The introduction of neuromuscular blocking drugs revolutionized the practice of anaesthesia. Before the advent of muscle relaxants, anaesthesia was induced and maintained by intravenous or inhalation agents. Tracheal intubation was uncommon, and muscle relaxation if needed was secured by deep inhalation anaesthesia with its attendant risks of respiratory or cardiac depression. After the introduction of muscle relaxants, anaesthesia underwent a conceptual change. Anaesthesia was redefined as a triad of narcosis, analgesia and muscle relaxation, specific drugs being used to produce each of these effects1.
Muscle relaxants belong to two groups, the depolarizers and the nondepolarizers. Depolarizers mimic the effect of acetylcholine at the neuromuscular junction, first causing muscle contractions (fasciculations) and then paralysing. Suxamethonium, the only depolarizer in use, has the advantage of acting within 60 seconds. Muscle relaxation lasts for under 5 minutes. It is not reversed by anticholinesterases such as neostigmine but plasma cholinesterase causes the effect to wear off quickly. Unwanted effects include malignant hyperpyrexia, increased intraocular pressure and life-threatening hyperkalaemia. Several deaths attributable to hyperkalaemic cardiac arrest have occurred following the use of suxamethonium in children with undiagnosed muscular dystrophies. There is a clinical need for a safer drug that works equally quickly. Nondepolarizers have slower onset (2-3 minutes) and are therefore unsuitable for rapid control of the airway. They work by competitive blockade of the neuromuscular junction and are reversed with anticholinesterases such as neostigmine. There is no initial muscle fasciculation. Important features of some of the nondepolarizers are listed in Table 1.
Table 1.
Features of nondepolarizing agents
| Onset/duration of action | Ganglion blockade | Histamine release | Cardiac effects | Elimination | |
|---|---|---|---|---|---|
| Tubocurarine | Slow/long | Yes +++ | Yes +++ | Hypotension | Renal |
| Gallamine | Slow/long | No | No | Tachycardia | Renal |
| Pancuronium | Slow/long | No | No | Tachycardia | Renal/hepatic |
| Vecuronium | Slow/intermediate | No | No | Nil | Hepatic/renal |
| Atracurium | Slow/intermediate | No | Yes + | No | Hofmann |
| Mivacurium | Slow/short | No | Yes + | No | Plasma cholinesterase |
| Rocuronium | Rapid/intermediate | No | No | No | Hepatic/renal |
CURARE—FROM AMAZON TO MERSEY
Curare was used for centuries by South American Indians to hunt game, and its evolution into the designer drugs of today began when tales of the mysterious ‘flying death’ were brought home to the Old World by Spanish conquistadors. Peter Martyr d'Anghera, a chronicler in the Court of King Ferdinand and Queen Isabella, first wrote of the poisoned arrows in his book De Orbe Novo, a collection of letters written in 15162. This was a blend of fact and fantasy that contributed to the mystique of curare and drew many men in its quest, some to their deaths.
In 1594, Sir Walter Raleigh visited Venezuela, and his book Discovery of the Large, Rich and Beautiful Empire of Guiana mentions the poisoned arrows. One of his lieutenants called the poison ‘ourari’3. Ourari is possibly a corruption of the Indian word uiraery from uria, meaning bird and eor to kill4. European attempts at rendering the Indian word led to several versions including ourara, urali, urare, woorari, wourali and curare5.
Wars between the English, the Spanish and the Portuguese prevented further exploration till the 18th century. Edward Bancroft, a physician, spent five years in South America and brought back samples of crude curare. Using these samples, Sir Benjamin Brodie demonstrated that small animals could be kept alive after being injected with curare by inflating their lungs with bellows6.
In 1804 Charles Waterton, an eccentric explorer, left the family seat Walton Hall in Yorkshire to manage the sugar estates owned by his family in Demerara, South America. He obtained several samples of wourali from a native tribe and tried it out on large animals. In 1814, he demonstrated to an audience that included Sir Benjamin Brodie the effects of wourali on three asses. The first ass was injected with wourali in the shoulder and died. The second had a tourniquet tied around its foreleg and wourali was injected below the tourniquet; the ass was alive and active as long as the tourniquet was in place but died soon after the tourniquet was removed. The third ass appeared to die after having wourali injected but was resuscitated by means of bellows and lived on in peace as Wouralia; for her travails in the cause of science she earned an obituary in the St James' Chronicle, a local newspaper7.
Claude Bernard published the details of his experiments on frogs in 1846. He showed that curare injected into a limb prevented the muscle contraction in response to nerve stimulation; the muscle continued to respond when stimulated directly. Curare when applied directly to the nerve failed to abolish muscle contraction in response to either nerve stimulation or direct muscle stimulation—proof that curare acted at the nerve-muscle junction.
In the 1860s the Edinburgh scientists Thomas Richard Fraser and Alexander Crum Brown, working on the relation between chemical structure and biological activity, discovered that when alkaloids such as atropine, brucine, codeine, morphine and nicotine had their nitrogen atoms changed from the tertiary to the quaternary form, they acquired curare-like activity8. This was the precursor to much of the work on neuromuscular blocking drugs that took place after the Second World War.
The turn of the century heralded several momentous developments. In Great Britain, Sir Henry Dale and colleagues working at the National Institute of Medical Research established the role of acetylcholine and the chemical basis of neuromuscular transmission9. Harold King isolated d-tubocurarine from a museum sample of curare. He established that it was a bulky rigid molecule with two quaternary ammonium groups at either end10.
Richard Gill, an American living in Ecuador, developed multiple sclerosis and returned to the United States in search of a cure. His neurologist Walter Freeman suggested that he might benefit from curare. Gill returned to the jungles of Ecuador and brought back twenty-five pounds of crude curare and several botanical samples11. These samples were identified as plants of two families—Menispermaceae, which include the genus Chondrodendron; and Loganiaceae, which include the genus Strychnos. Gill offered the curare to E R Squibb and Sons with the hope that their researchers would come up with a drug effective in multiple sclerosis. The crude curare was investigated in their laboratories. Oscar Wintersteiner and James Dutcher in 1942 were the first to isolate the alkaloid d-tubocurarine from biologically authenticated samples of Chondrodendron tomentosum12. A H Holladay of Squibb devised his rabbit ‘head-drop’ bioassay and standardized the commercial preparation of curare as Intocostrin.
A E Bennett, a neuropsychiatrist who was on the verge of abandoning convulsive shock therapy because of the high incidence of spinal fractures, decided to try and modify the convulsion with curare18. In June 1940, Bennett presented a film on the use of curare at the 91st annual session of the American Medical Association. Lewis Wright of Squibb saw this film and, thinking the drug might be of use to anaesthetists, donated some Intocostrin to E A Rovenstine of New York University to experiment with. Rovenstine passed it on to one of his residents, E M Papper, who tried it on two patients receiving ether anaesthesia. Both of them became apnoeic and had to be ventilated manually all through the night14. Endotracheal intubation was uncommon in those days.
Harold Randall Griffith, an anaesthetist at the Homeopathic Hospital in Montreal, was an ardent enthusiast for cyclopropane, and realized that the occasional episodes of apnoea induced by this agent could be overcome by mastering the skill of endotracheal intubation. He was confident of his ability to circumvent the major problem with the use of curare, and on 23 January 1942 he and his resident Enid Johnson administered curare to a young man undergoing appendicectomy15. The credit for introducing curare to anaesthetics belongs to him. Griffith and Johnson reported their use of curare in July 1942, and the introduction to their report is memorable: ‘Every anaesthetist has wished at times that he might be able to produce rapid and complete muscular relaxation in resistant patients under general anaesthesia16’. A commemorative stamp issued by Canada in 1991 honours Griffith's contribution to anaesthesia17.
The Second World War brought a halt to work on curare-like drugs at the UK National Institute of Medical Research, but American doctors stationed in England brought word of the use of curare as a muscle relaxant. John Halton, an anaesthetist from Liverpool, persuaded an American friend from the bomber squadron stationed at Burtonwood to bring back Intocostrin from the United States18. He and Cecil Gray used the drug on patients and were highly gratified by the results they obtained. Their experiences with curare were reported in 194619 and laid the basis of what became known as the Liverpool technique—a triad of narcosis, analgesia and muscle relaxation that in essence remains in use today.
SYNTHETIC BLOCKERS
The work of Crum Brown and Fraser on quaternary salts in the 1860s, and the subsequent research by King, provided the impetus for post-war developments in the pharmacology of synthetic neuromuscular blocking drugs. H R Ing in 1936 had reported the curariform activity of simple molecules such as tetramethylammonium iodide and trimethylsulphonium iodide20. Ing and Barlow in 1946, proceeding from the premise that the potency of curare was related in some way to the separation of two quaternary ammonium groups by an optimum distance, synthesized and tested several polymethylene bisquaternaries21. These are molecules with quaternary ammonium groups at either end separated by different lengths of polymethylene chains. At the same time another group of workers, W D M Paton and Eleanor J Zaimis, working from the UK National Institute of Medical Research, were testing similar compounds for histamine release and found that the octamethylene compound had curariform effects22. Both groups found that the decamethylene disquaternary salts, with a ten-carbon (C10) chain between the quaternary groups, had the most potent curariform action in the series of polymethylene bisquaternaries. Paton's group labelled this compound decamethonium and it was used clinically as a neuromuscular blocking drug for a short time. Both groups recognized that decamethonium was different from d-tubocurarine and that it produced a transient augmentation of contraction. The Paton group commented on the fasciculation that preceded the onset of block and speculated as to its cause: C10 produces neuromuscular block by initiating some active response in the endplate or muscle fibre. They also showed that, unlike d-tubocurare, decamethonium was not reversed by anticholinesterase agents.
Gallamine
Daniel Bovet, working at the Pasteur Institute in Paris, adopted a different approach: he began his search for neuromuscular blockers by synthesizing bulky curare-like molecules. In 1947 he synthesized gallamine, a trisquaternary compound and the first synthetic neuromuscular blocking drug used clinically23. Unlike decamethonium, gallamine did not cause initial stimulation of the neuromuscular junction. The effects of gallamine were reversible with anticholinesterases.
Suxamethonium
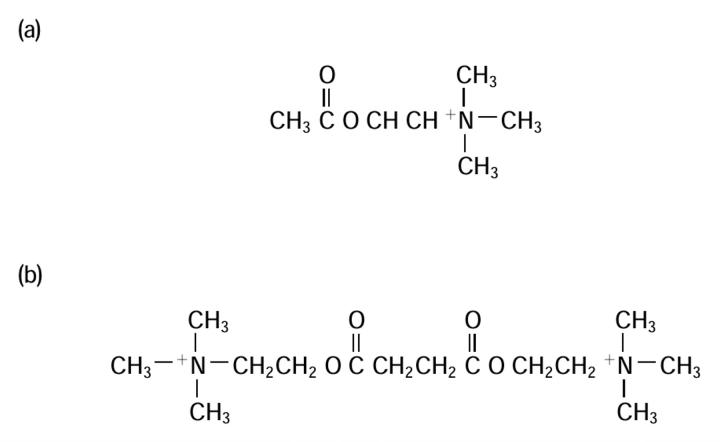
In 1951, Burns and Paton ascribed the neuromuscular blocking effects of decamethonium to depolarization of the motor end plate24. It was realized that bulky molecules, the pachycurares, caused nondepolarizing block and leptocurares, the slender molecules, caused depolarizing block. Three independent groups, from Italy, England and the United States, worked on simpler quaternary salts. Suxamethonium, two molecules of acetylcholine ranged back to back (Figure 1), was the result. Bovet, who had moved to Italy, published his work on suxamethonium in 1949. For his contribution to pharmacology, he was awarded the Nobel Prize for Medicine in 1957. By the end of the 1950s, d-tubocurarine and gallamine (the two nondepolarizers) and suxamethonium (the depolarizer) were available. All had several disadvantages.
Figure 1.
Acetylcholine (a) and suxamethonium (b)
Pancuronium
Nature provided further impetus when in the early 1960s malouetine, a disquaternary steroidal alkaloid, was isolated from the bark of a plant Malouetia bequaertiana. This was found to have curare-like effects. The race to design a steroid molecule with curare-like effects started. In 1964, Hewett and Savage synthesized an aminosteroidal with two quaternary ammonium groups25. In a remarkable feat of drug engineering they used the rigid steroidal androstane framework as the scaffolding for two acetylcholine molecules. Their empirical reasoning was that the rigid steroid molecule would provide the required separation between the quaternary groups, and the two acetylcholine molecules would provide affinity to receptors of the myoneural junction. This was a remarkable success, and pancuronium, as they named the new drug, displaced the other non-depolarizers of the day.
Vecuronium
The designing of pancuronium started the hunt for the ‘ideal neuromuscular blocking drug’. Savarese and Kitz in 1975 defined their ideal agent as one that would have ‘brief, noncumulative, nondepolarising neuromuscular action with rapid onset and recovery; it would be reversible by an appropriate antagonist and it would lack clinically important side effects’26. The perceived shortcomings of pancuronium were its slow onset, its cardiac vagolytic effects and its long duration of action. Pancuronium blocked the cardiac muscarinic receptors and caused tachycardia. Savage recognized that the muscarinic receptor activity resided in the quaternary group at one end (the A ring) and the neuromuscular blocking activity resided separately in the other quaternary group (the D ring)27. By demethylation of the A ring nitrogen Savage came up with vecuronium, a ‘clean drug’ devoid of cardiac effects. Vecuronium had an intermediate duration of action and a slow onset. The two aminosteroidals were both metabolized in the body, and the duration of their activity was unpredictable in patients with renal or hepatic failure. This was a disadvantage.
Atracurium
Others were working on benzylisoquinoline structures similar to d-tubocurarine. In 1956, a group of workers that included J B Stenlake, when studying the constituents of a plant, had identified petaline, a quaternary salt that underwent Hofmann elimination (spontaneous breakdown) in mildly alkaline conditions28. The challenge was to design a quaternary salt that would be a neuromuscular blocking drug and undergo Hofmann elimination under physiological conditions. In 1981 Stenlake and colleagues, in a collaborative enterprise between the University of Strathclyde and Wellcome Laboratories, synthesized atracurium, a benzylisoquinoline molecule that breaks down irreversibly at physiological pH and temperature and causes nondepolarizing block of the neuromuscular junction29. It has a slow onset and an intermediate duration of activity unaffected by renal or hepatic failure. Atracurium is a mixture of ten isomers and can release histamine. Cis-atracurium, an isomer of atracurium, is a ‘cleaner’ molecule more potent than atracurium and does not release histamine. Savarese and colleagues in the USA had developed mivacurium, an ester-linked benzylisoquinoline metabolized by plasma cholinesterase30 with a short duration of action and a slow onset. All the nondepolarizing neuromuscular blocking drugs had a slow onset. The only rapidly acting muscle relaxant was suxamethonium, a depolarizer, and it had several undesirable effects. There was therefore a clinical need for a rapidly acting nondepolarizing neuromuscular blocking drug.
Rocuronium, rapacuronium
The next major advance in the development of neuromuscular blockers came from the work of Bowman, who in 1988 established that with the aminosteroidals the speed of onset is related to the potency, with less potent drugs having a faster onset of action31. Rocuronium is a deacetoxy analogue of vecuronium. With vecuronium as the framework, substitutions on the single quaternary group produced a much less potent but faster acting molecule. Rocuronium, in appropriate doses, has a speed of onset only marginally slower than that of suxamethonium. It has an intermediate duration of action and depends on the kidney and liver for elimination. Rapacuronium is a recently developed analogue of vecuronium with a rapidity of onset that comes close to that of suxamethonium32. After its introduction into clinical practice several instances of severe bronchospasm were reported and the drug has now been withdrawn.
CONCLUSION
Neuromuscular blocking agents revolutionized the practice of anaesthesia. All the currently available agents have their limitations and the quest continues for an ideal drug. What is needed is an agent that is rapidly acting, non-cumulative, independent of renal or hepatic function for its elimination, easily and rapidly reversed and free from side-effects.
References
- 1.The era of relaxant anaesthesia [Editorial]. Br J Anaesth 1992;69: 551-3 [DOI] [PubMed] [Google Scholar]
- 2.Sykes K. Harold Griffith Memorial Lecture. The Griffith legacy. Can J Anaesth 1993;40: 351-74 [DOI] [PubMed] [Google Scholar]
- 3.Crul JA. Relaxant drugs: from native drugs to the selective agents of today. Acta Anaesthiol Scand 1982;26: 406-15 [DOI] [PubMed] [Google Scholar]
- 4.McIntyre AR. Curare Its History, Nature, and Clinical Use. Chicago: University of Chicago Press, 1947: 1
- 5.Birmingham AT. Waterton and Wouralia. Br J Pharmacol 1999;126: 1685-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brodie BC. Further experiments and observations on the action of poisons on the animal system. Phil Trans R Soc Lond 1812;102: 205-27 [Google Scholar]
- 7.Smith WDA. Waterton and Wouralia. Br J Anaesth 1983;55: 221-56338895 [Google Scholar]
- 8.Bynum WF. Chemical structure and pharmacological action: a chapter in the history of 19th century molecular pharmacology. Bull Hist Med 1970;44: 518-38 [PubMed] [Google Scholar]
- 9.Dale HH. Chemical transmission of the effects of nerve impulses. BMJ 1934;i: 835-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.King H. Curare alkaloids: 1, tubocurarine. J Chem Soc 1935: 1381-89
- 11.Humble RM. The Gill—Merrill Expedition. Penultimate chapter in the curare story. Anesthesiology 1982;57: 5159-26 [DOI] [PubMed] [Google Scholar]
- 12.Wintersteiner O, Dutcher JD. Curare alkaloids from Chondrodendron tomentosum. Science 1943;97: 467-70 [DOI] [PubMed] [Google Scholar]
- 13.Bennett AE. Curare: a preventive of traumatic complication in convulsive shock therapy. Am J Psychiatry 1994;151 (suppl): 249-58 [DOI] [PubMed] [Google Scholar]
- 14.Betcher AM. The civilizing of curare. Anesth Analg (Curr Res) 1977;56: 305-19 [DOI] [PubMed] [Google Scholar]
- 15.Gillies D, Wynands JE. Harold Randall Griffith, MD, CM, the pioneer of the use of muscle relaxants in anaesthesia. Br J Anaesth 1986;58: 943-5 [DOI] [PubMed] [Google Scholar]
- 16.Griffith HR, Johnson GE. The use of curare in general anaesthesia. Anesthesiology 1942;3: 418-20 [Google Scholar]
- 17.Kyle RA, Shampo MA. Harold R Griffith—introduction of muscle relaxants to anaesthesia. Mayo Clin Proc 1992;67: 237. [DOI] [PubMed] [Google Scholar]
- 18.Gray TC. Luck was a lady. In: Atkinson RS, Boulton RB, eds. The History of Anaesthesia. London: Royal Society of Medicine Press, 1989: 16-19
- 19.Gray TC, Halton J. A milestone in anaesthesia? (d-tubocurarine chloride). Proc R Soc Med 1946;39: 400-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ing HR. The curariform activity of onium salts. Physiol Rev 1936;16: 527-44 [Google Scholar]
- 21.Barlow RB, Ing HR. Curare-like action of polymethylene bisquaternary ammonium salts. Br J Pharmacol 1948;3: 289-304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paton WDM, Zaimis EJ. The pharmacological actions of polymethylene bistrimethyl ammonium salts. Br J Pharmacol 1949;4: 381-400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dorkins HR. Suxamethonium—the development of a modern drug from 1906 to the present day. Med Hist 1982;26: 145-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burns BD, Paton WDM. Depolarisation of the motor end plate by decamethonium and acetylcholine. J Physiol 1951;115: 41-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baird WLM, Reid AM. The neuromuscular blocking properties of a new steroid compound, pancuronium bromide. A pilot study in man. Br J Anaesth 1967;39: 775-80 [DOI] [PubMed] [Google Scholar]
- 26.Savarese JJ, Kitz RJ. Does clinical anaesthesia need new neuromuscular blocking agents? Anesthesiology 1975;42: 236-9 [DOI] [PubMed] [Google Scholar]
- 27.Buckett WR, Hewett CL, Savage DS. Pancuronium bromide and other neuromuscular blocking agents containing acetylcholine fragments. J Med Chem 1973;16: 1116-24 [DOI] [PubMed] [Google Scholar]
- 28.Stenlake JB, Waigh RD, Urwin J, Dewar GH, Coker GG. Atracurium: conception and inception. Br J Anaesth 1983;55: 3S-10S [PubMed] [Google Scholar]
- 29.Stenlake JB, Waigh RD, Dewar GH. Biodegradable neuromuscular blocking agents. Part 4 Atracurium besylate and related polyalkylene di-esters. Eur J Med Chem 1981;16: 515-24 [Google Scholar]
- 30.Savarese JJ, Miller RD, Lein CA, Caldwell JE. Pharmacology of muscle relaxants and their antagonists. In: Miller RD, ed. Anesthesia, 4th edn. New York: Churchill Livingstone, 1994: 417
- 31.Bowman WC, Rodger IW, Houston J, Marshall RJ, McIndewar I. Structure: action relationships among some desacetoxy analogs of pancuronium and vecuronium in the anaesthetised cat. Anesthesiology 1988;69: 57-62 [PubMed] [Google Scholar]
- 32.Rapacuronium (Org 9487): do we have a replacement for succinylcholine? [Editorial]. Br J Anaesth 1999;82: 489. [DOI] [PubMed] [Google Scholar]