Abstract
Pirfenidone [5-methyl-1-phenyl-2-(1H)-pyridone] down-regulates expression of cytokines and other mediators involved in the onset and development of pulmonary fibrosis. Pirfenidone also inhibits production of tumor necrosis factor alpha (TNF-α) from macrophages incubated with endotoxin and protects mice against endotoxin shock. Pirfenidone's ability to reduce cytokine expression in these disorders led us to investigate the drug's effect on another cytokine anomaly, superantigen-induced shock. BALB/c mice were exposed to staphylococcal enterotoxin B (SEB) either systemically or by aerosol and subsequently potentiated with a sublethal dose of lipopolysaccharide. In these experiments, pirfenidone given 2 to 4.25 h after SEB resulted in 80 to 100% survival versus only 0 to 10% survival among untreated control animals. Relative to serum cytokine levels from controls given toxin but no drug, there was a 35 to 80% decrease in TNF-α, interleukin 1, and other proinflammatory cytokines. In vitro experiments with human peripheral blood lymphocytes revealed that pirfenidone reduced SEB-induced cytokine levels 50 to 80% and inhibited 95% of SEB-induced T-cell proliferation. Overall, these studies demonstrated the potential utility of pirfenidone as a therapeutic against septic shock and the biological effects of SEB.
Staphylococcal enterotoxins (SEs), produced by the ubiquitous bacterium Staphylococcus aureus, are protein exotoxins that cause transient gastrointestinal problems following ingestion of SE-contaminated food (1, 19). SEs also possess superantigenic properties by cross-linking antigen-presenting cells and T cells, binding to the outer region of major histocompatibility complex class II molecules on antigen-presenting cells and to Vβ-specific determinants on the T-cell receptor (10, 21). The resulting complex triggers intense proliferation of T cells that eventually die by apoptosis (28, 46). Similar to antigen-activated T cells, cytokines such as interleukin 1 (IL-1), IL-2, gamma interferon (IFN-γ), and tumor necrosis factor alpha (TNF-α) are produced and released during T-cell proliferation (23). Under pathological conditions, these cytokine levels far exceed those required for normal physiologic homeostasis, resulting in severe tissue damage and often death (22, 47; J. Carlet, F. Taylor, M. Levi, A. Aritgas, H. ten Cate, and J. Marshall, abstract from the clinical expert round table (session 4) at the Margaux Conference on Critical Illness, Crit. Care Med. 29:S107-S108, 2001).
While many proinflammatory cytokines contribute to SE toxicity, TNF-α predominates as the precipitating factor in pathological manifestations (13, 29, 30). TNF-α belongs to the TNF superfamily of biologic effector molecules, and when released by antigen-activated cells, TNF-α in conjunction with other effector molecules modulates a wide array of cellular processes, including host defense, apoptosis, cell differentiation, and organogenesis (9, 27). However, the untoward release of high TNF-α levels results in organ dysfunction and systemic toxicity (23, 38).
The lungs are extremely sensitive to inflammatory reactions mediated by TNF-α, and two life-threatening syndromes, vascular leak and respiratory distress, develop during septic shock (16, 32). Further studies have indicated that cytokine-mediated acute respiratory distress syndrome and inflammatory lung disease occur during SE intoxication (14, 38). Without immediate intervention, these conditions are lethal (11, 22). Suppression of TNF-α and other proinflammatory cytokines limits acute symptoms, such as dyspnea, tachypnea, and hypoxemia, thereby reducing immediate respiratory dysfunction (34). However, residual damage resulting from inflammatory reactions can thicken alveolar walls and decrease alveolar function through a disease process known as pulmonary fibrosis (5). Without further treatment, these fibrotic lesions cause permanent and irreparable damage within the lungs.
Pirfenidone [5-methyl-1-phenyl-2-(1H)-pyridone], an antifibrotic agent, inhibits bleomycin-induced pulmonary fibrosis in hamsters as well as experimental peritonitis in rats (18, 41). Recently, pirfenidone has been shown to down-regulate production of intercellular adhesion molecule-1 in IL-1α-treated human synovial fibroblasts and to inhibit transforming growth factor-β at the transcription level in lung tissue from bleomycin-treated mice (17, 20). Pirfenidone also protects mice from endotoxin-induced shock and dramatically decreases TNF-α concentrations in endotoxin-treated macrophage cultures (6). In addition to these in vitro and in vivo studies, pirfenidone has been used in phase II clinical trials for treatment of pulmonary fibrosis (36).
Pirfenidone's ability to reduce pulmonary fibrosis and protect against endotoxic shock suggests that the drug could be used therapeutically against SE-induced biological effects, including shock. It is well known that bacterial superantigens like staphylococcal enterotoxin B (SEB) are naturally potentiated by endotoxin in vivo and in vitro (3, 4, 35, 40, 42). Therefore, an established lipopolysaccharide (LPS)-potentiated murine model and in vitro human peripheral blood lymphocyte assays were used to determine pirfenidone's potential for abrogating SE-induced biological effects (25, 39).
MATERIALS AND METHODS
Reagents.
A Limulus amoebocyte lysate assay (BioWhittaker, Walkersville, Md.) confirmed that purified SEB from Toxin Technologies (Sarasota, Fla.) contained <1 ng of endotoxin/mg of protein. Purified Escherichia coli 055:B5 LPS was purchased from Difco Laboratories (Detroit, Mich.). Human recombinant (hr) TNF-α, peroxidase-conjugated anti-rabbit immunoglobulin G (IgG) or anti-goat IgG was obtained from Boehringer Mannheim (Indianapolis, Ind.). Antibodies against hrTNF-α were purchased from R&D Systems (Minneapolis, Minn.), hrIL-1β was kindly provided by Joost Oppenheim (National Cancer Institute, Frederick, Md.), and antibodies against hrIL-1β and hrIL-6 were obtained from Endogen (Woburn, Mass.). Anti-hr IFN-γ IgG, with and without biotin, was obtained from Pharmingen (San Diego, Calif.). hrIFN-γ and hrIL-6 were obtained from Collaborative Research (Boston, Mass.). Mouse IL-1α, IL-6, IFN-γ, and TNF-α enzyme-linked immunosorbent assay (ELISA) kits were purchased from R&D Systems. Phosphate-buffered saline (PBS), sterile saline, and other reagents were purchased from Sigma Chemicals (St. Louis, Mo.). Pirfenidone was obtained from Marnac Inc. (Dallas, Tex.).
Animals.
Male BALB/c mice (18 to 25 g) were purchased from the National Cancer Institute Animal Facility (Frederick, Md.) and maintained in a pathogen-free environment. Research was conducted in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals, strictly adhering to principles stated in the Guide for Care and Use of Laboratory Animals (12). All animal experiments were performed in a facility accredited by the American Association for Accreditation of Laboratory Animal Care following a protocol approved by the Institute Laboratory Animal Care and Utilization Committee.
Murine model of superantigen-induced toxic shock.
The LPS-potentiated murine model for SE-induced shock has been previously characterized using intraperitoneal (i.p.) inoculations or aerosol exposure to SEs (25, 39). Briefly, for systemic exposure to SEB, each mouse received an i.p. inoculation of 1 μg of SEB (three 50% lethal doses [LD50]) in 200 μl of endotoxin-free PBS followed 4 h later by 80 μg of LPS given i.p. At specified times after SEB administration, the mice were given an i.p. inoculation of pirfenidone (200 mg/kg of body weight) resuspended in sterile saline (Sigma Chemicals) and checked twice daily until 96 h post-SEB injection.
For aerosol exposure to SEB, mice were placed in an exposure chamber within a biosafety level 3 hood line (25). The aerosols were generated using a Collison nebulizer (BGI Inc., Waltham, Mass.), and sampling results indicated consistent and reproducible exposures throughout all experiments. The mice received a whole-body dose of 0.12 mg/kg (approximately 7 LD50) followed 4 h later with 80 μg of LPS i.p. Another group was given aerosolized PBS and used for treated (LPS) or normal (PBS only) controls. At specified times after the SEB exposure, the animals received pirfenidone (200 mg/kg) i.p. and then were checked twice daily until 216 h post-SEB exposure.
Serum collection.
Previous murine studies determined that serum cytokine levels were highest 6 to 8 h after a whole-body SEB aerosol, and therefore, blood was collected at 7 h (25). The blood was allowed to clot and was then centrifuged in microtiter serum separator tubes (Becton Dickinson, Bedford, Mass.). The sera from each group of mice (n = 10) were pooled and stored at −70°C until they were analyzed.
Cell cultures.
Human peripheral blood lymphocytes (hPBL) were isolated by Ficoll-Hypaque density gradient centrifugation (24). The purified cells were cultured in vitro with or without pirfenidone (520 μg/ml) and SEB (150 ng/ml) for 16 h at 37°C. The culture fluids were analyzed for TNF-α, IL-1β, IL-6, and IFN-γ by ELISA as described previously (24).
Human T-cell proliferation assays.
Proliferation assays were performed as previously described (24). Briefly, SEB (150 ng/ml) was added to 96-well cultures of peripheral blood mononuclear cells (PBMC) (105 cells/well) with or without various concentrations of pirfenidone and then cultured at 37°C for 48 h. During the last 5 h, the cells were pulsed with 1 μCi of [3H]thymidine (New England Nuclear, Boston, Mass.)/well and then harvested onto glass fiber filters. The incorporated [3H]thymidine was measured by liquid scintillation. At various times during the experiment, cell viability was measured by trypan blue exclusion.
Cytokine detection.
The levels of TNF-α, IL-1β, IL-6, and IFN-γ in culture supernatant fluid from PBMC or TNF-α, IL-1α, IL-6, and IFN-γ in mouse sera were measured via sandwich ELISA by using cytokine-specific antibodies, according to the manufacturer's instructions. Recombinant cytokines (20 to 1,000 pg/ml) represented the calibration standards, and the detection limit for all assays was 20 pg/ml.
Statistical analysis.
The cytokine data were expressed as the mean reading ± standard deviation (SD) and were subsequently analyzed for significant difference (P ≤ 0.05) in Student's t test with Stata (Stata Corp., College Station, Tex.). The χ2 test was used to determine significant protection (P ≤ 0.05) by pirfenidone between treated and untreated groups.
RESULTS
Pirfenidone protects mice from SEB-mediated shock.
Cain and coworkers (6) showed that a 100- to 200-mg i.p. dose of pirfenidone/kg effectively lowers serum levels of TNF-α after endotoxin exposure. Preliminary studies performed in our laboratory also determined that a 200-mg/kg dose provided protection against SEB-induced shock (data not shown), and therefore that dose was used in the present studies.
Pirfenidone protected mice from SEB-mediated shock when given 1 to 4.25 h after an i.p. injection of SEB but did not afford protection if given 4.5 h after SEB (Table 1). All of the mice survived when pirfenidone was administered 2 or 3 h after SEB, and 80% survived if the drug was given 1 or 4.25 h after SEB. The mice were not protected if pirfenidone was administered 1 h before or 4.5 h after an i.p. SEB challenge.
TABLE 1.
Pirfenidone protects BALB/c mice from SEB-induced shock
| Treatmenta (h) | % Survival |
|---|---|
| PF | |
| −1 | 0 |
| +1 | 80 |
| +2 | 100 |
| +3 | 100 |
| +4.25 | 80 |
| +4.5 | 0 |
| Controls | |
| SEB + LPS | 0 |
| PBS + LPS | 100 |
| SEB + PBS | 100 |
Mice were given pirfenidone (PF) before (−) or after (+) SEB. Controls were not given pirfenidone, and percent survival was calculated 96 h after the SEB i.p. injection. n = 10 per group.
Within 2 h after LPS potentiation, all mice receiving SEB i.p., but not pirfenidone, exhibited clinical signs of intoxication, including ruffled fur, dyspnea, lethargy, and diarrhea. All of these animals died within 12 to 36 h. Although intoxicated animals given pirfenidone exhibited ruffled coats and dyspnea, the symptoms were transient with no observable diarrhea, and the survivors appeared healthy 96 h after the SEB inoculations. Mice given LPS only became lethargic but recovered within 48 h and were healthy throughout the remainder of the experiment.
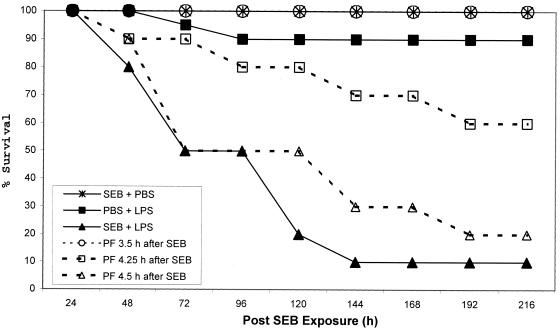
When SEB was administered by aerosol, only 10% of the mice receiving SEB plus LPS survived, while 100 or 60% of pirfenidone-treated mice survived if the drug was given 3.5 or 4.25 h, respectively, after SEB (Fig. 1). Although only 20% of the mice receiving pirfenidone 4.5 h after SEB survived, there was a delay in the time of death relative to those given aerosolized SEB plus i.p. LPS.
FIG. 1.
Survival of mice exposed to an SEB (7 LD50) aerosol. The group size (n = 20) is based on cumulative data from two experiments. All mice given SEB plus PBS survived (not shown on the graph). The remaining control groups included mice receiving PBS (aerosol) plus LPS or SEB (aerosol) plus LPS. At various times after receiving SEB, experimental groups exposed to SEB plus LPS were given an i.p. dose of 200 mg of pirfenidone (PF)/kg.
Clinical symptoms began within 6 to 24 h after exposure and were similar to those observed in mice receiving both SEB and LPS i.p. Ruffled fur, dyspnea, lethargy, and diarrhea developed between 12 and 72 h, and death occurred between 48 and 144 h. However, among toxin-treated animals given pirfenidone, symptoms varied depending upon the time of drug administration. If the mice received pirfenidone 3.5 h after SEB, they developed ruffled fur and lethargy between 6 and 24 h, but by 120 h, clinical signs had diminished significantly. Mice receiving pirfenidone 4.25 or 4.5 h after SEB again developed ruffled fur and lethargy between 6 and 24 h, and clinical signs among survivors diminished by 192 h, the time by which all mice given SEB and LPS had died.
Pirfenidone lowers serum cytokine levels following an SEB aerosol exposure and i.p. injection of LPS.
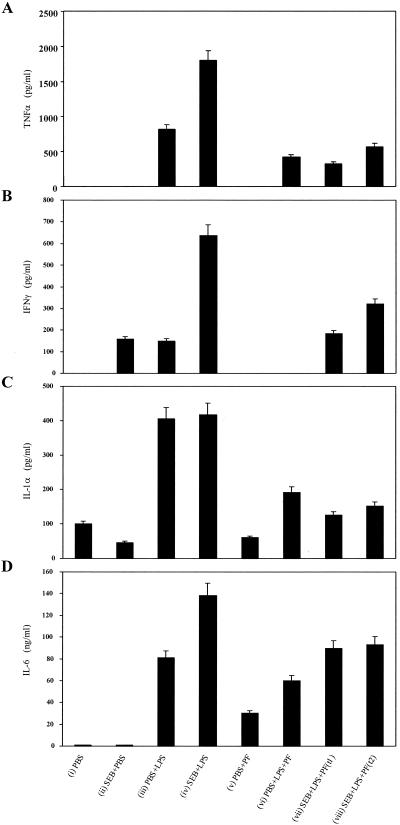
To determine if pirfenidone's protective effect was linked to decreased levels of serum proinflammatory cytokines, known to be intimately connected to lethal shock in this murine model (24, 25, 26, 39), blood was collected from mice 7 h after they received an aerosolized dose of SEB. TNF-α was not detected in the sera of controls receiving PBS, SEB plus PBS, or pirfenidone plus PBS (Fig. 2A). The TNF-α levels in sera from animals given PBS plus LPS were 750 pg/ml, 60% less than those in sera from animals given SEB plus LPS. Pirfenidone reduced serum TNF-α levels 70 to 80% among animals given SEB plus LPS. Pirfenidone, when administered to mice given PBS plus LPS, reduced TNF-α levels by 50%.
FIG. 2.
Levels of TNF-α (A), IFN-γ (B), IL-1α (C), and IL-6 (D), in pooled sera from mice exposed to PBS or SEB aerosols. The sera were from samples taken 7 h after the following treatments: (i) PBS, (ii) SEB plus PBS, (iii) PBS plus LPS, (iv) SEB plus LPS, (v) PBS plus pirfenidone (PF) given 3.5 h after PBS, (vi) PBS plus LPS with PF given 3.5 h after PBS, (vii) SEB plus LPS with PF (t1) given 3.5 h after SEB, and (viii) SEB plus LPS with PF (t2) given 4.25 h after SEB. The values represent the mean + SD for duplicate samples from three experiments.
Although pirfenidone decreased serum TNF-α levels most drastically among mice given SEB plus LPS, serum IFN-γ, IL-1α, and IL-6 levels were also decreased by 70, 60, and 40%, respectively (Fig. 2B to D). Pirfenidone alone had no effect upon TNF-α, IFN-γ, or IL-1α levels but caused a slight rise in IL-6 levels. The lowest serum cytokine levels were seen in animals given the drug 3.5 h after SEB exposure, with the greatest effect observed on TNF-α, IFN-γ, and IL-1α. Overall, these in vivo studies showed that among animals exposed to aerosolized SEB potentiated with LPS i.p., pirfenidone decreased the levels of various proinflammatory cytokines in the serum due to SEB, LPS, or SEB plus LPS.
Pirfenidone inhibits SEB-induced cytokines from PBMC.
Since elevated levels of proinflammatory cytokines, important for toxicity in this murine model (26, 39), were decreased in mice receiving pirfenidone, we next tested the effect of this drug on PBMC cultures incubated with SEB. Preliminary experiments showed that pirfenidone was not toxic at doses between 0.5 and 1,000 μg/ml and that SEB-induced cytokine levels were reduced in a dose-dependent manner with a drug concentration of 0.5 to 520 μg/ml (data not shown). Therefore, all subsequent experiments were performed using pirfenidone at a concentration of 520 μg/ml. When pirfenidone was added to cells with SEB, TNF-α levels were reduced 75% (Fig. 3). In PBMC incubated with medium only, IFN-γ, IL-1β, and IL-6 were detected at levels ≤20 pg/ml, but in cells incubated with SEB, they were detected at 450, 100, and 280 pg/ml, respectively. When pirfenidone was added to cultures with SEB, the levels of IFN-γ, IL-1β, and IL-6 were decreased 67, 100, and 50%, respectively.
FIG. 3.
Pirfenidone (520 μg/ml) decreased IFN-γ, TNF-α, IL-1β, and IL-6 levels from PBMC cultured for 16 h in vitro with SEB (150 ng/ml). Cytokine levels were measured by ELISA, and the groups included PBMC cultured with medium (med), SEB, and SEB plus pirfenidone (PF). The values represent the mean + SD for duplicate samples from three experiments.
Pirfenidone inhibits human T-cell proliferation induced by SEB.
By the classic definition, superantigens such as SEB induce massive T-cell proliferation (1). Therefore, we tested the ability of pirfenidone to inhibit SEB-induced T-cell proliferation (Fig. 4). Inhibition of SEB-induced proliferation by pirfenidone was dose dependent (12.5 to 1,250 μg/ml), and at higher drug concentrations, there was 70 to 90% inhibition versus cultures treated with SEB alone. The [3H]thymidine uptake among cultures incubated with SEB increased significantly over those of untreated cultures or those incubated with pirfenidone alone (data not shown). There was no evidence of drug-induced cytotoxicity, as determined by trypan blue exclusion, with >95% viability during these experiments, and there was no difference in cytotoxicity in PBMC cultured with or without pirfenidone for 2 days (data not shown).
FIG. 4.
Pirfenidone (PF) inhibited T-cell proliferation in PBMC cultured with SEB (150 ng/ml). Cultures were pulsed with 1 μCi of [3H]thymidine/ml and harvested onto glass fiber filters, and incorporated [3H]thymidine was measured by liquid scintillation. The values are the mean ± SD for triplicate cultures and represent data from three experiments.
DISCUSSION
Excessive production of TNF-α and other proinflammatory cytokines produced when SE activates T cells often results in severe tissue damage and sometimes death (8, 11, 13, 29). Inhibition of T-cell proliferation and reduction of proinflammatory cytokine levels is the underlying target for many therapeutic agents (2, 7, 26, 34). Previous investigations have shown that antibodies recognizing TNF-α decrease symptoms associated with toxic shock (30, 43). Compounds such as niacinamide or pentoxifylline that reduce circulating levels of TNF-α and other proinflammatory cytokines also prevent lethal shock caused by SEs (24, 26). These studies and numerous others reveal that inhibiting cytokine production, particularly that of TNF-α, is central to treatment of toxic shock (23, 30).
Pirfenidone inhibits TNF-α elicited when peritoneal macrophages are cultured with endotoxin. Additionally, mice injected with endotoxin and then treated with pirfenidone do not have elevated serum TNF-α levels and are protected against endotoxic shock (6). The ability of pirfenidone to inhibit TNF-α production in macrophages and protect mice from endotoxin-induced shock led us to investigate whether pirfenidone could lower TNF-α after SEB stimulation and protect mice from lethal effects of SEB exposure.
Numerous investigations show that LPS potentiates the biologic effects of SEs and that septic shock may reflect a culmination of excessive proinflammatory cytokines resulting from the actions of both toxins (3, 4, 24, 35, 39, 40, 42). Although SE and LPS often lead to different sequelae, acute shock caused by abnormally high levels of TNF-α and other proinflammatory cytokines results in life-threatening situations (8, 11, 38). Thus, an animal model in which the SEB effects are magnified by sublethal concentrations of LPS provides an in vivo system that can be used to evaluate the effectiveness of therapeutics against lethal shock (4, 23, 30).
Using this previously characterized model (24, 25, 26, 39, 40), our studies showed that pirfenidone protected mice when SEB was given i.p. (Table 1) or by aerosol (Fig. 1). All of the mice survived if pirfenidone was administered by either route 2 to 3.5 h after SEB, and 60 to 80% survived if pirfenidone was given 4.25 h after SEB. Pirfenidone also delayed death among animals exposed to aerosolized SEB. Within 48 h, 50% of mice challenged with an SEB aerosol and a sublethal dose of LPS had died in contrast to 0% of similarly treated animals given pirfenidone, although some mice given the drug died 72 h post-SEB exposure. Thus, delaying lethal shock may provide an opportunity for further intervention during which another dose of pirfenidone or other therapeutic agents can be administered and thereby provide additional protection against subsequent sequelae, including death.
In addition to the protective effect of pirfenidone against shock, the drug significantly reduced proinflammatory cytokine levels in serum (Fig. 2). Reduced cytokine levels correlated well with survival data (Fig. 1), supporting previous reports that TNF-α and other proinflammatory cytokines play an important role in human septic shock and in this murine model (13, 24, 29).
Our in vivo investigations were confirmed by in vitro experiments with PBMC cultures showing that pirfenidone inhibited SEB-induced T-cell proliferation in a dose-dependent manner (Fig. 4). Inhibition of T-cell proliferation correlated well with reduced cytokine levels (TNF-α, IL-1β, IFN-γ, and IL-6) in hPBL cultures incubated with SEB. Inhibition by pirfenidone of specific SEB-mediated effects was clearly demonstrated by suppression of T-cell proliferation and reduction of IFN-γ production by PBMC, because both T-cell proliferation and IFN-γ release are absent in LPS-stimulated hPBL (data not shown).
As shown by Cain and coworkers (6), the decrease in TNF-α levels among macrophage cultures was not dependent upon the inducing agent, since pirfenidone lowered TNF-α concentrations in macrophages following exposure to either endotoxin or mannosylated bovine serum albumin, both potent TNF-α inducers. Our studies showing that the drug reduced TNF-α concentrations in SEB-activated PBMC cultures provided further evidence that pirfenidone affects production of this cytokine and is not dependent upon cell type or stimulant. This finding agrees with previous reports showing that noncytotoxic concentrations of pirfenidone inhibit proliferation and cytokine synthesis in human retinal pigment epithelial cells cultured in vitro (48).
Pirfenidone produces effects that are similar to those of pentoxifylline in that both drugs inhibit production of TNF-α, IL-1, IFN-γ, and IL-6 and both prevent lethal shock in mice (24). Pentoxifylline has a biphasic effect that inhibits TNF-α at the transcription level and up-regulates production of cytokine receptors that are then shed from the cell surface to neutralize subsequent cytokine activity (15, 33). Investigations to determine whether pirfenidone causes similar responses are under way.
The ability of pirfenidone to prevent T-cell proliferation, as well as to reduce serum levels of TNF-α and other proinflammatory cytokines produced by T cells, macrophages, and presumably fibroblasts, makes the drug an excellent choice for treating inflammatory reactions in the lung. The lungs are extremely sensitive to inflammation caused by SE activation of local T-cell populations and subsequent production of TNF-α (16). TNF-α in turn induces cells to produce IL-6, IL-8, and proinflammatory peptides, such as neutrophil chemoattractant peptide (CINC), that subsequently attract neutrophils and other polymorphonuclear lymphocytes into the lung (44). Upon stimulation, these cells release cytokines, hydroxyl radicals, platelet-activating factor, and other chemokines that damage tissue (32, 45). Since pirfenidone inhibits lipid peroxidation and reduces hydroxyl radical formation, it may also inhibit the production of oxidants and thus prevent further lung injury (31).
Pirfenidone effectively reduces pulmonary fibrosis caused by a wide array of agents and facilitates the repair of fibrotic lesions (18, 36, 37, 41). In clinical situations, pirfenidone should prove to be an excellent therapeutic agent against septic or SE-induced shock, since effective systemic levels of the drug can be attained rapidly and steadily without adverse side effects. The experiments performed in this study were limited to one i.p. dose of pirfenidone. The drug is more efficacious when administered orally, and thus, giving the drug orally could presumably increase survival in our model (36). Future studies will investigate oral administration of pirfenidone in various animal models and the mode of action for protecting against SE-induced symptoms.
Editor: J. T. Barbieri
REFERENCES
- 1.Alber, G., D. K. Hammer, and B. Fleischer. 1990. Relationship between enterotoxic- and T lymphocyte-stimulating activity of staphylococcal enterotoxin B. J. Immunol. 144:4501-4506. [PubMed] [Google Scholar]
- 2.Arad, G., R. Levy, D. Hillman, and R. Kaempfer. 2000. Superantigen antagonist protects against lethal shock and defines a new domain for T-cell activation. Nat. Med. 6:414-421. [DOI] [PubMed] [Google Scholar]
- 3.Beno, D. W., M. R. Uhing, M. Goto, Y. Chen, V. A. Jiyamapa-Serna, and R. E. Kimura. 2001. Staphylococcal enterotoxin B potentiates LPS-induced hepatic dysfunction in chronically catheterized rats. Am. J. Physiol. Gastrointest. Liver Physiol. 5:G866-G872. [DOI] [PubMed]
- 4.Blank, C., A. Luz, S. Bendigs, A. Erdmann, H. Wagner, and K. Heeg. 1997. Superantigen and endotoxin synergize in the induction of lethal shock. Eur. J. Immunol. 27:825-833. [DOI] [PubMed] [Google Scholar]
- 5.Buckley, C. D., D. Pilling, J. M. Lord, A. N. Akbar, D. Scheel-Toellner, and M. Salmon. 2001. Fibroblasts regulate the switch from acute resolving to chronic persistent inflammation. Trends Immunol. 22:199-204. [DOI] [PubMed] [Google Scholar]
- 6.Cain, W. C., R. W. Stuart, D. L. Lefkowitz, J. D. Starnes, S. B. Margolin, and S. S. Lefkowitz. 1998. Inhibition of tumor necrosis factor and subsequent endotoxin shock by pirfenidone. Int. J. Immunopharmacol. 20:685-695. [DOI] [PubMed] [Google Scholar]
- 7.Calandra, T., B. Echtenacher, D. LeRoy, J. Pugin, C. N. Metx, L. Hultner, D. Heumann, D. Mannel, R. Bucala, and M. P. Glauser. 2000. Protection from septic shock by neutralization of macrophage migration inhibitory factor. Nat. Med. 6:164-170. [DOI] [PubMed] [Google Scholar]
- 8.Cannon, J. G., R. G. Tompkins, J. A. Gelfand, H. R. Michie, G. G. Stanford, J. W. van der Meer, S. Endres, G. Lonnemann, J. Corsetti, and B. Chernow. 1990. Circulating interleukin-1 and tumor necrosis factor in septic shock and experimental endotoxin fever. J. Infect. Dis. 161:79-84. [DOI] [PubMed] [Google Scholar]
- 9.Chan, F. K., R. M. Siegel, and M. J. Lenardo. 2000. Signaling by the TNF receptor superfamily and T cell homeostasis. Immunity 13:419-422. [DOI] [PubMed] [Google Scholar]
- 10.Choi, Y., B. Kotzin, L. Herron, J. Callahan, P. Marrack, and J. W. Kappler. 1989. Interaction of Staphylococcus aureus toxin “superantigens” with human T cells. Proc. Natl. Acad. Sci. USA 86:8941-8945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Das, U. N. 2000. Critical advances in septicemia and septic shock. Crit. Care 4:290-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Department of Health and Human Services. 1985. Guide for the care and use of laboratory animals, revised ed. NIH publication no. 86-23. Department of Health and Human Services, Washington, D.C.
- 13.Fast, D. J., P. M. Schlievert, and R. D. Nelson. 1989. Toxic shock syndrome-associated staphylococcal and streptococcal pyrogenic toxins are potent inducers of tumor necrosis factor production. Infect. Immun. 57:291-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujisawa, N., S. Hayashi, A. Kurdowska, J. M. Noble, K. Naitoh, and E. J. Miller. 1998. Staphylococcal enterotoxin A injury of human lung endothelial cells and IL-8 accumulation are mediated by TNF-α. J. Immunol. 161:5627-5632. [PubMed] [Google Scholar]
- 15.Han, J., P. Thompson, and B. Beutler. 1990. Dexamethosone and pentoxifylline inhibit endotoxin-induced cachectin/tumor necrosis factor synthesis at separate points in the signaling pathway. J. Exp. Med. 172:391-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herz, U., R. Ruckert, K. Wollenhaupt, T. Tschernig, U. Neuhaus-Steinmetz, R. Pabst, and H. Renz. 1999. Airway exposure to bacterial superantigen (SEB) induces lymphocyte-dependent airway inflammation associated with increased airway responsiveness--a model for non-allergic asthma. Eur. J. Immunol. 29:1021-1031. [DOI] [PubMed] [Google Scholar]
- 17.Iyer, S. N., G. Gurujeyalakshmi, and S. N. Giri. 1999. Effects of pirfenidone on transforming growth factor-β gene expression at the transcriptional level in bleomycin model of lung fibrosis. J. Pharmacol. Exp. Ther. 291:367-373. [PubMed] [Google Scholar]
- 18.Iyer, S. N., J. S. Wild, M. J. Schiedt, D. M. Hyde, S. B. Margolin, and S. N. Giri.1995. Dietary intake of pirfenidone ameliorates bleomycin-induced lung fibrosis in hamsters. J. Lab. Clin. Med. 125:779-785. [PubMed] [Google Scholar]
- 19.Johnson, H. M., B. A. Torres, and J. M. Soos. 1991. Superantigens: structure and relevance to human disease. Proc. Soc. Exp. Biol. Med. 198:765-771. [DOI] [PubMed] [Google Scholar]
- 20.Kaneko, M., H. Inoue, R. Nakazawa, N. Azuma, M. Suzuki, S. Yamauchi, S. B. Margolin, K. Tsubota, and I. Saito. 1998. Pirfenidone induces intercellular adhesion molecule-1 (ICAM-1) down-regulation on cultured human synovial fibroblasts. Clin. Exp. Immunol. 113:72-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kappler, J. W., A. Herman, J. Clements, and P. Marrack. 1992. Mutations defining functional regions of the superantigen staphylococcal enterotoxin B. J. Exp. Med. 175:387-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kasai, T., K. Inada, T. Takakuwa, Y. Yamada, Y. Inoue, T. Shimamura, S. Taniguchi, S. Sato, G. Wakabayashi, and S. Endo. 1997. Anti-inflammatory cytokine levels in patients with septic shock. Res. Commun. Mol. Pathol. Pharmacol. 98:34-42. [PubMed] [Google Scholar]
- 23.Krakauer, T. 1999. Immune response to staphylococcal superantigens. Immunol. Res. 20:163-173. [DOI] [PubMed] [Google Scholar]
- 24.Krakauer, T., and B. G. Stiles. 1999. Pentoxifylline inhibits superantigen-induced toxic shock and cytokine release. Clin. Diagn. Lab. Immunol. 6:594-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.LeClaire, R. D., R. E. Hunt, S. Bavari, J. E. Estep, G. O. Nelson, and C. L. Wilhelmsen. 1996. Potentiation of inhaled staphylococcal enterotoxin B-induced toxicity by lipopolysaccharide in mice. Toxicol. Pathol. 24:619-626. [DOI] [PubMed] [Google Scholar]
- 26.LeClaire, R. D., W. Kell, S. Bavari, T. J. Smith, and R. E. Hunt. 1996. Protective effects of niacinamide in staphylococcal enterotoxin-B-induced toxicity. Toxicology 107:69-81. [DOI] [PubMed] [Google Scholar]
- 27.Locksley, R. M., N. Killeen, and M. J. Lenardo. 2001. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 104:487-501. [DOI] [PubMed] [Google Scholar]
- 28.Marrack, P., and J. Kappler. 1990. The staphylococcal enterotoxins and their relatives. Science 248:705-709. [DOI] [PubMed] [Google Scholar]
- 29.Miethke, T., K. Duschek, C. Wahl, K. Heeg, and H. Wagner. 1993. Pathogenesis of the toxic shock syndrome: T cell mediated lethal shock caused by the superantigen TSST-1. Eur. J. Immunol. 23:1494-1500. [DOI] [PubMed] [Google Scholar]
- 30.Miethke, T., C. Wahl, K. Heeg, B. Echtenacher, P. H. Krammer, and H. Wagner. 1992. T cell-mediated lethal shock triggered in mice by the superantigen staphylococcal enterotoxin B: critical role of tumor necrosis factor. J. Exp. Med. 175:91-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Misra, H. P., and C. Rabideau. 2000. Pirfenidone inhibits NADPH-dependent microsomal lipid peroxidation and scavenges hydroxyl radicals. Mol. Cell. Biochem. 204:119-126. [DOI] [PubMed] [Google Scholar]
- 32.Neuman, B., B. Engelhardt, H. Wagner, and B. Holzmann. 1997. Induction of acute inflammatory lung injury by staphylococcal enterotoxin B. J. Immunol. 158:1861-1871. [PubMed] [Google Scholar]
- 33.Neuner, P., G. Klosner, E. Schauer, M. Pourmojib, W. Macheiner, C. Grunwald, R. Knobler, A. Schwarz, T. A. Luger, and T. Schwarz. 1994. Pentoxifylline in vivo down regulates the release of IL-1-beta, IL-6, IL-8 and tumor necrosis factor-alpha by human peripheral blood mononuclear cells. Immunology 83:262-267. [PMC free article] [PubMed] [Google Scholar]
- 34.Onai, H., and S. Kudo. 2001. Suppression of superantigen-induced lung injury and vasculitis by preadministration of human urinary trypsin inhibitor. Eur. J. Clin. Investig. 31:272-280. [DOI] [PubMed] [Google Scholar]
- 35.Petit, G. W., M. R. Elwell, and P. B. Jahrling. 1977. Possible endotoxemia in rabbits after intravenous injection of Staphylococcus aureus enterotoxin B. J. Infect. Dis. 4:646-648. [DOI] [PubMed] [Google Scholar]
- 36.Raghu, G., W. C. Johnson, D. Lockhart, and Y. Mageto. 1999. Treatment of idiopathic pulmonary fibrosis with a new antifibrotic agent, pirfenidone: results of a prospective, open-label phase II study. Am. J. Respir. Crit. Care Med. 159:1264-1267. [DOI] [PubMed] [Google Scholar]
- 37.Shetlar, M. R., D. J. Shetlar, D. J. Bloom, C. L. Shetlar, and S. B. Margolin. 1998. Involution of keloid implants in athymic mice treated with pirfenidone or with triamcinolone. J. Lab. Clin. Med. 132:491-496. [DOI] [PubMed] [Google Scholar]
- 38.Slifka, M. K., and J. L. Whitton. 2000. Clinical implication of dysregulated cytokine production. J. Mol. Med. 78:74-80. [DOI] [PubMed] [Google Scholar]
- 39.Stiles, B. G., S. Bavari, T. Krakauer, and R. G. Ulrich. 1993. Toxicity of staphylococcal enterotoxins potentiated by lipopolysaccharide: major histocompatability complex class II molecule dependency and cytokine release. Infect. Immun. 61:5333-5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stiles, B. G., T. Krakauer, and P. F. Bonventre. 1995. Biological activity of toxic shock syndrome toxin 1 and a site-directed mutant, H135A, in a lipopolysaccharide-potentiated mouse lethality model. Infect. Immun. 63:1229-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suga, H., S. Teraoka, K. Ota, S. Komemushi, S. Furutani, S. Yamauchi, and S. B. Margolin. 1995. Preventive effect of pirfenidone against experimental sclerosing peritonitis in rats. Exp. Toxicol. Pathol. 47:287-291. [DOI] [PubMed] [Google Scholar]
- 42.Sugiyama, H., E. M. McKissic, M. S. Bergdoll, and B. Heller. 1964. Enhancement of bacterial endotoxin lethality by staphylococcal enterotoxin. J. Infect. Dis. 114:111-118. [DOI] [PubMed] [Google Scholar]
- 43.Tracey, K. J., Y. Fong, D. G. Hesse, K. R. Manogue, A. T. Lee, G. C. Kuo, S. F. Lowry, and A. Cerami. 1987. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature (London) 330:662-664. [DOI] [PubMed] [Google Scholar]
- 44.Utsunomiya, I., M. Ito, and S. Oh-ishi. 1998. Generation of inflammatory cytokines in zymosan-induced pleurisy in rats: TNF induces IL-6 and cytokine-induced neutrophil chemoattractant (CINC) in vivo. Cytokine 10:956-963. [DOI] [PubMed] [Google Scholar]
- 45.Warner, R. L., L. Beltran, E. M. Younkin, C. S. Lewis, S. J. Weiss, J. Varnai, and K. J. Johnson. 2001. Role of stromelysin 1 and gelatinase B in experimental acute lung injury. Am. J. Respir. Cell Mol. Biol. 24:537-544. [DOI] [PubMed] [Google Scholar]
- 46.White, J., A. M. Pullen, R. Kubo, J. Kappler, and P. Marrack. 1989. The Vβ-specific superantigen staphylococcal enterotoxin B: stimulation of mature T cells and clonal deletion in neonatal mice. Cell 56:27-35. [DOI] [PubMed] [Google Scholar]
- 47.Wood, A. C., I. Todd, A. Cockayne, and J. P. Arbuthnott. 1991. Staphylococcal enterotoxins and the immune system. FEMS Microbiol. Immunol. 3:121-133. [DOI] [PubMed] [Google Scholar]
- 48.Zhang, S., I. A. Shiels, J. S. Ambler, and S. M. Taylor. 1998. Pirfenidone reduces fibronectin synthesis by cultured human retinal pigment epithelial cells. Aust. N. Z. Opthalmol. 26(Suppl. 1):S74-S76. [DOI] [PubMed]