Abstract
Monoclonal antibodies (MAbs) reactive with glucuronoxylomannan (GXM), the major capsular polysaccharide of the yeast Cryptococcus neoformans, produce distinct capsular reactions when viewed by differential interference contrast microscopy. These reactions depend on the epitope specificity of the antibody. Opsonic activities of immunoglobulin G1 (IgG1) MAbs that produce patterns termed rim and puffy were examined. Rim-pattern MAbs are reactive with an epitope shared by GXM serotypes A, B, C, and D. Puffy-pattern MAbs are reactive only with serotypes A and D. In phagocytosis assays, using serotype A cells and resident murine peritoneal macrophages, rim-pattern MAbs were markedly more opsonic than puffy-pattern MAbs. F(ab′)2 fragments of rim-pattern MAbs were synergistic with heat-labile factors in normal human serum for opsonization of the yeast. F(ab′)2 fragments of puffy-pattern MAbs were also synergistic with normal serum in opsonization but at a much lower level than fragments of rim-pattern MAbs. Normal serum alone was not opsonic. F(ab′)2 fragments of rim-pattern MAbs, but not puffy-pattern MAbs, stimulated phagocytosis of encapsulated cryptococci in the absence of serum. This serum-independent opsonic action of F(ab′)2 fragments was abrogated by pretreatment of macrophages with purified GXM, suggesting the involvement of a phagocyte GXM receptor. The results indicate that (i) there are multiple mechanisms by which anticapsular IgG MAbs facilitate phagocytosis of encapsulated cryptococci, (ii) some anti-GXM antibodies are opsonic in an Fc-independent manner, and (iii) opsonic activity correlates with the capsular reaction and occurs in an epitope-specific manner.
Cryptococcus neoformans is an encapsulated yeast that can produce a life-threatening meningitis in patients with defects in cellular immunity, especially those with AIDS. The capsular polysaccharide is an essential virulence factor; acapsular mutant strains are avirulent (4, 14). The major polysaccharide constituent of the capsule is glucuronoxylomannan (GXM). GXM has an α-1,3-linked mannose backbone that is O acetylated and substituted with single side chains of xylose and glucuronic acid. The degree of xylose substitution and O acetylation varies considerably, to produce four primary serotypes (A to D) and eight chemotypes (2, 6, 8, 39).
The cryptococcal capsule is a potent activator of the complement system, leading to binding of potentially opsonic fragments of C3, predominantly iC3b, within the capsular matrix and at the capsular surface (22, 25). Complement activation that occurs during incubation of encapsulated cryptococci in normal human serum (NHS) is due entirely to the action of the alternative complement pathway (24, 25). Despite the ability of the capsule to accumulate potentially opsonic ligands at the capsular surface, the capsule has potent antiphagocytic activity. Acapsular cryptococci are readily ingested by phagocytes such as macrophages and neutrophils; encapsulated cryptococci are poorly ingested (3, 19). Encapsulated cryptococci may be ingested at low levels if the yeast cells have been coated with heat-labile opsonins; however, the levels of phagocytosis are well below levels observed with acapsular cryptococci. In contrast, macrophages that have been treated with tumor necrosis factor alpha and granulocyte-monocyte colony-stimulating factor efficiently ingest encapsulated cryptococci that are serum opsonized (9, 10). The mechanism for the antiphagocytic action of the cryptococcal capsule is not known.
Several laboratories have developed monoclonal antibodies (MAbs) that are reactive with distinct epitopes on GXM. Studies by us and others found that the biological activities of anti-GXM MAbs may be dramatically influenced by the epitope specificity of the antibody. For example, MAbs of the immunoglobulin G1 (IgG1) isotype that are reactive with an epitope that is shared by GXM serotypes A, B, C, and D produce early activation of the classical complement pathway but suppress the overall rate and amount of C3 that would normally bind to the yeast as a consequence of activation of the alternative pathway (20). In contrast, IgG1 MAbs reactive with an epitope found only on serotypes A and D fail to activate the classical pathway and have no effect on deposition of C3 via the alternative pathway. In another example, IgM anti-GXM MAbs having distinct epitope specificities have quite different abilities to provide protection in a murine model of cryptococcosis (30, 31). Some antibodies are protective; other antibodies fail to protect.
In a recent study, we reported that antibodies with different epitope specificities produce distinct capsular reactions (similar to Neufeld's quellung reaction) when viewed by differential interference contrast (DIC) microscopy (29). The capsular reactions fell into two general categories. An annular pattern, termed rim, is produced on incubation of encapsulated serotype A cryptococci with MAbs reactive with an epitope shared by serotypes A, B, C, and D. Cryptococci with the rim pattern appear to have a transparent capsular interior with a highly refractive outer edge, suggesting the presence of an antibody-produced shell at the capsular surface. A second pattern, termed puffy, is produced by incubation of serotype A cells with MAbs reactive only with serotype A and D GXM. Importantly, the capsular reaction correlated with biological activities of the antibodies. Antibodies producing the rim pattern (i) activated the classical pathway when IgG1 antibodies were used, (ii) suppressed C3 deposition via the alternative pathway, and (iii) were protective in a murine model of cryptococcosis. In contrast, antibodies producing the puffy pattern (i) failed to activate the classical pathway when IgG1 antibodies were used, (ii) showed no suppression of C3 deposition via the alternative pathway, and (iii) failed to protect in a murine model of cryptococcosis.
The present study was designed to further understand the association between the epitope specificities of anti-GXM MAbs and biological activity. We examined the opsonic activities of antibodies producing rim and puffy capsular reactions. We also assessed the opsonic activities of Fab and F(ab)2 fragments of the antibodies. Fab and F(ab)2 fragments were examined because F(ab)2 fragments of rim-pattern antibodies produce a substantial change in the capsular surface whereas Fab fragments do not (29). The results showed that antibodies producing the rim pattern enhanced phagocytosis to a much greater extent than antibodies producing the puffy pattern. The results also found that antibodies producing the rim pattern have the potential for opsonic activity in an Fc-independent manner.
MATERIALS AND METHODS
C. neoformans cells and yeast polysaccharides.
Serotype A strain CN6 was used throughout and was provided by R. Cherniak (Georgia State University, Atlanta). Yeast cells were incubated in synthetic medium (7) supplemented with 24 mM sodium bicarbonate and 25 mM HEPES for 4 days at 37°C with 5% CO2. Cryptococci grown under these conditions produce large capsules (16). Preliminary studies determined that strain CN6 is particularly responsive to these growth conditions, producing uniformly large capsules (cell to cell) with a mean capsular width (outer edge of cell wall to outer capsular edge) of 4.5 μm. For one series of experiments, cells were grown at 30°C in synthetic medium without sodium bicarbonate, HEPES, and CO2. Cells grown under these conditions produced capsules with a mean capsular width of 0.8 μm. All yeast cells were killed by overnight incubation with 1% formaldehyde, washed with phosphate-buffered saline (PBS) and stored at 4°C.
GXM was isolated from supernatant fluids of CN6 cultures as described previously (7). Mannan was isolated from a strain of Candida albicans serotype A (ATCC 36801). C. albicans cells were grown on 2% glucose-1% peptone-0.3% yeast extract, the cells were collected by centrifugation, and the mannan was extracted by addition of hot water and autoclaving for 4 h. The mannan was precipitated as a copper complex by using Fehling's solution and then dissociated from the complex with Amberlite ion-exchange resin (Sigma, St. Louis, Mo.). The mannan was dialyzed against water, lyophilized, and stored at −20°C. GXM and mannan were solubilized in PBS, filter sterilized, and stored at 4°C.
MAbs and immunoglobulin fragments.
Anti-GXM MAbs 3C2, 471, and 302 are all of the IgG1 subclass and have been previously described in detail (1, 5, 13, 36). A new anti-GXM MAb, designated MAb 1326, was generated by immunizing BALB/c mice with serotype A GXM as previously described (13). MAb 1326 is an IgG1 that is reactive only with serotype A and D GXM. The characteristics of all MAbs used in the study are summarized in Table 1. MAbs 3C2, 1326, and 302 were produced by in vitro culture in a Tecnomouse system (Integra Biosciences, Ijamsville, Md.). MAb 471 was purified from mouse ascites by differential precipitation with caprylic acid and ammonium sulfate followed by immunoaffinity chromatography with a GXM-Sepharose column (21) and affinity chromatography with protein A. MAbs produced by in vitro culture were isolated by protein A affinity chromatography. Concentrations of MAbs were determined by UV spectroscopy, using an optical density at 280 nm of 1.43 for 1 mg of IgG/ml (34).
TABLE 1.
Characteristics of anti-GXM MAbs
| MAba | Molecular groupb | Serotype reactivityc | References |
|---|---|---|---|
| 3C2 | II | A, B, C, D | (1, 36) |
| 471 | II | A, B, C, D | (1, 36) |
| 302 | IV | A, D | (1, 13) |
| 1326 | NDd | A, D | This study |
All MAbs are of the IgG1 isotype.
Based on variable-region light- and heavy-chain sequences (5).
Determined by double immunodiffusion in agar and ELISA with GXM of known serotypes.
ND, not determined
F(ab′)2 and Fab fragments were generated by ficin digestion with the Immunopure IgG1 Fab and F(ab′)2 preparation kit (Pierce, Rockford, Ill.) and separated from intact IgG and Fc fragments by protein A affinity chromatography (Pierce). F(ab′)2 and Fab fragments were further purified by molecular sieve chromatography on Superdex 200 (Pharmacia, Princeton, N.J.). In an effort to eliminate contamination by intact IgG or other IgG fragments, fractions from the central two-thirds of the F(ab′)2 or Fab protein peaks from the Superdex 200 chromatography were pooled and concentrated, and the molecular sieve chromatography was repeated. The central two-thirds of the final peak was pooled, filter sterilized, and frozen at −80°C. Concentrations of the MAb fragments were determined by UV spectroscopy, using optical densities at 280 nm of 1.48 for 1 mg of F(ab′)2/ml and 1.53 for 1 mg of Fab/ml (34).
F(ab′)2 and Fab fragments were examined by enzyme-linked immunosorbent assay (ELISA) for the presence of undigested IgG. The ELISA was constructed using GXM-coated plates as described previously (13). Binding of intact IgG was assessed with a horseradish peroxidase-conjugated second antibody specific for the mouse Fc fragment (Sigma). As a positive control, binding of the F(ab′)2 and Fab fragments to immobilized GXM was confirmed with a horseradish peroxidase-conjugated secondary antibody specific for mouse kappa chains (Southern Biotechnology, Birmingham, Ala.).
Phagocytosis assays.
The use of laboratory animals in this study was approved by the University of Nevada, Reno, Institutional Animal Care and Use Committee and was compliant with relevant federal guidelines. Resident macrophages were harvested from the peritoneal cavities of 8- to 12-week-old female Swiss-Webster mice (Animal Production Program, Frederick Cancer Research and Development Center, Frederick, Md.). Cells were collected by centrifugation, resuspended in Iscove's modification of Dulbecco modified Eagle medium (Mediatech, Inc., Herndon, Va.) containing 10% heat-inactivated fetal bovine serum (HyClone, Logan, Utah), counted, added to eight-well Nunc Lab-Tek II chamber slides (Fisher Scientific, Pittsburgh, Pa.) at 2.5 × 105 cells per well, and incubated for 24 h at 37°C with 5% CO2. Nonadherent cells were removed by washing twice with Iscove's medium and incubated for an additional 24 h with Iscove's medium containing 10% fetal bovine serum.
Formaldehyde-killed CN6 cells were treated with (i) pooled NHS, (ii) heat-inactivated NHS (30 min at 56°C), (iii) MAbs or their F(ab′)2 or Fab fragments, or (iv) combinations of serum and MAbs. Treatment with NHS or heat-inactivated NHS was done by incubation of 5,000 yeast cells/μl of serum for 30 min followed by two washes with prewarmed Iscove's medium. Treatment with IgG, F(ab′)2, or Fab alone was accomplished by incubating yeast cells (3.3 × 106/ml) for 5 min at 37°C with MAbs or their fragments at 50 μg/ml. Some experiments required yeast cells treated with both NHS and MAbs. In this case, yeast cells were incubated with NHS for 30 min at 37°C, washed two times in Iscove's medium, and then incubated with MAbs for an additional 5 min at 37°C. This sequential treatment (NHS followed by MAb) took advantage of the intrinsic ability of encapsulated cryptococci to activate C3 and deposit potentially opsonic fragments of C3 via the alternative pathway and avoided any differences in deposition of C3 via either the classical or alternative pathway that would have occurred in the presence of rim or puffy antibodies (29).
For phagocytosis assays, monolayers of peritoneal macrophages were washed two times with fresh prewarmed Iscove's medium and overlaid with 150 μl of medium, and appropriately treated yeast cells (5 × 105 in 150 μl of Iscove's medium) were added to each chamber (approximately two yeast cells per macrophage) and incubated for 30 min at 37°C. Following incubation of yeast cells and peritoneal macrophages, the chambers were removed and the slides were washed with Iscove's medium, stained with Sure Stain Wright-Giemsa (Fisher Scientific), washed in water, air dried, and mounted with Permount (Fisher Scientific). One hundred macrophages were examined per well, and attachment or phagocytic indices (number of cells ingested per macrophage) were determined. DIC microscopy was utilized to distinguish ingested from attached cryptococci. Attached yeast cells were highly refractile with distinct cell walls; ingested yeast cells were not highly refractile, and the cell walls lacked clarity. Preliminary studies comparing attachment and ingestion by use of light microscopy and fluorescence microscopy with Uvitex (28) yielded similar results. To score a yeast cell as ingested, the entire cell had to be within the macrophage membrane. Any cryptococci that were not entirely engulfed were scored as attached. In all experiments using large-capsule cells, the level of attachment was 30 to 50% of the level of ingestion. There was no instance when the attachment results did not directly track the ingestion results. As a consequence, results from most experiments are reported only as the phagocytic (ingestion) index. The sole exception occurred with yeast cells grown to produce small capsules. In this instance, both attachment and ingestion data are reported. Unless otherwise noted, results are reported as the mean ± standard error of the mean (SEM) from three independent experiments. Each experiment had at least three replicate wells per data point.
The ability of GXM or C. albicans mannan to inhibit phagocytosis was assessed by addition of 20 or 100 μg of GXM or mannan in 150 μl of Iscove's medium to monolayers of macrophages. The macrophages were incubated for 5 min at 37°C; appropriately treated yeast cells (5 × 105 in 150 μl of Iscove's medium) were added and incubated for an additional 30 min at 37°C, and attachment and ingestion were assessed as described above.
DIC microscopy.
MAb-induced capsular reactions were done as previously described (29). Intact IgG, F(ab′)2, or Fab molecules at 50 μg/ml of PBS were incubated with CN6 cells (2 × 104) in a 50-μl reaction volume (PBS) for 5 min at 37°C. Capsular reactions were assessed with a Nikon Eclipse ES 800 microscope equipped with DIC optics. Images were acquired with a Photonic Sciences (Millham, United Kingdom) integrating charge-coupled device camera and Image Pro Plus 3.0 image analysis software (Media Cybernetics, Silver Spring, Md.). Capsular reactions were classified as rim or puffy (29). The rim pattern is characterized by a sharp increase in the optical gradient at the capsular edge followed directly by a decrease in the optical gradient. The hallmark of the puffy pattern is an increase in the optical gradient at the capsular surface and the absence of the immediate decrease that is characteristic of the rim pattern.
Statistical analysis.
Statistical analysis was done by analysis of variance with the assistance of SigmaStat version 2.03 (SPSS, Inc., Chicago, Ill.). Pairwise comparisons were done after the initial analysis of variance by using the Tukey post hoc test.
RESULTS
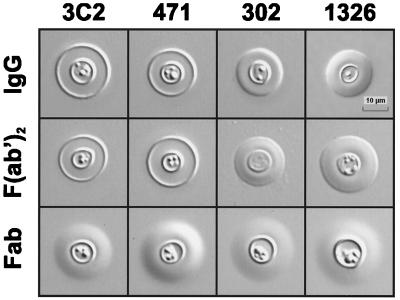
Our previous studies found that MAbs with different epitope specificities produce distinct capsular reactions with serotype A cryptococci (29). MAbs reactive with an epitope shared by serotypes A, B, C, and D produce an annular or rim pattern; MAbs reactive with only serotypes A and D produce a puffy pattern. Production of the rim pattern requires a bivalent antibody. An initial experiment verified the capsular pattern for the previously described MAbs (3C2, 471, and 302) and determined that MAb 1326 produced the puffy pattern, a result that is predicted on the basis of its reactivity with only serotypes A and D (Fig. 1). These studies also found that F(ab′)2 fragments of MAb 3C2 produced the rim pattern, whereas the Fab fragments produced a puffy pattern. The results shown in Fig. 1 demonstrate that MAb 471 and its F(ab′)2 and Fab fragments produced capsular reactions that were identical to patterns produced by MAb 3C2. In contrast, MAbs 302 and 1326 and their F(ab′)2 and Fab fragments produced patterns that represented variations on the puffy pattern. It is notable that MAbs 302 and 1326 produced a capsular reaction characterized by a rapid increase in refractive index at the capsular edge (seen as a change in light intensity from light to dark or dark to light), but the increase in refractive index was not nearly as precipitous as the increase seen with MAbs producing the rim pattern. Moreover, the capsular reaction produced by MAbs 302 and 1326 lacked the rapid decrease in refractive index that defines the rim pattern.
FIG. 1.
Capsular binding patterns produced by anti-GXM MAbs. MAbs 3C2, 471, 1326, and 302 [IgG, F(ab′)2, and Fab; 50 μg/ml] incubated with CN6 serotype A yeast cells produce two distinct capsular binding patterns, rim and puffy. Bivalent molecules of MAbs 3C2 and 471 [IgG and F(ab′)2] produce the rim pattern while the Fab fragments produce a form of the puffy pattern. MAbs 1326 and 302 produce variations of the puffy pattern with all antibody molecules. Images were acquired with a 100× oil immersion objective and DIC optics.
Opsonic activity of intact IgG1 MAbs.
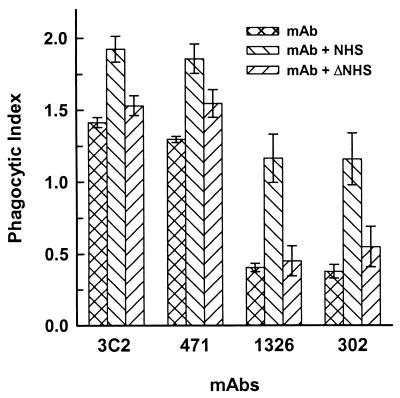
Serotype A cryptococci were opsonized with MAb 3C2, 471, 1326, or 302 alone or in the presence of NHS or heat-inactivated serum in an effort to evaluate the opsonic activity of MAbs that produce the rim and puffy capsular reactions. The results (Fig. 2) showed that the rim-pattern MAbs (MAbs 3C2 and 471) produced phagocytic indices that were threefold greater (P < 0.001) than those of MAbs that produce the puffy pattern (MAbs 1326 and 302; phagocytic indices of 1.4 and 1.3 versus 0.4 and 0.4, respectively). In all cases, attached or ingested cells appeared largely as individual yeast cells, indicating that agglutination of yeast cells by antibody had not occurred. In addition, microscopic examination of suspensions of yeast cells incubated with each of the antibodies showed an absence of agglutination under the conditions used in these experiments. Incubation of encapsulated cryptococci in NHS prior to treatment with the MAbs produced a significant (P < 0.001) enhancement in the opsonic activities of all MAbs. However, phagocytosis of cryptococci treated with puffy-pattern-producing MAbs 1326 and 302 in combination with NHS was well below phagocytosis of cryptococci incubated with NHS and rim-pattern producing MAbs 3C2 and 471. The opsonic activity of the NHS was synergistic with the MAbs rather than additive, because no measurable phagocytosis was observed with cryptococci opsonized by incubation with NHS alone (not shown). With the exception of MAb 471, treatment of encapsulated cryptococci with heat-inactivated serum prior to incubation with the MAbs produced no significant enhancement (P > 0.05) in phagocytosis over what was observed with the MAb alone, indicating that the synergistic action of NHS was dependent on heat-labile opsonins. Significant enhancement by heat-inactivated serum of the opsonic activity of MAb 471 was observed in one of three independent experiments and is probably not biologically significant.
FIG. 2.
Phagocytosis of encapsulated cryptococci treated with MAbs (50 μg/ml) that produce rim (MAbs 3C2 and 471) and puffy (MAbs 1326 and 302) capsular reactions. Serotype A cryptococci were treated with (i) IgG alone (ii) IgG and NHS, or (iii) IgG and heat-inactivated NHS (ΔNHS). Data are reported as the mean phagocytic index (number of ingested yeast cells per macrophage) from three independent experiments ± SEM.
The complete lack of ingestion of cryptococci opsonized with NHS was somewhat unexpected, because numerous previous studies found limited, but readily measurable, phagocytosis of NHS-opsonized cryptococci by neutrophils and macrophages. One important difference between the studies shown in Fig. 2 and studies described in many previous reports was our use of cryptococci grown under conditions that induce large capsules. As a consequence, we compared attachment and ingestion of cryptococci that were grown with bicarbonate-CO2 (capsule-inducing conditions) and without bicarbonate-CO2 (no induction of capsule). Cryptococci with small capsules that were treated with NHS could attach and be ingested by macrophages (Table 2). In contrast, large-capsule cryptococci treated with NHS neither attached nor were ingested.
TABLE 2.
Attachment and ingestion of NHS-opsonized cryptococci grown under conditions that produce large and small capsules
| Cryptococcal cells | Capsule width, μm (mean ± SD) | Attachment index (mean ± SD) | Ingestion index (mean ± SD) |
|---|---|---|---|
| Large capsule (grown with bicarbonate-CO2) | 4.5 ± 0.8 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Small capsule (grown without bicarbonate-CO2) | 0.80 ± 0.08 | 0.57 ± 0.30 | 0.55 ± 0.30 |
Opsonic activity of F(ab′)2 fragments.
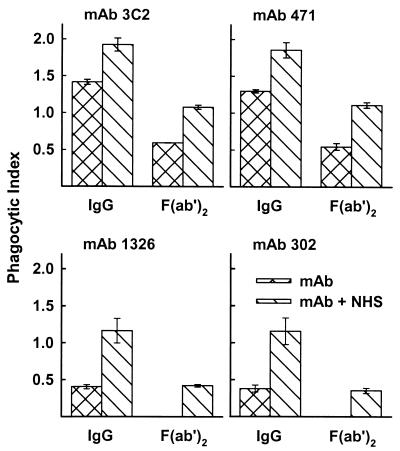
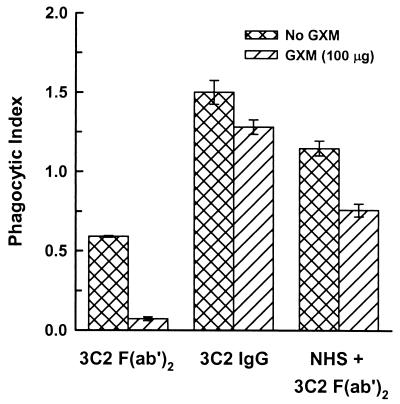
In a previous study we found that production of the rim pattern required cross-linking by a bivalent antibody or its F(ab′)2 fragments and produced a highly refractile shell at the capsular surface that prevented penetration of anticapsular antibodies to the capsular interior (29). Since opsonization by incubation in NHS alone failed to produce any phagocytosis of large-capsule cryptococci, we considered the possibility that the dramatic change in capsular structure produced by F(ab′)2 fragments of rim-pattern antibodies (Fig. 1) might facilitate phagocytosis of cryptococci opsonized by NHS. F(ab′)2 fragments of puffy-pattern MAbs alone (MAbs 1326 and 302) showed no opsonic activity in the absence of NHS (Fig. 3). Surprisingly, F(ab′)2 fragments of rim-pattern MAbs (3C2 and 471) alone induced phagocytosis of cryptococci, with phagocytic indices of 0.6 and 0.5. Such opsonic activity was not due to contamination of the F(ab′)2 fragments by undigested IgG. Undigested IgG had been removed by affinity chromatography over protein A and exhaustive molecular sieve chromatography. Analysis of the F(ab′)2 fragments by ELISA specific for murine Fc fragments showed undetectable levels of antibodies carrying Fc fragments (level of sensitivity, 1 ng/ml). The minimal level of intact IgG (MAbs 3C2 and 471) needed to show opsonic activity was 2.0 and 0.4 μg/ml, respectively, for cryptococci opsonized with IgG alone or with both IgG and NHS. As in the case of intact antibodies, microscopic examination of macrophages incubated with the various antibodies or suspensions of yeast cells and antibodies showed no evidence of agglutination of the yeast cells under the conditions used for these experiments.
FIG. 3.
Phagocytosis of cryptococci treated with intact IgG MAbs or the F(ab′)2 fragments of MAbs (50 μg/ml) that produce rim (MAbs 3C2 and 471) or puffy (MAbs 1326 and 302) capsular reactions. Cryptococci were treated with intact IgG or F(ab′)2 alone or in combination with NHS. Data are reported as the mean phagocytic index from three independent experiments ± SEM.
The phagocytic indices observed with cryptococci treated with both NHS and rim-pattern F(ab′)2 fragments (1.1 and 1.1 for MAbs 3C2 and 471, respectively) were significantly higher (P < 0.05) than those with cryptococci treated with both NHS and puffy-pattern MAbs (phagocytic indices of 0.4 and 0.4 for MAbs 1326 and 302, respectively) (Fig. 3). Phagocytosis of cryptococci opsonized by incubation with NHS and F(ab′)2 fragments of the puffy pattern was due to synergy between the two treatments, because neither treatment alone stimulated ingestion by macrophages. Phagocytosis of yeast cells treated with Fab fragments (3C2, 471, 1326, and 302) alone or in combination with NHS did not stimulate either attachment or ingestion of large-capsule cryptococci by peritoneal macrophages (not shown).
Inhibition of F(ab′)2-mediated phagocytosis by soluble GXM.
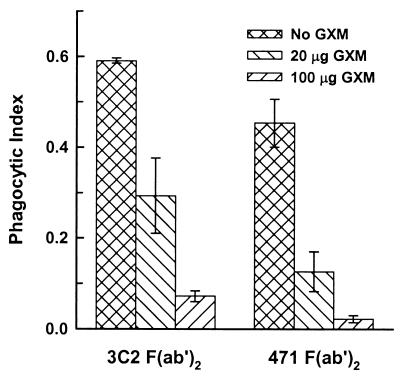
Facilitation of phagocytosis by F(ab′)2 fragments of the rim-producing MAbs 3C2 and 471 alone (Fig. 3) was unexpected because the immunoglobulin fragments lacked ligands for known receptors on macrophages. An alternative explanation is the possibility that capsular GXM itself is functioning as a ligand that acts in concert with the F(ab′)2 fragments to facilitate phagocytosis. To assess this possibility, we examined the ability of purified GXM to block phagocytosis of cryptococci opsonized with F(ab′)2 fragments of MAbs 3C2 and 471. Macrophage monolayers were preincubated for 5 min at 37°C with 20 or 100 μg of GXM (133 and 667 μg/ml, respectively) before the addition of cryptococci opsonized with F(ab′)2 fragments. The results (Fig. 4) showed significant inhibition (P < 0.001) of Fc-independent opsonization by 20 μg of GXM and almost complete inhibition by 100 μg of GXM.
FIG. 4.
Inhibition of F(ab′)2-mediated phagocytosis by soluble GXM. GXM (20 or 100 μg in 150 μl of medium), was added to macrophages and left for 5 min prior to addition of cryptococci opsonized with F(ab′)2 fragments of MAbs that produce the rim capsular reaction. Data are reported as the mean phagocytic index from three independent experiments ± SEM.
We also examined the effect of purified GXM on (i) phagocytosis of cryptococci opsonized by intact MAb 3C2 or (ii) the F(ab′)2-facilitated phagocytosis of cryptococci opsonized by incubation with NHS. Monolayers of macrophages were preincubated for 5 min with 100 μg of GXM, followed by addition of cryptococci treated with (i) intact IgG, (ii) F(ab′)2 fragments, or (iii) both NHS and F(ab′)2 fragments. The results (Fig. 5) showed a slight, but significant (P < 0.05), inhibition of phagocytosis of cryptococci opsonized with intact IgG and a modest, but significant (P < 0.05), inhibition of phagocytosis of cryptococci opsonized with both NHS and F(ab′)2 fragments. As with the results shown in Fig. 4, 100 μg of GXM produced a near complete inhibition of phagocytosis of cryptococci opsonized only with F(ab′)2 fragments of MAb 3C2.
FIG. 5.
Inhibition of Fc- and C3-mediated phagocytosis by soluble GXM. GXM (100 μg in 150 μl of medium) was added to macrophages 5 min before addition of cryptococci treated with (i) intact MAb 3C2, (ii) F(ab′)2 fragments of MAb 3C2, or (iii) both F(ab′)2 fragments of MAb 3C2 and NHS. Data are reported as the mean phagocytic index from three independent experiments ± SEM.
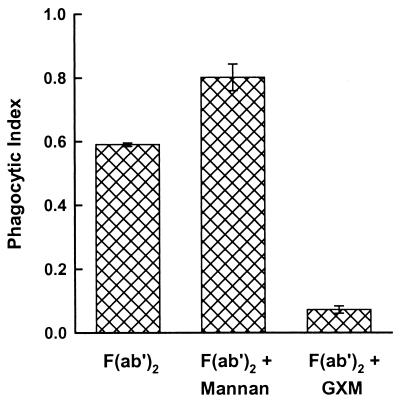
Finally, we considered the possibility that the ability of purified GXM to inhibit Fc-independent phagocytosis might be a general property of fungal cell wall polysaccharides. Accordingly, we compared the effects of preincubation of macrophages with 100 μg of GXM or C. albicans mannan for 5 min at 37°C on phagocytosis of cryptococci opsonized with F(ab′)2 fragments of MAb 3C2. The results (Fig. 6) showed that, once again, GXM produced almost complete inhibition. In contrast, pretreatment of macrophages with purified Candida mannan produced a modest, but significant (P < 0.001), enhancement of phagocytosis.
FIG. 6.
Effect of C. albicans mannan on Fc-independent opsonization of encapsulated cryptococci. Macrophages were untreated or preincubated for 5 min with Candida mannan (100 μg) or GXM (100 μg) prior to the addition of cryptococci opsonized with F(ab′)2 fragments of MAb 3C2. Data are reported as the mean phagocytic index from three independent experiments ± SEM.
DISCUSSION
Previous studies by ourselves and others identified biological functions of anti-GXM MAbs that were dependent on the epitope specificity of the antibody. These functions include protective efficacy in a murine model of cryptococcosis (30, 31), suppression of C3 deposition via the alternative complement pathway (20), and production of a capsular reaction when viewed by DIC microscopy (29). In the present study, we found that Fc-dependent and Fc-independent opsonization for phagocytosis by resident peritoneal macrophages are also influenced by epitope specificity. In addition, we report that there are two types of Fc-independent opsonization: opsonization that is facilitated by opsonins in NHS, presumably iC3b (22), and opsonization that is independent of NHS. Antibodies that exhibited high levels of Fc-dependent and Fc-independent opsonization were specific for an epitope that is shared by GXM serotypes A, B, C, and D and produced a distinct capsular reaction termed rim. In contrast, MAbs reactive with an epitope found only on serotypes A and D exhibited opsonic activity that was well below levels produced by antibodies reactive with serotypes A, B, C, and D. These poorly opsonic antibodies produced a capsular reaction termed puffy.
Macrophage Fc receptors are constitutively active for phagocytosis (reviewed in reference 33). Consequently, it is not surprising that anticapsular MAbs were effective opsonins for ingestion of encapsulated cryptococci. However, the differential opsonic activities of antibodies having distinct epitope specificities and producing different capsular reactions was unexpected. The mechanism for differential levels of phagocytosis following opsonization by intact IgG with different epitope specificities is not known. One possibility is that rearrangement of the capsular surface that occurs on binding of rim-pattern antibodies facilitates interaction between capsule-bound IgG and macrophage Fc receptors (see below).
One of the paradoxes of the antiphagocytic action of the cryptococcal capsule is the ability of the yeast to activate the complement system and deposit large amounts of iC3b within and at the capsular surface (22, 25). Despite the presence of an opsonic ligand at the capsular surface, phagocytosis of cryptococci coated with C3 fragments is limited at best. One factor that contributes to limited phagocytosis of C3-opsonized cryptococci with large capsules is suboptimal opsonization due to limiting amounts of serum in the opsonization milieu (23). We hypothesize that a further mechanism for the antiphagocytic nature of the cryptococcal capsule and the failure of iC3b to opsonize the yeast is the inherent fluidity of the polysaccharide capsule, which diminishes the efficiency of interaction between opsonic fragments of C3 and their receptors in the phagocyte plasma membrane. Such fluidity might also influence the opsonic action of anticapsular IgG, but to a lesser extent than the impact on opsonization by C3 fragments. Several pieces of data support this hypothesis. First, phagocytosis of cryptococci opsonized by incubation in NHS is enhanced to various degrees by treatment of phagocytes with procedures known to or likely to increase complement receptor mobility (9, 17, 27). Second, a study by Pierini and Doering used freeze-fracture electron microscopy to examine the architecture of the cryptococcal capsule (32). The results showed a tightly intertwined matrix near the cell wall that became progressively disordered with distance from the cell wall. This result would suggest greater mobility of ligands bound to cells with large capsules than of ligands bound to cells with small capsules.
The differential effects of F(ab′)2 fragments of rim- and puffy-producing MAbs on phagocytosis of cryptococci opsonized by incubation with NHS provide a third line of evidence in support of the hypothesis that the mobility of opsonic fragments of C3 influences phagocytosis of encapsulated cryptococci. Binding of rim-producing antibodies to encapsulated cryptococci produces a structural reordering at the capsular surface, resulting in a highly refractile shell at the perimeter of the capsule that is cross-linked to such an extent that macromolecules such as IgG cannot penetrate to the capsular interior (29). It is likely that polysaccharide at the capsular surface and any ligands bound to the cross-linked capsular surface, including iC3b, will have a markedly reduced mobility.
A comparison of the refractile outer edge of the capsular reaction produced by intact and F(ab′)2 fragments of puffy-pattern MAbs with capsular reactions of the corresponding Fab fragments (Fig. 1) suggests that bivalent puffy-pattern MAbs also produce some level of reordering of the capsular surface; however, the absence of the sharp decrease in refractive index characteristic of rim-pattern MAbs indicates that the surface cross-linking is much less effective in the case of puffy-pattern antibodies. As a consequence, the DIC microscopy predicts a limited synergy between F(ab′)2 fragments of puffy-pattern antibodies and surface-bound iC3b that would be significantly less effective than synergy between iC3b and F(ab′)2 fragments of rim-pattern antibodies. This is precisely the result that we observed (Fig. 3).
An unexpected finding from our study was the observation that F(ab′)2 fragments of rim-producing antibodies alone were opsonic. A somewhat similar result was described by Taborda and Casadevall, who found opsonization by anticapsular IgM alone (37). Because there are no cellular receptors for F(ab′)2 fragments or IgM, these results suggest the presence of a macrophage receptor for a constituent of the capsule itself. Given that the capsule is comprised primarily of GXM, we examined the ability of GXM to inhibit FcR- and complement receptor-independent phagocytosis. The results showed that purified GXM could almost completely inhibit F(ab′)2-mediated, complement receptor-independent phagocytosis. A preliminary report by Taborda and Casadevall similarly noted inhibition by soluble GXM of serum-independent opsonization by anticapsular IgM (C.P. Taborda and A. Casadevall, Abstr. 101st Gen. Meet. Am. Soc. Microbiol., abstr. F223, 2001).
An alternative explanation for the ability of soluble GXM to prevent phagocytosis is the possibility that the free GXM is producing an elution of the F(ab′)2 fragments. In the case of at least one of the antibodies, this is a very remote possibility. Specifically, MAb 3C2 binds to GXM in the solid phase with such tenacity that immunoaffinity purification of the antibody is impossible due to a failure of even the most extreme elution conditions to dissociate the antibody from GXM (unpublished results). In addition, if elution of the F(ab′)2 fragments were the mechanism for inhibition of opsonization by free GXM, a similar level of inhibition should have been observed for intact antibodies. Although soluble GXM produced a slight inhibition of IgG-mediated opsonization, the extent of inhibition was much less than that of the GXM-mediated inhibition of opsonization by F(ab′)2 fragments alone.
Several lines of evidence support the possibility that phagocytes have surface receptors for GXM. First, incubation of human monocytes with purified GXM elicits the secretion of interleukin-6 and interleukin-10 (11, 38). Second, GXM accumulates in macrophages in vivo (15, 18, 26). Third, MAbs specific for CD18 block binding of 14C-labeled CneF, a concentrated culture filtrate of C. neoformans, to neutrophils (12). Finally, GXM binds to Chinese hamster ovary cells that are transfected with human toll-like receptors 2 and 4 and/or CD14 (35). Although the present study did not attempt to identify the cellular receptor for GXM that mediated Fc- and complement-independent phagocytosis, previous reports suggest that CD18, CD14, toll-like receptor 2, or toll-like receptor 4, individually or in combination, may be responsible for this process.
Taken together, our results indicate that opsonization and phagocytosis of encapsulated cryptococci are multifactorial. At least three potential ligands (IgG antibody specific for GXM, C3b/iC3b bound to GXM, and GXM itself) and their known or putative receptors can participate in this process. Each can function independently, but optimal phagocytosis involves all three processes. An understanding of the interaction between each ligand and its receptor will contribute to our understanding of the antiphagocytic action of the cryptococcal capsule.
Acknowledgments
This work was supported by Public Health Service grants AI14209, AI37194, and AI44786 from the National Institute of Allergy and Infectious Diseases and a grant from the Foundation for Research.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Belay, T., R. Cherniak, T. R. Kozel, and A. Casadevall. 1997. Reactivity patterns and epitope specificities of anti-Cryptococcus neoformans monoclonal antibodies by enzyme-linked immunosorbent assay and dot enzyme assay. Infect. Immun. 65:718-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhattacharjee, A. K., J. E. Bennett, and C. P. J. Glaudemans. 1984. Capsular polysaccharides of Cryptococcus neoformans. Rev. Infect. Dis. 6:619-624. [DOI] [PubMed] [Google Scholar]
- 3.Bulmer, G. S., and M. D. Sans. 1967. Cryptococcus neoformans. II. Phagocytosis by human leukocytes. J. Bacteriol. 94:1480-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bulmer, G. S., M. D. Sans, and C. M. Gunn. 1967. Cryptococcus neoformans. I. Nonencapsulated mutants. J. Bacteriol. 94:1475-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casadevall, A., M. DeShaw, M. Fan, F. Dromer, T. R. Kozel, and L. Pirofski. 1994. Molecular and idiotypic analysis of antibodies to Cryptococcus neoformans glucuronoxylomannan. Infect. Immun. 62:3864-3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cherniak, R., V. Homayoun, L. C. Morris, and V. Faramarz. 1998. Cryptococcus neoformans chemotyping by quantitative analysis of 1H nuclear magnetic resonance spectra of glucuronoxylomannans with a computer-simulated artificial neural network. Clin. Diagn. Lab. Immunol. 5:146-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cherniak, R., E. Reiss, and S. H. Turner. 1982. A galactoxylomannan antigen of Cryptococcus neoformans serotype A. Carbohydr. Res. 103:239-250. [Google Scholar]
- 8.Cherniak, R., and J. B. Sundstrom. 1994. Polysaccharide antigens of the capsule of Cryptococcus neoformans. Infect. Immun. 62:1507-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collins, H. L., and G. J. Bancroft. 1992. Cytokine enhancement of complement-dependent phagocytosis by macrophages: synergy of tumor necrosis factor-α and granulocyte-macrophage colony-stimulating factor for phagocytosis of Cryptococcus neoformans. Eur. J. Immunol. 22:1447-1454. [DOI] [PubMed] [Google Scholar]
- 10.Cross, C. E., H. L. Collins, and G. J. Bancroft. 1997. CR3-dependent phagocytes by murine macrophages: different cytokines regulate ingestion of a defined CR3 ligand and complement-opsonized Cryptococcus neoformans. Immunology 91:289-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delfino, D., L. Cianci, E. Lupis, A. Celeste, M. L. Petrelli, F. Curro, V. Cusumano, and G. Teti. 1997. Interleukin-6 production of human monocytes stimulated with Cryptococcus neoformans components. Infect. Immun. 65:2454-2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong, Z. M., and J. W. Murphy. 1997. Cryptococcal polysaccharides bind to CD18 on human neutrophils. Infect. Immun 65:557-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eckert, T. F., and T. R. Kozel. 1987. Production and characterization of monoclonal antibodies specific for Cryptococcus neoformans capsular polysaccharide. Infect. Immun. 55:1895-1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fromtling, R. A., H. J. Shadomy, and E. S. Jacobson. 1982. Decreased virulence in stable, acapsular mutants of Cryptococcus neoformans. Mycopathologia 79:23-29. [DOI] [PubMed] [Google Scholar]
- 15.Goldman, D. L., S. C. Lee, and A. Casadevall. 1995. Tissue localization of Cryptococcus neoformans glucuronoxylomannan in the presence and absence of specific antibody. Infect. Immun. 63:3448-3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Granger, D. L., J. R. Perfect, and D. T. Durack. 1985. Virulence of Cryptococcus neoformans. Regulation of capsule synthesis by carbon dioxide. J. Clin. Investig. 76:508-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griffin, F. M., Jr. 1981. Roles of macrophage Fc and C3b receptors in phagocytosis of immunologically coated Cryptococcus neoformans. Proc. Natl. Acad. Sci. USA 78:3853-3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grinsell, M., L. C. Weinhold, J. E. Cutler, Y. Han, and T. R. Kozel. 2001. In vivo clearance of glucuronoxylomannan, the major capsular polysaccharide of Cryptococcus neoformans: a critical role for tissue macrophages. J. Infect. Dis. 184:479-487. [DOI] [PubMed] [Google Scholar]
- 19.Kozel, T. R. 1977. Non-encapsulated variant of Cryptococcus neoformans. II. Surface receptors for cryptococcal polysaccharide and their role in inhibition of phagocytosis by polysaccharide. Infect. Immun. 16:99-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kozel, T. R., B. C. H. deJong, M. M. Grinsell, R. S. MacGill, and K. K. Wall. 1998. Characterization of anticapsular monoclonal antibodies that regulate activation of the complement system by the Cryptococcus neoformans capsule. Infect. Immun. 66:1538-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kozel, T. R., and C. A. Hermerath. 1988. Benzoquinone activation of Cryptococcus neoformans capsular polysaccharide for construction of an immunoaffinity column. J. Immunol. Methods 107:53-58. [DOI] [PubMed] [Google Scholar]
- 22.Kozel, T. R., and G. S. T. Pfrommer. 1986. Activation of the complement system by Cryptococcus neoformans leads to binding of iC3b to the yeast. Infect. Immun. 52:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kozel, T. R., A. Tabuni, B. J. Young, and S. M. Levitz. 1996. Influence of opsonization conditions on C3 deposition and phagocyte binding of large- and small-capsule Cryptococcus neoformans cells. Infect. Immun. 64:2336-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kozel, T. R., M. A. Wilson, and J. W. Murphy. 1991. Early events in initiation of alternative complement pathway activation by the capsule of Cryptococcus neoformans. Infect. Immun. 59:3101-3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kozel, T. R., M. A. Wilson, G. S. T. Pfrommer, and A. M. Schlageter. 1989. Activation and binding of opsonic fragments of C3 on encapsulated Cryptococcus neoformans by using an alternative complement pathway reconstituted from six isolated proteins. Infect. Immun. 57:1922-1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lendvai, N., A. Casadevall, Z. Liang, D. L. Goldman, J. Mukherjee, and L. S. Zuckier. 1998. Effect of immune mechanisms on the pharmacokinetics and organ distribution of cryptococcal polysaccharide. J. Infect. Dis. 177:1647-1659. [DOI] [PubMed] [Google Scholar]
- 27.Levitz, S. M., and T. P. Farrell. 1990. Growth inhibition of Cryptococcus neoformans by cultured human monocytes: role of the capsule, opsonins, the culture surface, and cytokines. Infect. Immun. 58:1201-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levitz, S. M., and A. Tabuni. 1991. Binding of Cryptococcus neoformans by human cultured macrophages: requirements for multiple complement receptors and actin. J. Clin. Investig. 87:528-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MacGill, T. C., R. S. MacGill, A. Casadevall, and T. R. Kozel. 2000. Biological correlates of capsular (quellung) reactions of Cryptococcus neoformans. J. Immunol. 164:4835-4842. [DOI] [PubMed] [Google Scholar]
- 30.Mukherjee, J., G. Nussbaum, M. D. Scharff, and A. Casadevall. 1995. Protective and non-protective monoclonal antibodies to Cryptococcus neoformans originating from one B-cell. J. Exp. Med. 181:405-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mukherjee, J., M. D. Scharff, and A. Casadevall. 1992. Protective murine monoclonal antibodies to Cryptococcus neoformans. Infect. Immun. 60:4534-4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pierini, L. M., and T. L. Doering. 2001. Spatial and temporal sequence of capsule construction in Cryptococcus neoformans. Mol. Microbiol. 41:105-115. [DOI] [PubMed] [Google Scholar]
- 33.Ravetch, J. V., and J.-P. Kinet. 1991. Fc receptors. Annu. Rev. Immunol. 9:457-492. [DOI] [PubMed] [Google Scholar]
- 34.Segal, D. M. 1995. Antibody detection and preparation, p. 2.0.1-2.13.16. In J. E. Coligan, A. M. Kruisbeek, D. H. Margulies, E. M. Shevach, and W. Strober (ed.), Current protocols in immunology. John Wiley and Sons, Inc., New York, N.Y.
- 35.Shoham, S., C. Huang, J.-M. Chen, D. T. Golenbock, and S. M. Levitz. 2001. Toll-like receptor 4 mediates intracellular signaling without TNF-α release in response to Cryptococcus neoformans polysaccharide capsule. J. Immunol. 166:4620-4626. [DOI] [PubMed] [Google Scholar]
- 36.Spiropulu, C., R. A. Eppard, E. Otteson, and T. R. Kozel. 1989. Antigenic variation within serotypes of Cryptococcus neoformans detected by monoclonal antibodies specific for the capsular polysaccharide. Infect. Immun. 57:3240-3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taborda, C. P., and A. Casadevall. 2001. Immunoglobulin M efficacy against Cryptococcus neoformans: mechanism, dose dependence, and prozone-like effects in passive protection experiments. J. Immunol. 166:2100-2107. [DOI] [PubMed] [Google Scholar]
- 38.Vecchiarelli, A., C. Retini, C. Monari, C. Tascini, F. Bistoni, and T. R. Kozel. 1996. Purified capsular polysaccharide of Cryptococcus neoformans induces interleukin-10 secretion by human monocytes. Infect. Immun. 64:2846-2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilson, D. E., J. E. Bennett, and J. W. Bailey. 1968. Serologic grouping of Cryptococcus neoformans. Proc. Soc. Exp. Biol. Med. 127:820-823. [DOI] [PubMed] [Google Scholar]