Abstract
Shigella dysenteriae type 1-induced apoptotic cell death in rectal tissues from patients infected with Shigella dysenteriae type 1 was studied by the terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) technique and annexin V staining. Expression of proteins and cytokines participating in the apoptotic process (caspase-1, caspase-3, Fas [CD95], Fas ligand [Fas-L], perforin, granzyme A, Bax, WAF-1, Bcl-2, interleukin-2 [IL-2], IL-18, and granulocyte-macrophage colony-stimulating factor) in tissue in the acute and convalescent stages of dysentery was quantified at the single-cell level by in situ immunostaining. Apoptotic cell death in the lamina propria was markedly up-regulated at the acute stage (P < 0.05), where an increased number of necrotic cells were also seen. Phenotypic analysis of apoptotic cells revealed that 43% of T cells (CD3), 10% of granulocytes (CD15), and 5% of macrophages (CD56) underwent apoptosis. Increased activity of caspase-1 persisted in the rectum up to 1 month after onset. More-extensive expression of Fas, Fas-L, perforin, caspase-3, and IL-18, but not IL-2, at the acute stage than at the convalescent stage was observed. Increased expression of caspase-3 and IL-18 in tissues with severe inflammation compared to expression in those with mild inflammation was evident, implying a possible role in the perpetuation of inflammation. Significantly reduced cell death during convalescence was associated with a significant up-regulation of Bcl-2, Bax, and WAF-1 expression in the rectum compared to that in the acute phase of infection. Thus, induction of apoptosis at the local site in the early phase of S. dysenteriae type 1 infection was associated with a significant up-regulation of Fas/Fas-L and perforin and granzyme A expression and a down-regulation of Bcl-2 and IL-2, which promote cell survival.
Shigella spp cause bacillary dysentery by invading the human colonic mucosa (25). Following entry through M cells, the bacteria spread intracellularly in the epithelium and cause massive infiltration of the mucosa by granulocytes, macrophages, lymphocytes, and natural killer cells (10, 11, 17). Studies with experimental models and cell lines strongly suggest that pathogenesis involves both apoptosis and necrosis of the epithelial cells and the lamina propria cells (4, 14, 19, 20). Shigella flexneri is capable of inducing programmed cell death, or apoptosis, in macrophages in vitro as well as in vivo (28, 29, 30). One of the cytotoxic genes, the invasion plasmid antigen B gene (ipaB), localized to the ipa operon of Shigella's virulence plasmid, was shown to be essential and sufficient to induce apoptosis (28). The induction of apoptosis occurred after IpaB bound to caspase-1 and induced caspase-1 activation. Activation of caspase-1 results in the induction of apoptosis and the cleavage of the proinflammatory cytokines interleukin-1β (IL-1β) and IL-18 (6, 27). Both IL-1β and IL-18 were required to mediate inflammation in S. flexneri-infected caspase-1−/− knockout mice (21). In line with this, extensive production of IL-1β was also evident in severely inflamed rectal biopsy samples and stools of adult patients with shigellosis during acute and convalescent stages (15, 18). Apoptosis was shown to occur in the rectum during acute Shigella infection in adults (7). Apoptosis was related to the histological grade of inflammation; however, the enzymes and genes responsible for apoptosis were not studied. Shigellosis was accompanied by a persistent up-regulation of proinflammatory cytokines and other innate mediators at the local site for more than 1 month after the onset of dysentery and even long after clinical resolution of the disease (16, 18). This indicates that the inflammatory responses and the subsequent apoptotic process are not able to cause the required rapid elimination of the infected cells.
Another explanation for the histological evidence of persistence of inflammation in the convalescent stage may be a delay in the normal resolution mechanisms. There may be at least two proapoptotic events going on, one induced by Shigella (29) and the other subsequent to the polyclonal activation involved in the clearance of inflammatory cells from the site of infection. Thus, we hypothesized that Shigella infection disturbed the intricate balance in the apoptosis-regulatory protein network at the local site, leading to apoptosis and a chain of inflammatory reactions, while the host acts in response to confine the inflammation and regulate cell cycle progression when irreparable DNA damage is detected. The present study aimed to determine whether natural Shigella infection-induced apoptotic cell death in the rectal mucosa was partly due to a disturbance in the apoptosis-regulatory pro- and antiapoptotic protein networks. We therefore studied the expression of Bcl-2, Bax, WAF-1, caspase-1, caspase-3, Fas/Fas ligand (Fas-L), granzyme A, perforin, IL-2, IL-18, and granulocyte-macrophage colony-stimulating factor (GM-CSF) at the local site of infection. All these molecules may play a critical role in the regulation of cell death and survival.
MATERIALS AND METHODS
Patients.
Adult patients (male; age range, 18 to 45 years) with occult blood and mucus in stools were initially selected for the study at the outpatient section of the Clinical Research Service Center of the International Centre for Diarrhoeal Diseases Research, Bangladesh (ICDDR,B) in Dhaka. All patients with presumptive cases of Shigella infection with a history of 0 to 4 days of diarrhea were initially enrolled. Stool samples were examined by direct microscopy for the presence of cysts and vegetative forms of intestinal parasites and ova of helminths and cultured for Salmonella, Shigella, and Aeromonas species, Vibrio cholerae 01 and 0139, and Campylobacter jejuni. Stool samples were plated on MacConkey and SS (Shigella-Salmonella) agar plates, and, after overnight incubation of plates, serological confirmation of suspected Shigella colonies was done by slide agglutination. Only those patients who had confirmed Shigella dysenteriae type 1 (n = 25) in stools were included in the study.
Patients underwent careful investigations, which included physical examination; assessment of fever, blood pressure, pulse, and stool frequency; and stool microscopy for red blood cells, pus cells, and state of dehydration. All patients received pivmecillinam immediately after admission as empirical therapy. Drugs were changed when necessary after obtaining the antimicrobial sensitivity pattern. Patients were released from the hospital when diarrhea subsided (usually 4 to 5 days) and were requested to return for follow-up visits. Twenty-five patients with culture-confirmed S. dysenteriae type 1 infection were enrolled in the study. Permission to conduct the study was obtained from the ethical review committee of ICDDR,B. Signed informed consent was obtained from each patient and control according to the guidelines of the ethical review committee.
Healthy controls.
Fifteen healthy adult subjects living in an area where Shigella is endemic in Bangladesh were recruited as controls. Physical and clinical investigations were carried out to exclude those with a history of enteric infection or fever within the previous 3 months. Signed informed consent was obtained from each control.
Biopsy sample collection.
Rectal biopsy samples, 10 to 12 cm from anus, were obtained by sigmoidoscopy (Olympus, Tokyo, Japan; biopsy forceps were from Megabite Microvasive, Boston Scientific Corporation) from patients on the day of admission (3 to 5 days after the onset of diarrhea) and 30 days later (30 to 35 days after onset). At each time point, a total of six pieces of rectal pinch biopsy samples were obtained. Two pieces were fixed in buffered formalin and embedded in paraffin, and sectioning was done in a microtome (RM 2055; Leica, Nussloch, Germany) (thickness, 3 μm). Sections were mounted on glass slides (Superfrost*/plus; Menzel-Glaser), dried overnight at 37°C, and kept at room temperature for use. Two pieces were collected in Histocon (Histolab, Goteberg, Sweden), snap-frozen in liquid nitrogen, and kept at −70°C until used for cryostat sectioning (Leica; CM 3000). Frozen rectal tissues were sectioned at a thickness of 6 μm and mounted on glass slides (Superfrost*/plus). These sections were fixed with freshly prepared 2% (wt/wt) formaldehyde (Sigma Chemical Co., St. Louis, Mo.) dissolved in phosphate-buffered saline (pH 7.4) for 15 min followed by a few washes with Ca2+ and Mg2+ containing Earle's balanced salt solution (BSS) (Gibco Ltd., Paisley, Scotland) supplemented with 0.01 M HEPES buffer (Gibco). Following the washing, the slides were air dried and kept at −20°C until used. Two pieces of tissue were fixed in 2.5% glutaraldehyde, postfixed in osmium tetroxide, and embedded in Araldite. Ultrathin sections were cut on an LKB UM4 ultramicrotome with a diamond knife (Diatome), stained with uranyl acetate and lead citrate, and examined with a Philips (Eindhoven, The Netherlands) EM201C electron microscope. The ultrastructural analysis of these biopsy samples is being carried out in a parallel study.
Antibodies.
The following antibodies were used for immunohistochemical staining: anti-human Fas receptor (anti-CD95), anti-human Fas-L, rabbit polyclonal anti-Bax (Santa Cruz Biotechnology, Inc., Santa Cruz, Calif.), anti-human WAF-1 (a kind gift from Novakemi AB, Stockholm, Sweden), anti-human perforin (Endogen, Woburn, Mass.), anti-human granzyme A (Pharmingen International, San Diego, Calif.), and anti-human Bcl-2 (Dako A/S, Glostrup, Denmark), anti-caspase-1 (recognizes precursor as well as the mature forms; a kind gift from Merck Co.), anti-caspase-3 (recognizes the active form, 17 to 22 kDa; Pharmingen). The following surface marker monoclonal antibodies were used: anti-human CD3 (pan-T cells), CD68 (macrophages) (Becton Dickinson, San Jose, Calif.), and CD15 (granulocytes) (Dako). In addition, antibodies to cytokines used were anti-IL-18, anti-GM-CSF (Endogen), and anti-IL-2 (Pharmingen).
Detection of apoptotic cells in tissues.
Apoptotic cells were visualized by the terminal deoxynucleotidyltransferase (TdT)-mediated dUTP-biotin nick end labeling (TUNEL) method using the ApoTACS in situ diaminobenzidine (DAB) apoptosis detection kit (R&D Systems, Minneapolis, Minn.). Briefly, the sections were blocked with 2% H2O2 for 5 min. Slides were incubated with an equilibration buffer for 5 min. A reaction mixture containing TdT and dUTP-digoxigenin was added, and slides were incubated for 1 h at 37°C. The reaction was then stopped with stop/wash buffer, and slides were washed with PBS for 5 min. The slides were then incubated with peroxidase-labeled antidigoxigenin for 30 min at room temperature. The color reaction was developed by using DAB (Vector Laboratories, Burlingame, Calif.) for 4 to 7 min. The slides were counterstained with hematoxylin for 3 to 10 s and mounted with phosphate-buffered glycerol. Early stages of apoptotic cells in unfixed tissue were detected by staining with annexin V to identify translocation of phosphatidylserine (a marker for the early stage of apoptosis). Cryopreserved frozen slides were brought to room temperature, rehydrated in BSS, and blocked for endogenous peroxidase activity, and nonspecific binding was blocked with 1% goat serum, after which the slides were incubated overnight with a mouse anti-human annexin V antibody (Pharmingen) at a concentration of 4 μg/ml in BSS containing 0.1% saponin (sap-BSS; Sigma Chemical Co.). Slides were fixed and washed and incubated for 1 h with a biotinylated goat anti-mouse antibody followed by washing and incubation with an avidin-biotin-horseradish peroxidase complex (DAKO Laboratories A/S) for 30 min. After a washing, substrate DAB (Sigma Chemical Co.) was added for development of color. Slides were washed in water, counterstained with hematoxylin, and mounted.
Immunohistochemistry.
Immunohistochemical staining for cytokines and protein markers was performed by the saponin procedure as previously described for cytokines (15). Briefly, the slides were rehydrated in BSS followed by incubation in 1% H2O2 in BSS for 30 min to remove endogenous peroxidase activity. Tissue sections were incubated overnight with specific antibodies at concentrations of 2 to 4 μg/ml in sap-BSS. Subsequently, after the slides were washed with sap-BSS, biotinylated goat anti-mouse immunoglobulin G1 (IgG1) or IgG2b (1:300) (Caltag Laboratories, South San Fransisco, Calif.) supplemented with 1% normal goat serum or biotinylated goat anti-rabbit Ig (1:600) (Vector Laboratories) supplemented with 1% normal rabbit serum in sap-BSS was added, and the slides were incubated for 30 min. This was followed by incubation for 30 min with the avidin-biotin-horseradish peroxidase complex (DAKO Laboratories A/S) after a washing. The color reaction was developed with DAB (brown) for 5 to 10 min. As a control, specific antibodies were replaced by irrelevant isotype-matched antibodies. The above procedure was repeated for double staining using specific antibodies of different isotypes and IgG subclasses and biotinylated Ig conjugates. The step of blocking endogenous peroxidase activity was omitted. Slides were incubated with ExtrAvidin alkaline phosphatase conjugate (1:800; Sigma Chemical Co.) and were subsequently developed by incubation in a fast red substrate system (Dako) for 20 to 30 min, counterstained with hematoxylin for 3 to 10 s, and mounted. Two-color staining was performed for phenotyping apoptotic cells and for simultaneous detection of (i) caspase-1-, caspase-3-, and Fas (CD95)-expressing apoptotic cells, (ii) double-positive (caspase-1+ CD15+) cells, (iii) perforin-expressing CD8+ lymphocytes, and (iv) Fas-L-expressing CD68+ cells. Double-stained slides were counted manually with an ocular micrometer (Olympus) fitted into the eyepiece, and data were calculated as numbers of cells per 100-μm2 field area. Results were expressed as percentages of double-positive cells among cells with a specific phenotype.
Computerized image analysis for detection of immunostaining.
Immunohistochemical staining of specific enzymes in rectal tissues was examined with a DMLB microscope (Leica Wetzlar GmbH) equipped with a 3CCD color camera (Sony Corporation, Tokyo, Japan). Each image was examined in a Qwin image analysis system (Leica Cambridge Ltd., Cambridge, United Kingdom) that was directed by a personal computer system. The standards were set for positive as well as negative cells. The positive staining of enzymes in rectal tissue sections was defined by computer-assisted analysis of video microscopic images as described earlier (16). Each image was analyzed with a program routine (Tissue Includer) especially designed for these experiments. The acquired image was divided into 512 by 512 pixels, and each pixel was expressed in square micrometers (area) after calibration at the magnification being used. The data acquired were imported to Microsoft Excel and Sigma Stat. Positive immunostaining, as assessed by computer-assisted analysis of video microscopic images, was determined for the studied proteins and enzymes in patients and in healthy controls. For each tissue section, at least 20 0.4- by 105-μm2 fields were investigated at ×40 magnification and the average was used for each protein or enzyme staining. The automated video microscopic analysis allowed for quantification of positive immunoreactivity relative to the total cell area of the tissue section, and the results, the ratios of positive pixels to total pixels, were expressed as percentages.
Statistical analyses.
The data were processed with Excel 2000 (Office 2000; Microsoft Corporation, Redmond, Wash.). Statistical analyses were performed using the Wilcoxon/Kruskal-Wallis test and the Mann-Whitney U test with Sigma Stat for Windows, version 2.03 (SPSS Inc.). Probabilities were regarded as significant if they reached a 95% level of confidence (P < 0.05).
RESULTS
Acute shigellosis was accompanied by extensive apoptosis and necrosis in the rectal mucosa.
Biopsy samples obtained from patients 3 to 5 days from the onset of dysentery exhibited extensive numbers of apoptotic cells (Fig. 1a; Table 1); the numbers decreased during convalescence. The frequency of annexin V-expressing cells in the rectal biopsy samples was also highest during the acute stage. However, the quantitative expression of apoptotic cells in tissue detected by the TUNEL method was significantly higher than the expression of annexin V-positive cells, suggesting that the number of cells at the early stages of apoptosis detected by the annexin V staining was smaller than the number of cells undergoing full-blown apoptosis. The reason for this could be the inability to obtain rectal biopsy samples from these patients earlier than 3 to 5 days after onset of dysentery. Extensive necrotic erosion of the surface epithelium was evident at various places (Fig. 1b). Healthy subjects had significantly lower incidences of apoptotic cells (P < 0.01) and no necrosis in the rectal biopsy samples (Table 1).
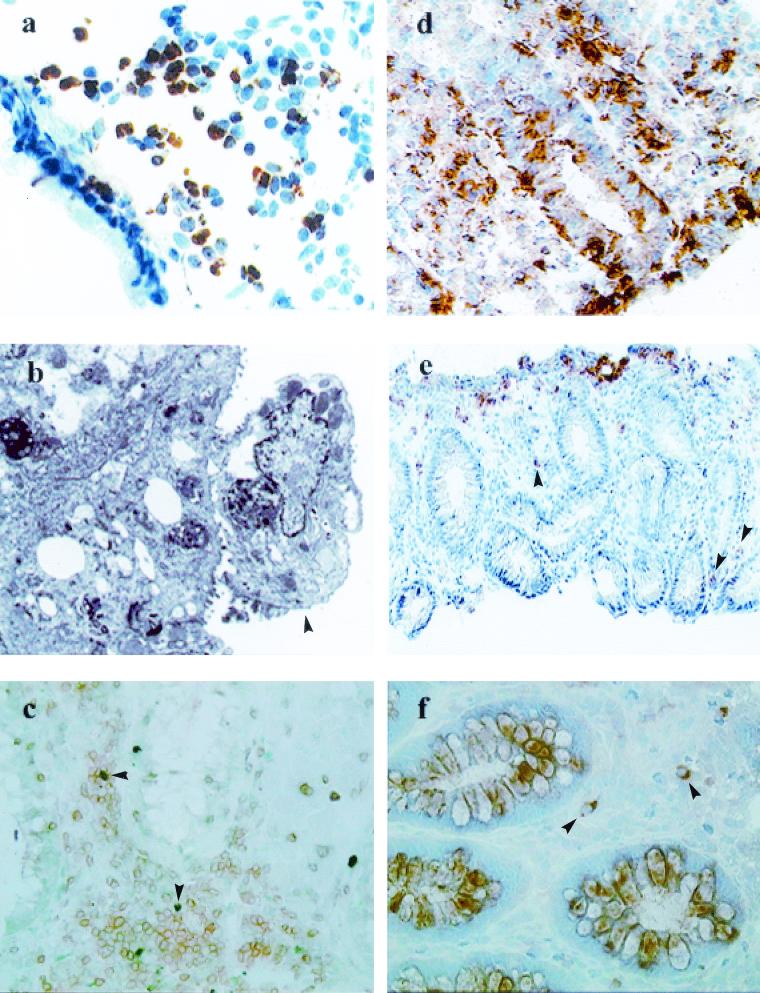
FIG. 1.
In acute shigellosis, notable increases in apoptotic T cells, granulocytes, and macrophages, along with an increased number of necrotic cells in the rectal mucosa, are seen. A significant up-regulation of noncaspase regulators of apoptosis (Fas/Fas-L and perforin and granzyme A expression) is also evident. (a) Immunohistochemistry of the rectal mucosa of a patient with Shigella infection. Visible is extensive expression of TUNEL-positive cells (brown) and debris in the lamina propria during the acute stage of shigellosis. Magnification, ×320. (b) Electron micrograph of a rectal-surface epithelial cell (arrowhead) from an adult patient with shigellosis showing focal necrosis. Evidence of degeneration includes loss of microvilli, dilated rough endoplasmic reticulum, vesiculation, large autolysosomes, and nuclear changes. Magnification, ×5,440. (c) Double immunostaining showing TUNEL-positive (green; arrowheads) CD3 T cells (brown) in the lamina propria in a rectal biopsy sample from an acute Shigella-infected patient. Magnification, ×320. (d) Extensive expression of CD95 (Fas) in the lamina propria and in the epithelial lining of the rectal mucosa in patients during the acute stage of shigellosis. Magnification, ×320. (e) Expression of Fas-L in cells mostly located close to the surface epithelium, with some scattered in the deeper lamina propria (arrowheads), in rectal biopsy samples from a patient with shigellosis. Magnification, ×80. (f) Perforin-expressing CD8+ lymphocytes in the lamina propria in the rectum during the acute stage of Shigella infection. Arrowheads, double-positive cells (brown and pink). Magnification, ×320.
TABLE 1.
Presence of apoptotic cells in the rectal mucosa in patients with shigellosis during acute and convalescent stages and in healthy controls
| Type of apoptotic cells | % Expression of positive staining/tissue sectiona in:
|
||
|---|---|---|---|
| Patientsb (n = 25) at:
|
Healthy controls (n = 15) | ||
| Acute stage (3-5) | Convalescent stage (30-40) | ||
| TUNEL+ | 10 (6-21)∗† | 2.5 (2-5) | 1.9 (1.5-2.4) |
| Annexin-V+ | 1.9 (0.5-5.6)∗† | 0.43 (0.15-0.75) | 0.4 (0.28-0.6) |
The average studied area for each section was 13.5 × 105 μm2 (±10%). Quantification of immune-positive areas (in square micrometers) in relation to the total tissue section was determined by a computerized image-analyzing technique, and the results were expressed as the percentages of the ratios of positive areas to total area. Data are medians with 25th and 75th percentiles in parentheses. Probability values were determined by the Wilcoxon signed rank test for comparing patients in the acute stage and the convalescent stage (∗, P < 0.05) and the Mann-Whitney test for comparing patients in the acute stage and healthy controls (†, P < 0.05).
Numbers of days after the onset of diarrhea are in parentheses.
The phenotype of apoptotic cells was determined by two-color immunostaining. The majority of the apoptotic cells consisted of infiltrating mononuclear cells localized in the lamina propria. Approximately 43% of CD3+ T lymphocytes, 10% of CD15+ granulocytes, and 5% of CD68+ macrophages/monocytes underwent apoptosis (Fig. 1c; Table 2). Thus, a great number of CD3-positive cells underwent apoptosis, while the apoptotic macrophages were less prevalent. The frequency of TUNEL-positive T cells and neutrophils/granulocytes decreased significantly from the acute to the convalescent stage (Table 2). We were unable to stain apoptotic B cells or Ig-producing cells in the rectal tissues. Electron-microscopic examination, however, showed evidence of apoptotic plasma cells (figure not shown) in the lamina propria.
TABLE 2.
Distribution of subtypes of apoptotic cells (detected by the TUNEL method) in the rectal mucosa in patients with shigellosis during acute and convalescent phases of the disease
| Phenotypic marker | % of apoptotic cells ina:
|
|||
|---|---|---|---|---|
| Patientsb in:
|
Healthy controls | |||
| Acute phase (3-5) | Convalescent phase (30-40) | P | ||
| CD3 | 43 (29-63) | 18 (15-29) | 0.02 | 0 (0-0) |
| CD15 | 10 (4-14) | 0.44 (0.09-2) | 0.003 | 0 (0-0.015) |
| CD68 | 5.4 (3-9) | 4 (2-7.6) | 0.98 | 0.4 (0-1.2) |
Numbers of double-stained (TUNEL and subtype) cells per hundred cells of a specific subtype were determined by manual quantification using graded scales (in 100 μm2). The average studied tissue area of each section was 13.5 × 105 μm2 (±10%). Probability values were determined by the Wilcoxon rank sum test for comparing data between biopsy samples obtained at 3 to 5 days and 30 to 40 days after onset of disease. P values <0.05 are significant. CD3, pan-T cells, CD68, macrophages, CD15, granulocytes.
Numbers of days after onset of disease are in parentheses.
Expression of apoptosis-related proteins in the rectal tissues.
Rectal biopsy samples were examined for the expression of CD95, Fas-L, perforin, granzyme A, WAF-1, Bax, and Bcl-2. A marked increase in the expression of CD95 was evident in acute-stage biopsy samples (P < 0.05); expression declined to control levels during convalescence (Table 3, Fig. 1d). Expression of Fas-L in tissue was short-lived, with a transient appearance in the acute stage (Fig. 1e). There was a total absence of expression during convalescence and in healthy controls (P < 0.005) (Table 3). The up-regulated expression of Fas-L was predominantly on the CD3+ T cells but was also on CD68+ cells (see Fig. 3a). The frequency of perforin-expressing CD8+ cells in the acute-stage biopsy samples was significantly increased compared to the frequencies observed in the late recovery phase (Table 3; see Fig. 3b) (P = 0.003). Compared to that in healthy controls, expression of granzyme A in lymphocytes was significantly elevated in patients and remained elevated throughout the study period (Table 3).
TABLE 3.
Comparative analysis of apoptosis-related proteins in the rectal mucosa in patients with S. dysenteriae type 1 infection during the acute and the convalescent stages and in healthy controls
| Apoptosis-related protein | Median % expression of positively stained tissue area (μm2)/total tissue areaa in:
|
||
|---|---|---|---|
| Acute-stage patients | Convalescent-stage patients | Healthy controls | |
| Fas receptor | 11.7 (10.5-13)∗† | 0.15 (0.1-0.2) | 1.35 (0.9-1.6) |
| Fas-L | 0.25 (0.1-1.9)∗† | 0 | 0 |
| Caspase-1 | 1.2 (0.4-1.8)† | 0.44 (0.16-0.8) | 0.2 (0.13-0.2) |
| Caspase-3 | 0.1 (0.048-0.2)∗† | 0.02 (0.015-0.06) | 0.02 (0.005-0.03) |
| Perforin | 1.32 (1-2.7)∗† | 0.8 (0.7-1) | 0.2 (0.1-0.9) |
| Granzyme A | 1.7 (0.8-4)† | 2.2 (1.6-3)‡ | 0.38 (0.2-0.48) |
| Bax | 0.47 (0-2)∗ | 4.6 (2.6-6)‡ | 0.4 (0.15-0.6) |
| WAF-1 | 1 (0-1.6)∗ | 3 (2-4.5)‡ | 0.2 (0.08-0.8) |
| Bcl-2 | 1.75 (1-1.9)∗† | 12 (5.6-18)‡ | 0.4 (0.22-1.4) |
| IL-2 | 0.45 (0.35-1)∗ | 0.6 (0.3-1)‡ | 1.2 (0.85-1.7) |
| IL-18 | 2.2 (1.56-2.9)∗† | 0.56 (0.4-1.2) | 0.7 (0.4-0.7) |
| GM-CSF | 1.35 (1-3.8)∗† | 0.24 (0.08-0.3) | 0.4 (0.19-0.5) |
Quantification of immunoreaction-positive areas in relation to total tissue area was determined by a computerized image-analyzing technique. The 25th and 75th percentile values are in parentheses. Probability values were determined by the Wilcoxon signed rank test for comparing patients in the acute stage and the convalescent stage (∗, P < 0.05) (3 to 5 days and 30 to 40 days after onset of the disease, respectively) and the Mann-Whitney test for comparing patients in the acute stage and healthy controls (†, P < 0.05) and patients in the convalescent stage and healthy controls (‡, P < 0.05).
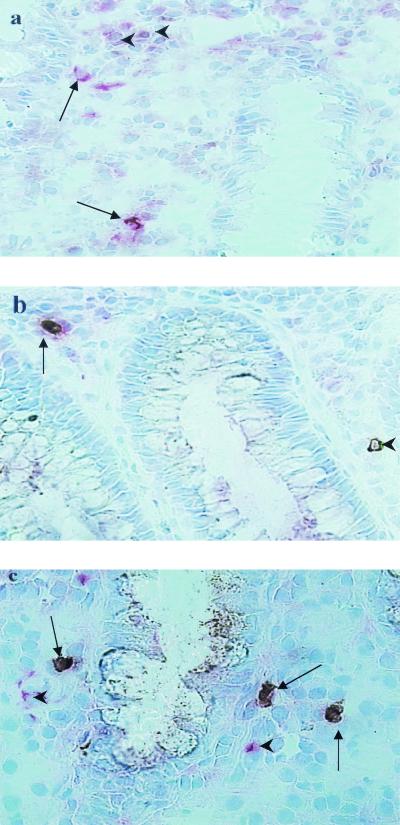
FIG. 3.
Various inflammatory cells infiltrate the local site of infection in the rectum during acute shigellosis and express proapoptotic molecules. (a) Fas-L-expressing CD68+ macrophages (deep pink and brown; arrows) were seen in close proximity to the luminal crypts in the lamina propria. Some Fas-L-expressing cells (brown only; arrowheads) that did not stain for CD68+ macrophages were also present in the vicinity. (b) Perforin-expressing CD8+ T lymphocyte (arrow) in an inflamed rectum during the acute stage of Shigella infection. A single perforin-expressing cell (arrowhead) close to a crypt was also seen. (c) A number of CD15-expressing granulocytes that coexpressed proapoptotic enzyme caspase-1 (arrows) were present in the rectal tissue during the early stage of shigellosis. Some other cell types that also expressed caspase-1 (single staining, pink; arrowheads) were seen near the double-positive granulocytes.
The WAF-1 protein was localized to the surface and crypt epithelium throughout the course of the disease, but more cells expressing WAF-1 were present in the lamina propria in the convalescent stage than in the acute stage and in healthy controls (Fig. 2a; Table 3). Similarly, the number of Bax-expressing cells also increased in the convalescent phase of shigellosis (Table 3). The staining pattern was cytoplasmic, and the positive cells were predominantly found in the surface epithelium and in the mononuclear cells in the lamina propria (Table 3). The expression of membrane-bound forms of Bcl-2 in tissue of patients with acute shigellosis was similar to that of healthy controls (Table 3; Fig. 2b). However, a sharp increase in the expression of this protein was seen in the late recovery phase (Table 3; Fig. 2c).
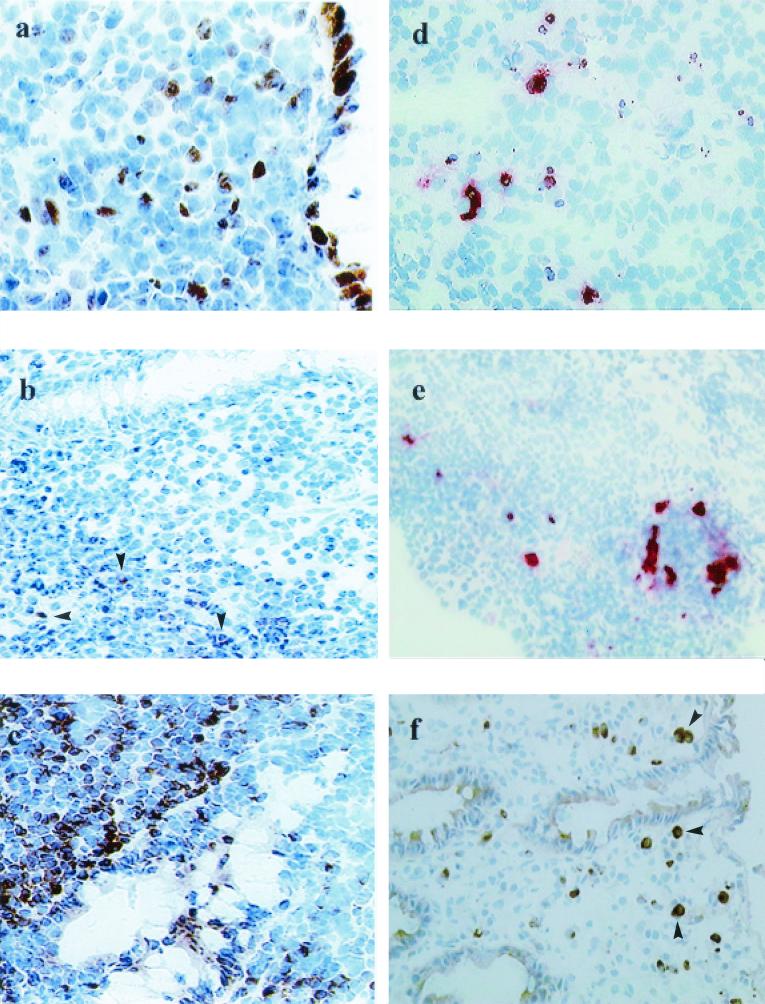
FIG. 2.
During acute Shigella infection, there was up-regulation of proapoptotic enzymes (caspase-1 and caspase-3) and substrate IL-18 and a down-regulation of an antiapoptotic marker (Bcl-2) in the local site of infection. During convalescence, there was increased expression of antiapoptotic protein Bcl-2 in parallel with a reduction in cell death in the rectum. (a) Expression of WAF-1 in tissue was localized mainly to the cells in the epithelium and some cells in the lamina propria in the rectum during the course of shigellosis. The staining pattern was cytoplasmic. Magnification, ×320. (b) Expression of the membrane-bound form of Bcl-2 was markedly reduced in the rectum in the acute stage of shigellosis. There were few Bcl-2-expressing cells present in the lamina propria close to the crypts (arrowheads). Magnification, ×320. (c) The expression of Bcl-2 in tissue increased considerably during recovery from acute shigellosis, with massive numbers of cells expressing the Bcl-2 protein. Magnification, ×320. (d) Double immunohistochemical staining showing apoptotic (TUNEL-positive, brown) caspase-3 (red)-expressing cells in the lymphoid aggregates. Magnification, ×320. (e) Apoptotic cells (TUNEL-positive, brown) expressing caspase-1 (red) were localized in the same lymphoid aggregate as that seen in Fig. 3a. Magnification, ×320. (f) IL-18-expressing cells (arrowheads) infiltrating the rectal mucosa in acute shigellosis. Mostly, IL-18-expressing cells were in close proximity to eosinophils. Magnification, ×320.
Induction of caspase-1, caspase-3, IL-18, and GM-CSF expression in the acute stage.
Since both antibodies recognize active forms of caspase-1 and caspase-3, a significant increase in expression of caspase-1 in the rectum in the acute stage compared to that in controls (Table 3) suggested increased caspase-1 activity. The activity gradually declined during convalescence though the tissue expression was not significantly lower than that in the acute stage (P < 0.1). Expression of caspase-1 occurred in CD68+ macrophages, CD15+ granulocytes, and CD3-positive lymphocytes in the lamina propria, as was evident from double immunostaining (Fig. 3c). Similarly, expression of the active form of caspase-3 in tissue was significantly elevated in the acute stage compared to that in the convalescent stage or in the healthy controls (P < 0.01; Table 3). When double staining was performed, TUNEL-positive caspase-3-expressing cells (Fig. 2d) as well as caspase-1-expressing cells (Fig. 2e) were observed in the lamina propria and in lymphoid aggregates during the acute stage. These double-positive cells were virtually absent in the convalescent stage. The expression of IL-18, a substrate of caspase-1, in the rectal tissues was seen mostly in macrophage-like cells in the lamina propria in close proximity to eosinophils (Fig. 2f). Extensive expression of IL-18 in the acute stage was observed, and expression gradually declined during convalescence (Table 3). Healthy controls showed fewer cells expressing IL-18 than patients in the acute stage (P < 0.003). The level of expression of GM-CSF in tissue was increased in the acute stage, and expression declined to control levels during recovery (P < 0.04) (Table 3).
Reduced expression of IL-2 in shigellosis.
When stained for IL-2, a growth factor for T cells and a promoter of lymphocyte survival, rectal tissues exhibited very few IL-2-expressing cells in the lamina propria in the acute stage. Surprisingly, healthy controls showed a significantly increased expression of IL-2 in the rectum compared to the patients in the acute and convalescent stages. Thus, increased apoptosis of CD3 cells in the rectum was accompanied by reduced IL-2 expression in the acute stage, but the reduction in apoptotic cell death was not associated with increased IL-2 expression (Table 3).
DISCUSSION
This study illustrates that acute S. dysenteriae type 1 infection in adult patients was associated with extensive apoptotic cell death in the rectal mucosa, predominantly in CD3-positive T cells infiltrating the lamina propria and lymphoid aggregates (Fig. 1C; Table 2), combined with a down-regulation of IL-2 and Bcl-2. Necrotic erosion of the surface epithelium was also observed during the acute stage of shigellosis; this may have occurred as a result of invasion by Shigella as well as excessive production of reactive oxygen species that are key mediators of necrotic cell death (14, 16, 26) or secondary necrosis of apoptotic cells due to an ineffective clearance mechanism. We do not have definite cellular markers of apoptotic and necrotic cells with which to dissect these processes in situ in inflamed tissues. Imbalance between apoptosis and necrosis may be important in the immunopathogenesis of such inflammatory diseases.
In vitro studies showed that IpaB of virulent Shigella spp. mediates apoptosis of invaded macrophages via activation of caspase-1, which in turn cleaves pro-IL-1β and pro-IL-18 into mature forms (6), which were important for induction of inflammation (21). In the present study, we also observed increased activity of caspase-1 as well as caspase-3 (which is involved in both Fas-L- and granzyme-induced apoptosis) in the lamina propria and lymphoid aggregates during acute shigellosis, with a substantial reduction during convalescence. Expression of caspase-1 and caspase-3 was significantly increased in tissues with moderate-to-severe inflammation compared to that in tissues with mild inflammation (Table 4) and thus may have contributed to the persistence of inflammation. In accordance, we have shown persistent up-regulated expression of IL-1β (a substrate of caspase-1)-producing cells in the rectum up to 1 month after clinical recovery from acute shigellosis, which perpetuated the inflammation (15).
TABLE 4.
Expression of apoptosis-related proteins in tissue from the rectums of patients: comparison between mild and severe histology
| Protein or cytokine | Median % expression of positively stained tissue area (μm2)/total tissue areaa for indicated histology in:
|
|||||
|---|---|---|---|---|---|---|
| Acute stage
|
Convalescent stage
|
|||||
| Severe | Mild | P | Moderate | Mild or normal | P | |
| Caspase-1 | 1.2 (0.7-1.5) | 0.2 (0.12-1.6) | 0.2 | 0.7 (0.46-3)∗ | 0.2 (0.11-0.3) | 0.03 |
| Caspase-3 | 0.35 (0.16-0.4)∗ | 0.05 (0.02-0.15) | 0.02 | 0.06 (0.01-0.1) | 0.02 (0.01-0.05) | 0.5 |
| IL-18 | 2.5 (2-3.3) | 1.8 (1.3-2.4) | 0.3 | 0.6 (0.29-1.2) | 0.56 (0.5-1.1) | 0.8 |
Quantification of immunoreaction-positive areas in relation to total tissue area was determined by a computerized image-analyzing technique. The 25th and 75th percentile values are in parentheses. Probability values were determined by the Wilcoxon signed rank test for comparing patients in the acute stage and the convalescent stage (∗, P < 0.05) (3 to 5 days and 30 to 40 days after onset of the disease, respectively).
Induction of both CD95 and Fas-L expression in the acute stage of shigellosis and the presence of TUNEL-positive Fas-expressing cells in the lamina propria and the lymphoid aggregates indicated that the Fas-mediated lytic pathway was up-regulated. The presence of TUNEL-positive cells bearing the CD3 marker in the rectum in the same locations was suggestive of activation-induced T-cell death in acute shigellosis and was not due to direct infection of the lymphocytes (9). A recent study by Aliprantis et al. has shown that the bacterial lipoprotein is capable of inducing activation-induced apoptosis in epithelial cells and monocytes through Toll-like receptor 2 (2). This may also hold true in the present study. Again, reduced expression of IL-2 at the local site in the acute stage may have contributed to a lack of lymphocyte survival (1). However, we observed significantly increased frequency of CD3 and CD8 cells infiltrating the mucosa in the acute stage compared to that for healthy controls, and the numbers of cells decreased during convalescence (17). When and why the down-regulation of IL-2 occurs in shigellosis are not known. The finding that the majority of the apoptotic cells were CD3+ cells suggested that there might be a selective deletion of T lymphocytes and NK cells (Table 1). T-cell deletion could also be a strategy utilized by the pathogen to prevent development of specific immune responses of the host directed against the infecting organism. It was proposed that selective elimination of IgG- and IgA-committed B cells by Shiga-like toxin produced by virulent Escherichia coli was responsible for the lack of long-term immunity to dysenteric diseases (5). In the present study, we were unable to colocalize TUNEL+ cells and B lymphocytes in the mucosal tissues even though electron-microscopic examination of tissue sections showed numerous apoptotic plasma cells.
Cytotoxic T cells trigger apoptosis in the target cells by the granule exocytosis pathway by releasing perforin and granzymes or by the membrane-associated Fas/Fas-L pathway. Expression of perforin was up-regulated in the acute stage of shigellosis even when apoptotic cell death of CD3+ T lymphocytes was evident and there was reduced expression of IL-2 and gamma interferon (IFN-γ) (15). Protein Bcl-2 is capable of protecting lymphocytes from perforin-mediated as well as from Fas/Fas-L-mediated cell death (22, 24). Up-regulation of perforin was accompanied by a reduction in Bcl-2 expression, showing an inverse relationship between expression of perforin and granzyme A and that of Bcl-2 in tissue in Shigella infection. Thus, both the death pathways, the perforin/granzyme and Fas/Fas-L pathways, mediated by cytotoxic T cells were up-regulated in the acute stage of shigellosis.
Neutrophils play a major role in the tissue damage associated with an inflammatory response during shigellosis (13, 14). Resolution of neutrophil-mediated inflammation is achieved, in part, through induction of neutrophil apoptosis. Both neutrophils and eosinophils express Fas receptor, and apoptosis occurs by ligation of the Fas receptor (23). Since granulocyte apoptosis was evident in the acute stage of shigellosis together with the up-regulation of Fas/Fas-L, it may not be incorrect to speculate that the Fas pathway may be one of the mechanisms by which apoptosis of these granulocytes occurs in shigellosis. Neutrophils exposed in vitro to IL-1, GM-CSF, or prostaglandins are inhibited in their constitutive rate of apoptosis and are resistant to death signals by Fas-L and tumor necrosis factor alpha (3, 12). In fact, in an earlier study we have seen high levels of IL-1 and GM-CSF and prostaglandins in stools from patients during acute shigellosis (16, 18). Thus, concomitantly increased expression of IL-1 and GM-CSF in tissue in the wake of high numbers of neutrophils undergoing apoptosis in acute shigellosis may delay the apoptotic process to some extent either to provide “functional longevity” to counteract the pathogens (8) or to decrease the burden on the already-overwhelmed phagocytic cells. GM-CSF may also promote growth, differentiation, and activation of granulocytes and growth of T cells. This in turn may increase the load of these cells in tissue.
IL-18 is expressed in a variety of cells including macrophages, epithelial cells, gut-associated lymphoid tissues, etc. In adult patients with shigellosis, huge numbers of cells were found to express IL-18 as well as IL-1β in the acute stage (15). Both IL-18 and IL-1β released from Shigella-infected macrophages promoting inflammation required caspase-1 processing for activation (21). IL-18 is also a potent IFN-γ-inducing cytokine, and concomitant expression of IL-18 and IFN-γ in tissue was evident at later time points of the study (15).
In our study, there was no evidence that physiological apoptosis regulators WAF-1 and Bax influenced the susceptibility to apoptosis in acute Shigella infection. Extensive apoptosis in the acute stage was not associated with an up-regulation of apoptosis-regulatory proteins WAF-1 and Bax (Table 3). On the contrary, the expression increased during convalescence. Cell growth arrest is a common response to DNA damage. WAF-1 controls multiple cell cycle phases in normal development and elicits cell cycle arrest in G0/G1 stage but not the induction of apoptosis. An earlier study of adult patients with shigellosis showed marked increase in expression of a cell proliferation marker (Ki67) in the acute and the convalescent stages compared to that in healthy controls (7). Therefore, increased expression of WAF-1 in tissue during shigellosis may serve as a cell cycle checkpoint, restricting the accelerated cell cycle progression into terminal differentiation in case of DNA damage, and may regulate uncontrolled proliferation of cells during convalescence. Overexpression of Bax during recovery may be one mechanism for terminating cell proliferation when tissue repair is concluded.
In conclusion, the data show that both apoptotic and necrotic cell death was evident in the rectal mucosa in acute Shigella infection in the adult patients. Extensive apoptosis during acute shigellosis was accompanied by increased activity of apoptosis promoters such as caspase-1 and caspase-3 and a down-regulation of the cell survival promoters, Bcl-2 and IL-2. There was induction of T-cell-mediated death pathways by perforin and granzyme and Fas/Fas-L cytolytic effector molecule expression in the rectum in the acute stage of shigellosis. The specificity of CD3+ perforin-, granzyme A-, and Fas-L-expressing cells remains to be elucidated. Perhaps there was a lack of Shigella antigen-specific cytotoxic T lymphocytes, while the overexpression of these molecules resulted in a non-Shigella-specific apoptotic process of infiltrating CD3+ T cells?
Acknowledgments
R. Raqib and C. Ekberg contributed equally to this work.
We gratefully acknowledge the participation of the healthy controls and patients in this study. We thank Minnie Mathan for the electron micrography.
This study was conducted with the support of grants from the Swedish Agency for Research Cooperation with Developing Countries (Sida/SAREC; grant 98-05440).
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Akbar, A. N., and M. Salmon. 1997. Cellular environments and apoptosis: tissue microenvironments control activated T-cell death. Immunol. Today 18:72-76. [DOI] [PubMed] [Google Scholar]
- 2.Aliprantis, A. O., R. B. Yang, M. R. Mark, S. Suggett, B. Devaux, J. D. Radolf, G. R. Klimpel, P. Godowski, and A. Zychlinsky. 1999. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science 285:736-739. [DOI] [PubMed] [Google Scholar]
- 3.Brach, M. A., S. deVos, H. J. Gruss, and F. Herrmann. 1992. Prolongation of survival of human polymorphonuclear neutrophils by granulocyte-macrophage colony-stimulating factor is caused by inhibition of programmed cell death. Blood 80:2920-2924. [PubMed] [Google Scholar]
- 4.Clerc, P., B. Baudry, and P. J. Sansonetti. 1988. Molecular mechanisms of entry, intracellular multiplication and killing of host cells by shigellae. Curr. Top. Microbiol. Immunol. 138:3-13. [PubMed] [Google Scholar]
- 5.Cohen, A., V. Madrid-Marina, Z. Estrov, M. H. Freedman, C. A. Lingwood, and H. M. Dosch. 1990. Expression of glycolipid receptors to Shiga-like toxin on human B lymphocytes: a mechanism for the failure of long-lived antibody response to dysenteric disease. Int. Immunol. 2:1-8. [DOI] [PubMed] [Google Scholar]
- 6.Hilbi, H., J. E. Moss, D. Hersh, Y. Chen, J. Arondel, S. Banerjee, R. A. Flavell, J. Yuan, P. J. Sansonetti, and A. Zychlinsky. 1998. Shigella-induced apoptosis is dependent on caspase-1 which binds to IpaB. J. Biol. Chem. 273:32895-32900. [DOI] [PubMed] [Google Scholar]
- 7.Islam, D., B. Veress, P. K. Bardhan, A. A. Lindberg, and B. Christensson. 1997. In situ characterization of inflammatory responses in the rectal mucosae of patients with shigellosis. Infect. Immun. 65:739-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee, A., M. K. Whyte, and C. Haslett. 1993. Inhibition of apoptosis and prolongation of neutrophil functional longevity by inflammatory mediators. J. Leukoc. Biol. 54:283-288. [PubMed] [Google Scholar]
- 9.Leite-de-Moraes, M. C., A. Herbelin, C. Gouarin, Y. Koezuka, E. Schneider, and M. Dy. 2000. Fas/Fas ligand interactions promote activation-induced cell death of NK T lymphocytes. J. Immunol. 165:4367-4371. [DOI] [PubMed] [Google Scholar]
- 10.Mathan, M. M., and V. I. Mathan. 1991. Morphology of rectal mucosa of patients with shigellosis. Rev. Infect. Dis. 13(Suppl. 4):S314-S318. [DOI] [PubMed] [Google Scholar]
- 11.Mathan, M. M., and V. I. Mathan. 1986. Ultrastructural pathology of the rectal mucosa in Shigella dysentery. Am. J. Pathol. 123:25-38. [PMC free article] [PubMed] [Google Scholar]
- 12.Murray, J., J. A. Barbara, S. A. Dunkley, A. F. Lopez, X. Van Ostade, A. M. Condliffe, I. Dransfield, C. Haslett, and E. R. Chilvers. 1997. Regulation of neutrophil apoptosis by tumor necrosis factor-alpha: requirement for TNFR55 and TNFR75 for induction of apoptosis in vitro. Blood 90:2772-2783. [PubMed] [Google Scholar]
- 13.Perdomo, J. J., P. Gounon, and P. J. Sansonetti. 1994. Polymorphonuclear leukocyte transmigration promotes invasion of colonic epithelial monolayer by Shigella flexneri. J. Clin. Investig. 93:633-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perdomo, O. J., J. M. Cavaillon, M. Huerre, H. Ohayon, P. Gounon, and P. J. Sansonetti. 1994. Acute inflammation causes epithelial invasion and mucosal destruction in experimental shigellosis. J. Exp. Med. 180:1307-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raqib, R., A. A. Lindberg, B. Wretlind, P. K. Bardhan, U. Andersson, and J. Andersson. 1995. Persistence of local cytokine production in shigellosis in acute and convalescent stages. Infect. Immun. 63:289-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raqib, R., S. M. Mia, F. Qadri, T. I. Alam, N. H. Alam, A. K. Chowdhury, M. M. Mathan, and J. Andersson. 2000. Innate immune responses in children and adults with shigellosis. Infect. Immun. 68:3620-3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raqib, R., F. P. Reinholt, P. K. Bardhan, A. Karnell, and A. A. Lindberg. 1994. Immunopathological patterns in the rectal mucosa of patients with shigellosis: expression of HLA-DR antigens and T-lymphocyte subsets. APMIS 102:371-380. [DOI] [PubMed] [Google Scholar]
- 18.Raqib, R., B. Wretlind, J. Andersson, and A. A. Lindberg. 1995. Cytokine secretion in acute shigellosis is correlated to disease activity and directed more to stool than to plasma. J. Infect. Dis. 171:376-384. [DOI] [PubMed] [Google Scholar]
- 19.Sansonetti, P. J. 1992. Molecular and cellular biology of Shigella flexneri invasiveness: from cell assay systems to shigellosis. Curr. Top. Microbiol. Immunol. 180:1-19. [DOI] [PubMed] [Google Scholar]
- 20.Sansonetti, P. J. 1998. Molecular and cellular mechanisms of invasion of the intestinal barrier by enteric pathogens. The paradigm of Shigella. Folia Microbiol. 43:239-246. [DOI] [PubMed] [Google Scholar]
- 21.Sansonetti, P. J., A. Phalipon, J. Arondel, K. Thirumalai, S. Banerjee, S. Akira, K. Takeda, and A. Zychlinsky. 2000. Caspase-1 activation of IL-1beta and IL-18 are essential for Shigella flexneri-induced inflammation. Immunity 12:581-590. [DOI] [PubMed] [Google Scholar]
- 22.Schroter, M., B. Lowin, C. Borner, and J. Tschopp. 1995. Regulation of Fas(Apo-1/CD95)- and perforin-mediated lytic pathways of primary cytotoxic T lymphocytes by the protooncogene bcl-2. Eur. J. Immunol. 25:3509-3513. [DOI] [PubMed] [Google Scholar]
- 23.Simon, H. U. 2001. Regulation of eosinophil and neutrophil apoptosis--similarities and differences. Immunol. Rev. 179:156-162. [DOI] [PubMed] [Google Scholar]
- 24.Sutton, V. R., D. L. Vaux, and J. A. Trapani. 1997. Bcl-2 prevents apoptosis induced by perforin and granzyme B, but not that mediated by whole cytotoxic lymphocytes. J. Immunol. 158:5783-5790. [PubMed] [Google Scholar]
- 25.Takeuchi, A., H. Sprinz, E. H. LaBrec, and S. B. Formal. 1965. Experimental bacillary dysentery. An electron microscopic study of the response of the intestinal mucosa to bacterial invasion. Am. J. Pathol. 47:1011-1044. [PMC free article] [PubMed] [Google Scholar]
- 26.Wyllie, A. H. 1997. Apoptosis: an overview. Br. Med. Bull. 53:451-465. [DOI] [PubMed] [Google Scholar]
- 27.Zychlinsky, A., C. Fitting, J. M. Cavaillon, and P. J. Sansonetti. 1994. Interleukin 1 is released by murine macrophages during apoptosis induced by Shigella flexneri. J. Clin. Investig. 94:1328-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zychlinsky, A., B. Kenny, R. Menard, M. C. Prevost, I. B. Holland, and P. J. Sansonetti. 1994. IpaB mediates macrophage apoptosis induced by Shigella flexneri. Mol. Microbiol. 11:619-627. [DOI] [PubMed] [Google Scholar]
- 29.Zychlinsky, A., M. C. Prevost, and P. J. Sansonetti. 1992. Shigella flexneri induces apoptosis in infected macrophages. Nature 358:167-169. [DOI] [PubMed] [Google Scholar]
- 30.Zychlinsky, A., K. Thirumalai, J. Arondel, J. R. Cantey, A. O. Aliprantis, and P. J. Sansonetti. 1996. In vivo apoptosis in Shigella flexneri infections. Infect. Immun. 64:5357-5365. [DOI] [PMC free article] [PubMed] [Google Scholar]