 108 parasites in 2 ml of PBS containing 1% Triton X-100 at 4°C for 5 min, followed by centrifugation at 10,000 × g for 20 min at 4°C (27). Prefixed Vero cells (106) were then incubated in 250 μl of Triton X-100 extracts for 2 h at 4°C. Subsequently the preparations were centrifuged at 10,000 × g for 5 min, and the supernatants were collected and processed for SDS-PAGE and immunoblotting. The pellets were washed in PBS three times and were finally taken up in SDS-PAGE sample buffer. Equal amounts of nonbound (supernatants) and bound (pellets) proteins were loaded onto gels. Immunoblotting was performed as described above.
For assays of binding to adherent cells, freshly trypsinized Vero cells were grown at 37°C with 5% CO2 in 96-well tissue culture plates at 105 cells/well. In some experiments, cells were treated either with 2 mM p-nitrophenyl-β-xylopyranoside (β-xyloside) for 48 h to inhibit the addition of glycosaminoglycans to surface proteoglycan core proteins or with 60 mM NaClO3 for 24 h to reduce cell surface proteoglycan sulfation prior to fixation. The cells were then washed three times with ice-cold PBS and fixed in 2.5% glutaraldehyde in EBSS for 30 min at 4°C. Subsequently, free aldehyde groups were blocked by incubation in 100 mM ethanolamine (pH 8) at 4°C overnight. Prior to use, monolayers were washed in PBS, and nonspecific binding sites were blocked in PBS-1.5% BSA for 1 h at room temperature. The cells were then incubated either with freshly purified N. caninum tachyzoites (105/well) or with the secreted fraction of N. caninum tachyzoites (see above) at 100 μl/well for 2 h at room temperature, followed by three washes in PBS. Adherent parasites were detected following fixation and blocking of nonspecific binding sites with a MAb directed against the major immunodominant tachyzoite antigen NcSRS2 (27), and bound NcMIC1 was detected using affinity-purified anti-recNcMIC1 antibodies (diluted 1:50 in antibody dilution buffer), followed by the respective secondary antibodies conjugated to alkaline phosphatase. Antibody binding was visualized by using p-nitrophenylphosphate as a substrate and measuring absorbance values at 405 nm on a Dynatech MR7000 enzyme-linked immunosorbent assay (ELISA) reader. Each assay was carried out in quadruplicate, and the outcome of one representative experiment of at least three independent experiments producing virtually identical results is shown.
Solid-phase binding assays involving defined glycosaminoglycans.
Ninety-six-well plates were coated with 5 mg of heparin, heparan sulfate, dextran, dextran sulfate, or chondroitin sulfate A, B, or C per ml for 12 h at 4°C. ELISA plates were then washed and incubated with 100 μl of N. caninum secreted fraction for 2 h at 4°C. Specimens were then washed in PBS, and nonspecific binding sites were blocked for 2 h in PBS-1.5% BSA. Antibody labeling and detection of bound antibodies were performed as described above.
The nucleotide sequence data reported in this paper are available in the EMBL, GenBank, and DDJB databases under accession number AF421187.
RESULTS
Identification and molecular characterization of the gene coding for NcMIC1.
We had earlier reported on the identification of glycoproteins on the surface of N. caninum tachyzoites by using lectin blotting. One of the protein bands in a Triton X-100-soluble tachyzoite extract which was reactive with concanavalin A, wheat germ agglutinin, and Jacalin following lectin blotting had an Mr of 60,000. This band largely comigrated with an immunoreactive band obtained by immunoblotting with a polyclonal anti-N. caninum antiserum (data not shown). The reactive immunoglobulins were affinity purified on this 60-kDa band, and these affinity-purified antibodies were used for immunoscreening of an N. caninum tachyzoite cDNA expression library constructed in the phage λgt22A. Following screening and PCR as outlined in Materials and Methods, the full-length cDNA coding for NcMIC1, which is comprised of 1,380 nucleotides, was obtained. The correct ATG most likely initiating translation was identified through (i) the sequence homology with the TgMIC1 gene (14) and (ii) the correct positioning of an A at position −3 and a G at position +4, which is in agreement with sequences relevant for initiation of translation. In addition, the sequence features closely resemble those previously observed for microneme proteins in T. gondii (reviewed in reference 35)
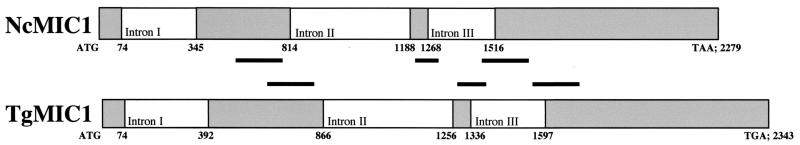
Primers NcMIC1SigPepB (binding to the 5′ end of the open reading frame) and NcMIC1revH (annealing to the 3′ end) were used for PCR amplification of the corresponding genomic DNA coding for NcMIC1. The genomic DNA fragment exhibited a significantly larger size (2.3 kb, compared to 1.3 kb for the cDNA-derived fragment [data not shown]), which was indicative of the presence of introns. Indeed, sequence analysis revealed the presence of three introns of 272, 375, and 249 nucleotides, respectively (Fig. 1).
FIG. 1.
Schematic presentation of NcMIC1 and TgMIC1 genomic sequences. The numbers refer to the overall lengths and positions of the intron-exon splice sites. The positioning of the domains of high similarity (>70%) with respect to the nucleotide sequence is indicated with black bars.
In the corresponding T. gondii gene (GenBank accession no. Z71786), the three introns exhibit slightly different lengths, i.e., 319, 391, and 262 nucleotides, respectively. Thus, with the exception of intron I, the intron-exon splicing sites are located at different points. This results in some variation with regard to the positioning of the two latter introns. The genomic DNA coding for NcMIC1 (from the start codon to the stop codon) amounts to 2,279 bp, and the corresponding gene coding for TgMIC1 consists of 2,343 bp. The NCMIC1 and TGMIC1 genes were aligned using BLASTN 2.2.1 software (National Center for Biotechnology Information), and three regions with significant percent identities (more than 70%) were identified. The first region of high similarity (comprised of 209 nucleotides and 75% identity) found on the two genomic sequences is entirely associated with the second exon in both genes (Fig. 1) and, when translated, corresponds to a 70-aa polypeptide sequence in both proteins which contains fragments of internal repeats of TgMIC1 and of NcMIC1 (corresponding to aa 100 to 170 in NcMIC1). It includes a first CXXXCG motif, indicative of the presence of a member of the TSP-like family of adhesive proteins (28) (Fig. 2). The second domain of high similarity at the genomic level (120 nucleotides and 80% identity) is comprised of both exon and intron sequences, and the coding part corresponds to a region on the two polypeptides (aa 186 to 206 in NcMIC1) which contains a second CXXXCG (thrombospondin related anonymous protein [TRAP]) motif. The third genomic sequence also contains both intron and exon sequences (200 nucleotides and 72% identity), and the coding portion corresponds to aa 207 to 252 on the NcMIC1 polypeptide.
FIG. 2.
Deduced polypeptide sequence of NcMIC1 and alignment with the homologous protein in T. gondii (TgMIC1). Hydrophobic signal peptide sequences are marked in italics. Internal repeats (TgRPT1/2 and NcRPT1/2) are underlined. Conserved cysteine residues are indicated with asterisks, potential N-glycosylation sites are marked with gggg, and CXXXCG motifs are boxed. The TSP-like domain (14) is underlined with a solid bar.
NcMIC1 cDNA and deduced polypeptide sequences.
Figure 2 shows the polypeptide sequence of NcMIC1 as deduced from the corresponding cDNA and the alignment and comparison with the corresponding T. gondii homologue, TgMIC1. The overall sequence similarity is high, with 47% identical and 63% similar amino acids shared between the two proteins. The N-terminal portion of NcMIC1 contains a hydrophobic signal peptide sequence of 20 aa, which is slightly longer than the signal peptide sequence found on TgMIC1 (17 aa). NcMIC1 contains two potential N-glycosylation sites, located at positions 119 to 122 and positions 363 to 366, which are not conserved in TgMIC1. Like for TgMIC1, two tandemly repeated domains were identified in NcMIC1 within the N-terminally located domain (aa 20 to 234) of the molecule. However, these internal repeats are significantly shorter (44 and 48 aa) than the repeats previously found in TgMIC1 (127 and 135 aa) by Fourmaux et al. (14). In addition, the repeats are partially overlapping in TgMIC1 but are clearly separated in NcMIC1.
The degree of homology within the region of these repeated domains (aa 20 to 234 in NcMIC1 and aa 17 to 236 in TgMIC1) is much higher (57% identity and 74% similarity) than that within the residual C-terminal domains of the two molecules, which share only 36% identity and 52% similarity. Another striking feature is the high degree of conservation with regard to the positioning of cysteine residues: of the 18 cysteines found in NcMIC1 and TgMIC1, all are identically positioned and 14 are located within the region comprised of the repeated domains, potentially forming intrachain disulfide bonds. This indicates that this domain within the two molecules not only is conserved through its overall polypeptide sequence but also could be structurally very closely related.
A potentially adhesive, TSP-like domain had been earlier identified in TgMIC1 as an integral part of the second internal repeat (14). This domain of 28 aa, delineated in Fig. 2, is highly conserved in NcMIC1, both with regard to its positioning and with regard to its amino acid sequence (71% identity and 85% similarity). In NcMIC1 this TSP-like sequence is not completely integrated into the tandemly repeated domains but is found to be partially located downstream of the second repeat. The TSP-associated peptide sequence CXXXCG, which is conserved throughout the phylum (28), is identically positioned and integrated into the internal repeats of both NcMIC1 and TgMIC1 (Fig. 2).
Immunolocalization of NcMIC1.
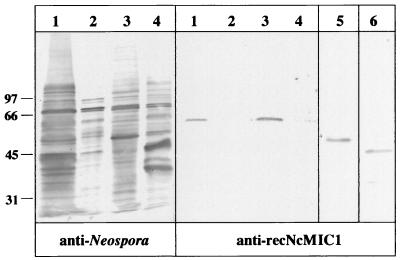
In order to obtain specific antibodies directed against NcMIC1, the insert corresponding to the original cDNA clone, corresponding to aa 182 to 460, was expressed in E. coli as a polyhistidine-tagged recombinant protein. Attempts to express larger fragments in E. coli were not successful (data not shown). Antibodies were affinity purified from a polyclonal anti-N. caninum antiserum, and the specificity of these anti-recNcMIC1 antibodies is demonstrated in Fig. 3. Upon partitioning of N. caninum tachyzoite extracts using Triton X-114, Western blot analysis employing anti-recNcMIC1 antibodies showed that NcMIC1 is sequestered almost exclusively into the Triton X-114-soluble, hydrophilic fraction. The reactive band comigrated at around 60 kDa when extracts were separated under reducing conditions but exhibited a slightly lower molecular mass of 50 kDa when separated under nonreducing conditions. The recombinant protein recNcMIC1 migrates at 45 kDa when separated under reducing conditions (Fig. 3).
FIG. 3.
Immunoblots of N. caninum tachyzoite fractions obtained by extraction of parasites with the nonionic detergent Triton X-114. SDS-PAGE was carried out under reducing conditions with whole tachyzoite extract (lanes 1), Triton X-114-insoluble proteins (lanes 2), Triton X-114-soluble hydrophilic proteins (lanes 3), and hydrophobic detergent-soluble proteins (lanes 4). Lane 5, soluble hydrophilic proteins separated under nonreducing conditions; lane 6, E. coli extract expressing recNcMIC1 separated under reducing conditions. Immunoblots were stained with either anti-N. caninum antiserum or antibodies affinity purified on recNcMIC1. Numbers on the left indicate molecular masses in kilodaltons.
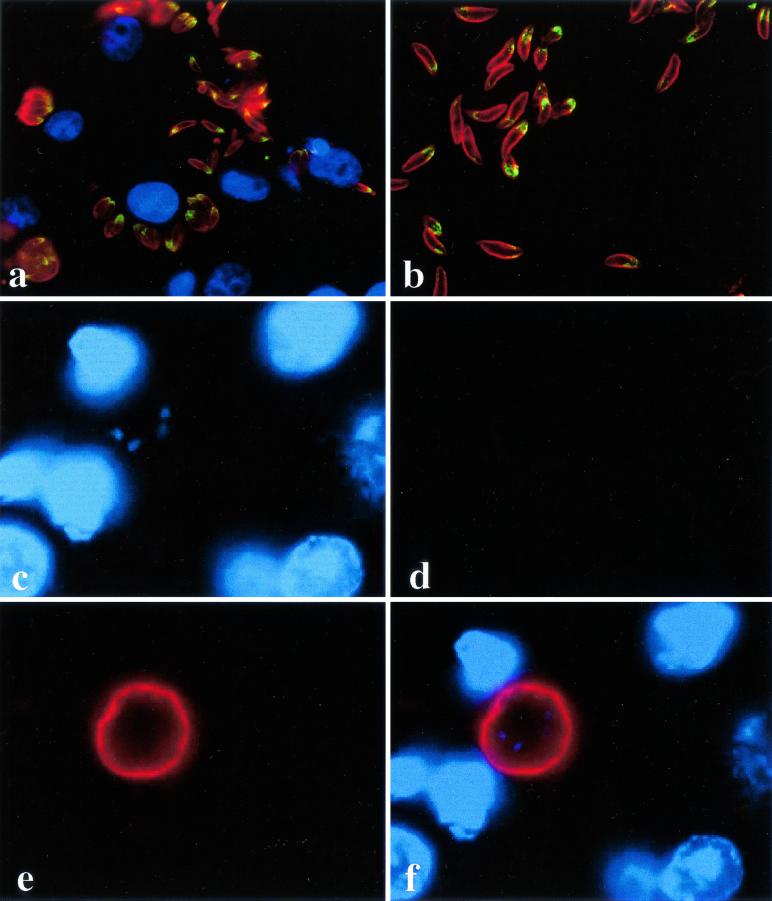
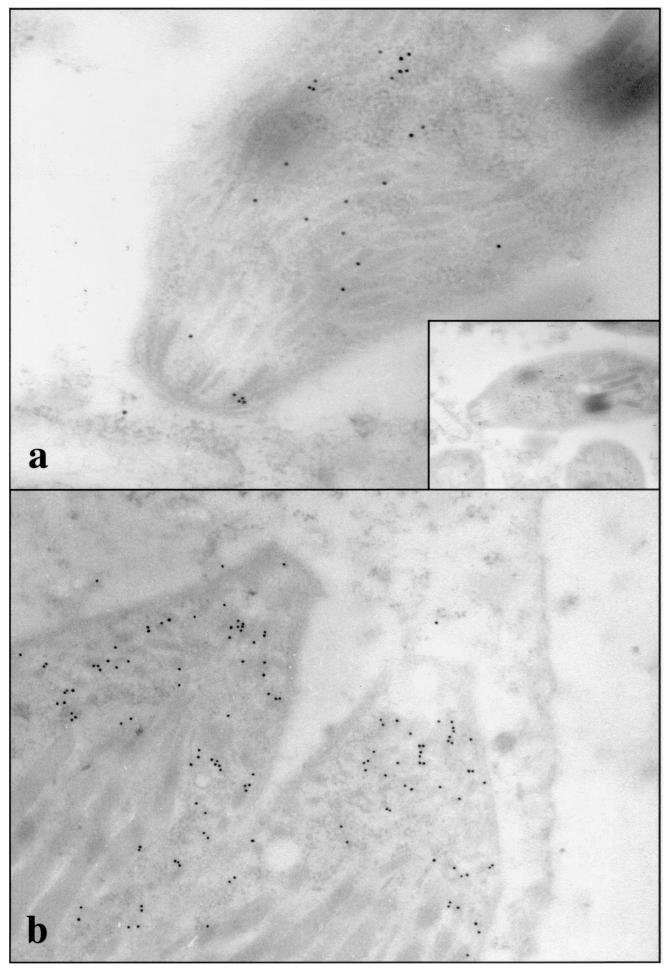
These affinity-purified antibodies were used to localize NcMIC1 in N. caninum tachyzoites. Double immunofluorescence staining, employing anti-recNcMIC1 antibodies and a polyclonal anti-N. caninum antiserum, was performed on infected Vero cells (Fig. 4a). Both intracellular parasites and tachyzoites which were in the process of liberating themselves from the host cells were stained exclusively at the apical tip. The host cells remained unstained. Apical staining was unchanged when isolated and purified N. caninum tachyzoites were labeled (Fig. 4b). Immunogold TEM analysis, performed on LR-White sections of both extracellular and intracellular tachyzoites, confirmed that NcMIC1 was indeed associated with the micronemes (Fig. 5). Anti-recNcMIC1 antibodies were employed in combination with MAb CC2 (15a), directed against a T. gondii bradyzoite cyst wall protein, to analyze expression in the bradyzoite stage in paraffin sections taken of mouse brain infected with N. caninum tissue cysts. In these cysts, MAb CC2 labeled exclusively the cyst wall, and NcMIC1 expression could no longer be observed (Fig. 4c to f).
FIG. 4.
Immunofluorescent staining of N. caninum tachyzoites obtained from in vitro cultures (infected Vero cells [a] and isolated tachyzoites [b] and of an N. caninum tissue cyst in a paraffin-embedded brain tissue section obtained from an infected mouse (c to f). Panels a and b were labeled with anti-recNcMIC1 antibodies and fluorescein isothiocyanate-conjugated anti-rabbit immunoglobulin G, followed by staining with anti-N. caninum antiserum and tetramethyl rhodamine isocyanate-conjugated anti-rabbit immunoglobulin G. In panel a, nuclei are stained with Hoechst 23558. Panel c shows DNA staining of a tissue cyst using Hoechst 23558, panel d shows the absence of staining with anti-recNcMIC1 antibodies, and panel e shows that labeling with MAb CC2 is confined to the cyst wall. In panel f, the overlay is presented.
FIG. 5.
Immunogold TEM of LR-White-embedded N. caninum tachyzoites, either located extracellularly (a) or residing within the parasitophorous vacuole (b). Sections were stained with anti-recNcMIC1 antibodies and secondary goat anti-rabbit antibodies conjugated to 10-nm-diameter gold particles. Note preferential staining of the micronemes at the apical portion of the cells.
Secretion and host cell surface binding of NcMIC1.
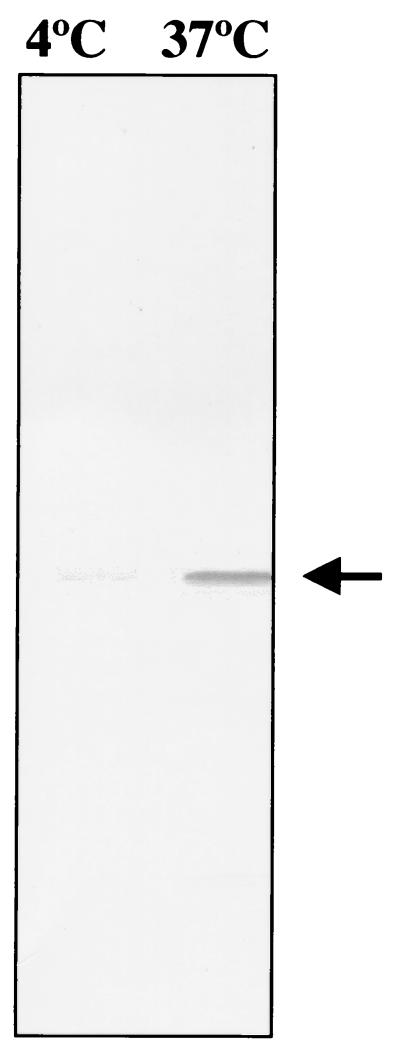
The localization of NcMIC1 within the micronemes implied that this protein would be secreted by N. caninum tachyzoites. In order to investigate this, parasites were isolated and purified at 4°C, under conditions which would not favor secretion (27), and were then incubated at 37°C for 10 min in EBSS. This procedure has previously been shown to induce the release of proteins from secretory organelles of N. caninum tachyzoites (27). Following centrifugation, the supernatant was analyzed by immunoblotting with anti-recNcMIC1 antibodies. Under these conditions, a substantial amount of NcMIC1 appeared in the supernatant compared to the control, which remained at 4°C at all times (Fig. 6).
FIG. 6.
Immunoblots of proteins which are secreted from N. caninum tachyzoites. Tachyzoites were maintained at either 4 or 37°C for 10 min in EBSS. Western blots of secreted fractions were stained with anti-recNcMIC1 antibodies.
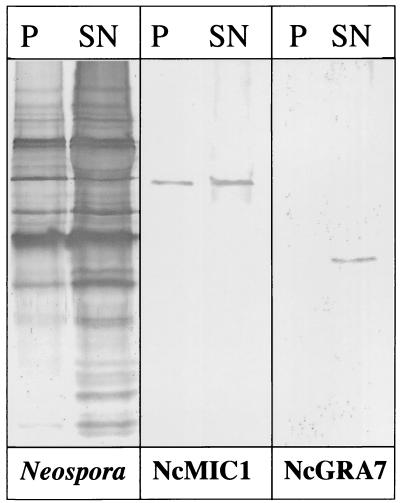
Soluble NcMIC1 was assessed for binding to the Vero cell surface by using two different assays. First, prefixed Vero cells were incubated with Triton X-100 extracts of freshly purified N. caninum tachyzoites. Following incubation of these extracts with Vero cells, coprecipitated proteins and the supernatants containing unbound proteins were analyzed by SDS-PAGE and immunoblotting (Fig. 7). Staining with the anti-N. caninum antiserum revealed that several parasite proteins readily bound to Vero cells, while others did not. NcMIC1 was largely associated with the Vero cells after centrifugation, indicating that it had the capacity to bind to the Vero cell surface. Similar results were obtained when secreted supernatants were assessed for Vero cell binding. No protein was detected in the pellet after centrifugation without addition of Vero cells (data not shown). In contrast, NcGRA7, a previously identified dense granule antigen (19), remained entirely in the supernatant following the incubation with Vero cells (Fig. 7).
FIG. 7.
Immunoblots of coprecipitation assays using prefixed Vero cells incubated in the presence of Triton X-100-soluble extracts of N. caninum tachyzoites. Immunoblots were stained with anti-N. caninum antiserum, anti-recNcMIC1 antibodies, and anti-NcGRA7 antibodies. Lanes P (pellet), fraction cosedimenting with Vero cells; lanes SN (supernatant), nonbound proteins.
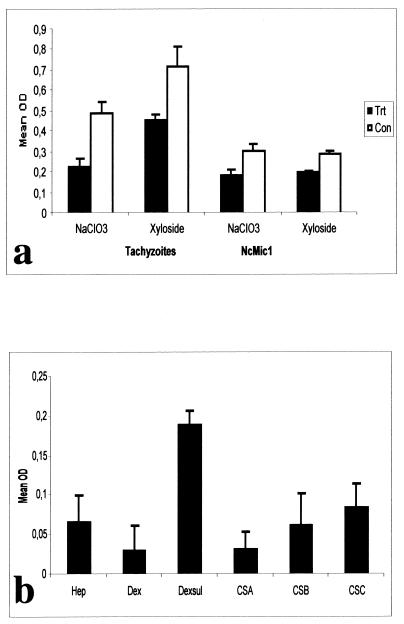
Using a second, ELISA-based approach, Vero cell monolayers were assessed for adhesion of live tachyzoites and NcMIC1 binding. These monolayers were grown in 96-well ELISA plates and were incubated with either live tachyzoites or secreted protein fractions (Fig. 8). N. caninum tachyzoites readily adhered to the Vero cell monolayers, and NcMIC1 also efficiently bound to these Vero cells, with bound NcMIC1 being readily detectable with anti-recNcMIC1 antibodies and an alkaline phosphatase conjugate. However, binding of both tachyzoites and NcMIC1 was significantly reduced when Vero cells were treated with β-xyloside, a soluble acceptor for glycosaminoglycan polymerization that competes with the endogenous proteoglycan core protein acceptor, resulting in diminished cell surface proteoglycan content (6, 30). This indicates that a substantial portion of tachyzoites, as well as of NcMIC1, associates with host surface glycosaminoglycans. Pretreatment of Vero cell monolayers with NaClO3, which interferes with sulfation reactions (6, 30), prior to incubation with secreted fractions also resulted in a significant decrease of both parasite adhesion and NcMIC1 binding (Fig. 8). Thus, N. caninum tachyzoites adhere to Vero cell monolayers largely through sulfated proteoglycans, and NcMIC1 binds to sulfated glycosaminoglycans on the host cell surface. In order to confirm these findings, secreted fractions obtained from N. caninum tachyzoites were exposed to a panel of defined sulfated or nonsulfated glycosaminoglycans. As shown in Fig. 8b, NcMIC1 exhibited a high binding activity for the highly sulfated dextran sulfate, as well as for other sulfated glycosaminoglycans (heparin, chondroitin sulfate B, and chondroitin sulfate C), while chondroitin sulfate A and the nonsulfated dextran did not exhibit comparable binding to NcMIC1. Therefore, highly sulfated proteoglycans can be regarded as potential host cell receptors which mediate binding of NcMIC1 to the host cell surface.
FIG. 8.
(a) Binding of live N. caninum tachyzoites and NcMIC1 to Vero cell monolayers. Untreated, NaClO3-treated, and β-xyloside-treated Vero cell monolayers were incubated with freshly purified N. caninum tachyzoites or with the secreted fraction of N. caninum tachyzoites. Bound tachyzoites were detected using anti -N. caninum antiserum, and bound NcMIC1 was detected using anti-recNcMIC1 antibodies. Note the reduction in parasite adherence as well as NcMIC1 binding to Vero cells following treatments which affect the host cell surface glycosaminoglycan composition. OD, optical density; Trt, treated; Con, control. Error bars indicate standard deviations. (b) Binding of NcMIC1 to defined, solid-phase-bound glycosaminoglycans. Secreted fractions were incubated with heparin (Hep), dextran (Dex), dextran sulfate (Dexsul), and chondroitin sulfates A, B, and C (CSA, CSB, and CSC, respectively). Bound NcMIC1 was detected using anti-recNcMIC1 antibodies. Note the preferential binding of NcMIC to the highly sulfated glycosaminoglycan dextran sulfate, but only low binding is detected with dextran and chondroitin sulfate A.
DISCUSSION
Adhesion to and invasion of host cells represent crucial steps in the life cycle of N. caninum. Thus, the identification and characterization of molecules involved in these processes is important and could lead to novel means of intervention.
Molecular characterization of NcMIC1.
In this study, we have characterized an N. caninum microneme protein (NcMIC1) which is homologous to the microneme protein TgMIC1 from the closely related T. gondii. The close relationship between these two proteins is first demonstrated at the genetic level. Both genes contain three introns (introns I to III), which do not share significant similarity with regard to their nucleotide sequences. The relative placements and approximate lengths of the exons are highly similar, suggesting that these are true homologues, derived from a common ancestor that already contained the introns (Fig. 1). Regions with high nucleotide identity are largely located within the N-terminal half of the deduced polypeptide (see below) which is most highly conserved between NcMIC1 and TgMIC1. Thus, the high degree of conservation at the genomic level is also reflected in the high degree of conservation at the protein level (Fig. 2).
Although the genomic sequence coding for NcMIC1 is slightly shorter than the genomic sequence coding for TgMIC1, the MIC1 gene in N. caninum codes for a 460-aa polypeptide, which is slightly larger than TgMIC1 (456 aa). Similar to TgMIC1, the NcMIC1 protein sequence can be divided into three distinct domains. The N-terminal domain is characterized by a leader sequence of 20 aa, which would target the protein into the secretory pathway. All microneme proteins identified so far exhibit an N-terminal signal peptide sequence, which represents only one of several signals which are necessary for accurate trafficking and sorting of microneme proteins during their transit through the endoplasmic reticulum and the Golgi network (33, 35). The signal peptide sequence is followed by a domain of approximately 220 aa which contains two tandemly repeated sequences. Both repeats contain the CXXXCG motif, which is a hallmark of the TSP-like family of adhesive proteins found in most apicomplexan parasites (28). This motif is identically positioned in both NcMIC1 and TgMIC1. More recently, the TRAP family of micronemal proteins in Plasmodium has been found to play a crucial role in the process of host cell attachment and invasion (29). Thus, it is likely that the N. caninum TRAP homologue, NcMIC2 (25), as well as the corresponding T. gondii protein, TgMIC2 (4), and other related molecules such as NcMIC1 could also be crucially involved in establishing the physical contact between parasite and host cell. In addition to the striking similarity with regard to the primary sequences of NcMIC1 and TgMIC1 within the N-terminal half, there is most likely also a high degree of similarity with regard to their secondary structure, since 14 out of 18 cysteine residues are found to be identically positioned within this region of the two proteins spanning the two internal repeats. Thus, due to the likeliness of intrachain disulfide bonding, these domains containing the internal repeats are also conformationally closely related. It is known that proper folding, with the correct pairs of cysteine residues involved, is a prerequisite for the correct targeting, secretion, and other functional characteristics of microneme proteins (15). We found that the electrophoretic mobility of NcMIC1 is indeed increased when SDS-PAGE is carried out under nonreducing conditions (Fig. 3), thus indicating that disulfide bridges are crucially involved in largely determining the conformation of the NcMIC1 polypeptide. Finally, the C-terminal half represents the one part of the two proteins which is not as strictly conserved. The previously identified PXK and PKT motifs found in TgMIC1, which could potentially be involved in forming β-sheets (14), are not conserved in NcMIC1.
NcMIC1 was originally identified as a protein comigrating with a concanavalin A-, wheat germ agglutinin-, and Jacalin-reactive band of 60 kDa following lectin blotting. Two potential N-glycosylation sites are found in the deduced NcMIC1 protein sequence, one located within the N-terminal region and the other located in the C-terminal domain. However, these glycosylation sites are not found in the TgMIC1 sequence, and at present it is not clear whether NcMIC1 is glycosylated or not.
Expression and localization of NcMIC1.
Attempts to express the entire NcMIC1 polypeptide as a recombinant protein in E. coli were not successful (N. Keller and A. Hemphill, unpublished data). However, one part of the molecule, corresponding to aa 183 to 460, was expressed in E. coli, and anti-recNcMIC1 antibodies were obtained by affinity purification on nitrocellulose-bound proteins. These antibodies were used to localize NcMIC1 by immunofluorescence in N. caninum-infected Vero cells and within parasites isolated and purified from cell cultures. In all cases the localization was identical, being largely confined to the apical part of the tachyzoites (Fig. 4 and 5).
Immunohistochemistry was performed to investigate whether NcMIC1 is also expressed in the bradyzoite stage of the parasite. Paraffin-embedded mouse brain tissue sections, which were previously shown to harbor N. caninum tissue cysts containing bradyzoites (26), were used. Labeling with anti-recNcMIC1 antibodies and MAb CC2, directed against a T. gondii bradyzoite cyst wall protein (15a), was performed and revealed that NcMIC1 was not detectable in those tissue cysts which exhibited distinct cyst wall labeling with MAb CC2 (Fig. 4). However, a molecular analysis of this lack of expression in the bradyzoite stage was not performed. Expression of N. caninum antigens in both tachyzoite and bradyzoite stages was found to occur for most N. caninum antigens identified to date, including another microneme protein (NcMIC3 [34]), the 33-kDa dense granule antigen NcGRA7 (19), and the 35-kDa major surface antigen NcSRS2 (17). In contrast, NcSAG1, another major immunodominant N. caninum surface antigen, was found to be expressed exclusively in the tachyzoite stage (20).
NcMIC1 and its interaction with the host cell surface.
The presence of an N-terminal signal peptide sequence and the fact that NcMIC1 was sequestered almost exclusively into the Triton X-114 hydrophilic fraction following detergent extraction suggested that, in analogy to other microneme proteins, NcMIC1 was secreted and released by the parasite into the environment as a soluble molecule. In T. gondii, it has been shown that initial contact of tachyzoites to host cells is followed by Ca2+ signaling and that this rise in intracellular Ca2+ is required for microneme secretion and invasion (2, 4, 9, 22, 25, 36). We found that NcMIC1 is indeed secreted into the medium as a soluble molecule upon incubation of tachyzoites at 37°C (Fig. 6); however, the role of Ca2+ in this process needs to be investigated in more detail.
The presence of potentially adhesive TSP-like motifs suggested that NcMIC1 could interact with the host cell surface. Thus, the host cell surface binding activity of NcMIC1 was investigated. We performed coprecipitation assays, in which Vero cells were incubated in the presence of Triton X-100-soluble N. caninum proteins and bound proteins were sedimented by centrifugation. These assays showed that NcMIC1 indeed coprecipitated with Vero cells, while NcGRA7, a dense granule protein, did not cosediment with Vero cells, serving as a negative control (Fig. 7).
Further binding experiments, employing an assay which used host cell monolayers as binding matrices, showed that N. caninum tachyzoites bind to highly sulfated glycosaminoglycans found on cell surface proteoglycans, and interference in the synthesis of glycosaminoglycans (by use of β-xyloside) or inhibition of the sulfation process (through NaClO3 treatment) resulted in a significant decrease of tachyzoite adherence (Fig. 8). Sulfated proteoglycans are one class of receptors used for host cell attachment by T. gondii (6), and the widespread distribution of these molecules on the surfaces of mammalian cells may contribute to the broad specificity of host cells susceptible to invasion by T. gondii and possibly also N. caninum. Monitoring of the cell binding activity of NcMIC1 by using secreted fractions obtained from N. caninum tachyzoites showed that NcMIC1 binding to Vero cells was also diminished by altering the host cell surface proteoglycan composition with β-xyloside and NaClO3 (Fig. 8). This suggested that NcMIC1 plays an important functional role with regard to the physical interaction between the parasite and host cell surface by interacting with specific proteoglycans.
How such a functional role as a potential adhesin can be fulfilled by a protein which is secreted as a soluble molecule is not known. However, more recent evidence, which showed that microneme proteins do not act a sole players but are secreted as multiprotein complexes, has been obtained (24). For instance, Reiss et al. (32) have shown that the correct trafficking of the soluble microneme proteins TgMIC1 and TgMIC4 depends strongly on the presence of a transmembrane escorter protein, TgMIC6, and immunoprecipitation experiments have confirmed that these three microneme proteins form a stable complex. In addition, the absence of TgMIC1 in T. gondii MIC1 knockout mutants had a drastic effect on its two interaction partners, as they remained stuck in the endoplasmic reticulum-Golgi complex (13, 33). Nevertheless, none of the microneme knockout mutants described to date (13, 32, 33) exhibited a marked impaired infectivity compared to wild-type T. gondii parasites. Thus, the adhesion and invasion machinery is characterized by a high degree of redundancy, which is also reflected by the large and diverse arsenal of potential adhesive domains expressed within the multiple microneme proteins identified to date. Furthermore, a novel TgMIC2-associated protein (TgM2AP) has been recently identified in T. gondii tachyzoites, which participates in tachyzoite host cell entry, is tightly bound to TgMIC2, and is regarded as a fundamental component for T. gondii invasion (31). Homologues of TgM2AP have been found Eimeria tenella and N. caninum.
Proteoglycans are composed of core proteins which are modified by the addition of glycan moieties collectively termed glycosaminoglycans (1). Glycosaminoglycans consist of linear polymers composed of disaccharide repeating units of uronic acid and hexosamine. As the chains polymerize, a multitude of sulfation and epimerization reactions occur, and together with the length of the polymer, the proteoglycans are rendered highly heterogenous in composition and structure (1). By solid-phase assays using specific glycosaminoglycans, we found that NcMIC1 exhibited a binding activity for the artificial highly sulfated glycosaminoglycan dextran sulfate and a much less pronounced binding activity for the nonsulfated form, dextran. In addition, other naturally occurring glycosaminoglycans, including heparin and chondroitin sulfates B and C, show a marked NcMIC1 binding activity (Fig. 8b). Recently, T. gondii tachyzoites were reported to bind to heparin, dextran sulfate, and chondroitin sulfates A and C (6). Ortega-Barria and Boothroyd (30) have also demonstrated the importance of sulfated sugars on the surface of host cells and have identified three proteins which represent candidate adhesins with lectin-like activity. Thus, further work will be performed in order to elucidate the interaction partners of NcMIC1 on the parasite and host cell sides, aiming towards the characterization of potential targets for intervention against N. caninum infection.
Acknowledgments
Many thanks are addressed to Norbert Müller and Bruno Gottstein (Institute of Parasitology, University of Bern) for helpful suggestions throughout the work and for carefully reading the manuscript. We also thank Maja Suter (Institute of Animal Pathology) and Phillippe Tregenna-Piggott and Beatrice Frey (Department of Chemistry and Biochemistry, University of Bern) for access to their electron microscopy facilities and Volker Heussler and Dirk Dobbelaere (Institute of Animal Pathology) for access to their immunofluorescence unit. J. P. Dubey is gratefully acknowledged for providing the N. caninum Nc-1 isolate, and Milton McAllister (University of Illinois) is gratefully acknowledged for generously providing paraffin blocks of tissue cyst-infected mouse brain.
This study was financially supported largely by the Swiss National Science Foundation (grant no. 3200-056486.99), the Foundation Research 3R, and a European Union grant (QLK2-CT-2001-01050) provided by the Swiss Ministry for Education and Science (BBW no. 00.0498). A.N. is a recipient of a stipend from the Swiss Federal Commission of Foreign Students and was supported by the Roche Foundation.
Editor: J. M. Mansfield
REFERENCES
- 1.Bork, P., A. K. Downing, B. Kieffer, and I. D. Campbell. 1996. Structure and distribution of modules in extracellular proteins. Q. Rev. Biophys. 29:119-167. [DOI] [PubMed] [Google Scholar]
- 2.Brydges, S. D., G. D. Sherman, S. Nockemann, A. Loyens, W. Daubener, J. F. Dubremetz, and V. B. Carruthers. 2000. Molecular characterization of TgMIC5, a proteolytically processed antigen secreted from the micronemes of Toxoplasma gondii. Mol. Biochem. Parasitol. 111:51-66. [DOI] [PubMed] [Google Scholar]
- 3.Carruthers, V. B., and L. D. Sibley. 1997. Sequential protein secretion from three distinct organelles of Toxoplasma gondii accompanies invasion of human fibroblasts. Eur. J. Cell Biol. 73:114-123. [PubMed] [Google Scholar]
- 4.Carruthers, V. B., and L. D. Sibley. 1999. Mobilization of intracellular calcium stimulates microneme discharge in Toxoplasma gondii. Mol. Microbiol. 31:421-428. [DOI] [PubMed] [Google Scholar]
- 5.Carruthers, V. B., Giddings, O. K., and L. D. Sibley. 1999. Secretion of micronemal proteins is associated with Toxoplasma invasion of host cells. Cell. Microbiol. 1:225-235. [DOI] [PubMed] [Google Scholar]
- 6.Carruthers, V. B., S. Hakansson, O. K. Giddings, and L. D. Sibley. 2000. Toxoplasma gondii uses sulfated proteoglycans for substrate and host cell attachment. Infect. Immun. 68:4005-4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dobrowolski, J. M., and L. D. Sibley. 1996. Toxoplasma invasion of mammalian cells is powered by the actin cytoskeleton of the parasite. Cell. 84:933-939. [DOI] [PubMed] [Google Scholar]
- 8.Dobrowolski, J. M., V. B. Carruthers, and L. D. Sibley. 1997. Participation of myosin in gliding motility and host cell invasion by Toxoplasma gondii. Mol. Microbiol. 26:163-173. [DOI] [PubMed] [Google Scholar]
- 9.Donahue, C. G., V. B. Carruthers, S. D. Gilk, and G. E. Ward. 2000. The Toxoplasma homolog of Plasmodium apical membrane antigen-1 (AMA-1) is a microneme protein secreted in response to elevated intracellular calcium levels. Mol. Biochem. Parasitol. 111:15-30. [DOI] [PubMed] [Google Scholar]
- 10.Dubey, J. P., A. L. Hattel, D. S. Lindsay, and M. J. Topper. 1988. Neonatal Neospora caninum infection in dogs: isolation of the causative agent and experimental transmission. J. Am. Vet. Med. Assoc. 193:1259-1263. [PubMed] [Google Scholar]
- 11.Dubey, J. P., and D. S. Lindsay. 1996. A review of Neospora caninum and neosporosis. Vet. Parasitol. 67:1-59. [DOI] [PubMed] [Google Scholar]
- 12.Dubremetz, J. F., N. Garcia-Reguet, V. Conseil, and M. N. Fourmaux. 1998. Apical organelles and host-cell invasion by Apicomplexa. Int. J. Parasitol. 28:1007-1013. [DOI] [PubMed] [Google Scholar]
- 13.Ellgaard, L., M. Molinari, and A. Helenius. 1999. Setting the standards: quality control in the secretory pathway. Science 286:1882-1888. [DOI] [PubMed] [Google Scholar]
- 14.Fourmaux, M. N., A. Achbarou, O. Mercereau-Puijalon, C. Biderre, L. Briche, A. Loyens, C. Odberg-Ferrgut, D. Camus, and J. F. Dubremetz. 1996. The MIC1 microneme protein of Toxoplasma gondii contains a duplicated receptor-like domain and binds to host cell surface. Mol. Biochem. Parasitol. 83:201-210. [DOI] [PubMed] [Google Scholar]
- 15.Frand, A. R., J. W. Cuozzo, and C. A. Kaiser. 2000. Pathways for protein disulphide bond formation. Trends Cell. Biol. 10:203-209. [DOI] [PubMed] [Google Scholar]
- 15a.Gross, U., H. Bormuth, C. Gaissmaier, C. Dittrich, V. Krenn, W. Bohne, and D. J. P. Ferguson. 1995. Monoclonal rat antibodies directed against Toxoplasma gondii suitable for studying tachyzoite-bradyzoite interconversion in vivo. Clin. Diagn. Lab. Immunol. 2:542-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hemphill, A., B. Gottstein, and H. Kaufmann. 1996. Adhesion and invasion of bovine endothelial cells by Neospora caninum tachyzoites. Parasitology 112:183-197. [DOI] [PubMed] [Google Scholar]
- 17.Hemphill, A., R. Felleisen, B. Connolly, B. Gottstein, B. Hentrich, and N. Müller. 1997. Characterization of a cDNA clone encoding Nc-p43, a major Neospora caninum surface protein. Parasitology 115:581-590. [DOI] [PubMed]
- 18.Hemphill, A., and S. L. Croft. 1997. Electron microscopy in parasitology, p 227-268. In M. Rogan (ed.), Analytical parasitology. Springer Verlag, Heidelberg, Germany.
- 19.Hemphill, A., N. Gajendran, S. Sonda, N. Fuchs, B. Gottstein, B. Hentrich, and M. Jenkins. 1998. Identification and characterization of a dense granule-associated protein in Neospora caninum tachyzoites. Int. J. Parasitol. 28:429-438. [DOI] [PubMed] [Google Scholar]
- 20.Hemphill, A. 1999. The host-parasite relationship in neosporosis. Adv. Parasitol. 43:47-104. [DOI] [PubMed] [Google Scholar]
- 21.Hemphill, A., and B. Gottstein. 2000. A European perspective on Neospora caninum. Int. J. Parasitol. 30:877-924. [DOI] [PubMed] [Google Scholar]
- 22.Hoff, E. F., S. H. Cook, G. D. Sherman, J. M. Harper, D. J. P. Ferguson, J. F. Dubremetz, and V. B. Carruthers. 2001. Toxoplasma gondii: molecular cloning and characterization of a novel 18-kDa secretory antigen, TgMIC10. Exp. Parasitol. 97:77-88. [DOI] [PubMed] [Google Scholar]
- 23.Howe, D. K., A. C. Crawford, D. S. Lindsay, and L. D. Sibley. 1998. The p29 and p35 immunodominant antigens of Neospora caninum tachyzoites are homologous to the family of surface antigens of Toxoplasma gondii. Infect. Immun. 66:5322-5328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaasch, A. J., and K. A. Joiner. 2000. Protein targeting determinants in the secretory pathway of apicomplexan parasites. Curr. Opin. Microbiol. 3:422-428. [DOI] [PubMed] [Google Scholar]
- 25.Lovett, J. L., D. K. Howe, and L. D. Sibley. 2000. Molecular characterization of a thrombospondin-related anonymous protein homologue in Neospora caninum. Mol. Biochem. Parasitol. 107:33-43. [DOI] [PubMed] [Google Scholar]
- 26.McAllister, M. M., J. P. Dubey, D. S. Lindsay, W. R. Jolley, R. A. Wills, and A. M. McGuire. 1998. Dogs are definitive hosts of Neospora caninum. Int. J. Parasitol. 28:1473-1478. [PubMed] [Google Scholar]
- 27.Naguleswaran, A., A. Cannas, N. Keller, N. Vonlaufen, G. Schares, F. J. Conraths, C. Björkman, and A. Hemphill. 2001. Neospora caninum microneme protein NcMIC3: secretion, subcellular localization, and functional involvement in host cell interaction. Infect. Immun. 69:6483-6494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naitza, S., F. Spano, K. J. H. Robson, and A. Crisanti. 1998. The thrombospondin related protein family of apicomplexan parasites. The gears of the cell invasion machinery. Parasitol. Today 14:479-484. [DOI] [PubMed] [Google Scholar]
- 29.Nussenzweig, V., and R. Menard. 2000. Analysis of a malaria sporozoite protein family required for gliding motility and cell invasion. Trends Microbiol. 8:94-95. [DOI] [PubMed] [Google Scholar]
- 30.Ortega-Barria, E., and J. C. Boothroyd. 1999. A Toxoplasma lectin like activity specific for sulfated polysaccharides is involved in host cell infection. J. Biol. Chem. 274:1267-1276. [DOI] [PubMed] [Google Scholar]
- 31.Rabenau, K. E., A. Sohrabi, A. Tripathy, C. Reitter, J. W.Aijoka F. M. Tomley, and V. B. Carruthers. 2001. TgM2AP participates in Toxoplasma gondii invasion of host cells and is tightly associated with the adhesive protein TgMIC2. Mol. Microbiol. 41:537-547. [DOI] [PubMed] [Google Scholar]
- 32.Reiss, M., N. Viebig, S. Brecht, M. Fourmaux, M. Soete, M. M. Di Cristina, J. F. Dubremetz, and D. Soldati. 2001. Identification and characterization of an escorter for two secretory adhesins in Toxoplasma gondii. J. Cell Biol. 152:563-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soldati, D., J. F. Dubremetz, and M. Lebrun. 2001. Microneme proteins: structural and functional requirements to promote adhesion and invasion by the apicomplexan parasite Toxoplasma gondii. Int. J. Parasitol. 31:1293-1302. [DOI] [PubMed] [Google Scholar]
- 34.Sonda, S., N. Fuchs, B. Gottstein, and A. Hemphill. 2000. Molecular characterization of a novel microneme antigen in Neospora caninum. Mol. Biochem. Parasitol. 108:39-51. [DOI] [PubMed] [Google Scholar]
- 35.Tomley, F. M., and D. Soldati. 2001. Mix and match modules: structure and function of microneme proteins in apicomplexan parasites. Trends Parasitol. 17:81-88. [DOI] [PubMed] [Google Scholar]
- 36.Vieira, M. C., and S. N. Moreno. 2000. Mobilization of intracellular calcium upon attachment of Toxoplasma gondii tachyzoites to human fibroblasts is required for invasion. Mol. Biochem. Parasitol. 84:203-214. [DOI] [PubMed] [Google Scholar]
- 37.Wessel, D., and U. I. Flügge. 1984. A method for the quantitative recovery of protein in dilute solution in the presence of detergent. Anal. Biochem. 138:141-143. [DOI] [PubMed] [Google Scholar]