Abstract
Lipopolysaccharide (LPS) has recently been shown to facilitate macrophage foam cell formation and has been suggested to be a proatherogenic factor. The mechanism of LPS induced cholesterol accumulation, however, is unclear. In this report, using the macrophage-like RAW 264.7 cell line, we provide experimental evidence that LPS's proatherogenic effects may at least in part reflect altered cholesterol metabolism. Data presented demonstrate that in a dose-dependent manner, LPS is able to down regulate the mRNA expression of the two primary high-density lipoprotein (HDL) receptors, scavenger receptor B1 (SR-B1) and ATP binding cassette A1 (ABCA1), with a 50% inhibitory concentration of less than 0.2 ng/ml, as well as to decrease SR-B1 protein expression by 80%. We also found that LPS treatment resulted in a significant decrease (to 20% of the control level) of the specific 125I-HDL binding as well as in 50% inhibition of the HDL-mediated cholesterol efflux compared to untreated cells. In addition, we compared the potencies of various modified LPS preparations and demonstrated that the phosphorylated lipid A portion of LPS, which is highly conserved among gram-negative microorganisms, including Chlamydia, is primarily responsible for the effects of LPS on SR-B1 and ABCA1 expression. Inhibitors of NF-κB activation were observed to efficiently block the suppressive effect of LPS on SR-B1 and ABCA1, suggesting a mechanism involving NF-κB. These data indicate that the LPS effects on cholesterol metabolism may contribute to the proatherogenic properties of LPS.
Lipopolysaccharides (LPS) are major integral components of the outer membrane of gram-negative bacteria. When released from bacteria, LPS elicit in higher organisms a broad spectrum of biological activities, especially activation of immune and inflammatory cells, including macrophages, monocytes, and endothelial cells (17, 42). The vascular endothelial cells, like macrophages, play a central role in a host's defense against bacterial infection and are a major cellular target for LPS action. LPS has multiple effects on macrophages, including the induction of secreted inflammatory mediators such as leukocyte adhesion molecules, soluble cytokines, and chemokines (28, 42, 56). In addition to the role of LPS in gram-negative sepsis (28), recent studies demonstrate that LPS induces immune, proinflammatory, and other as-yet-undetermined mechanisms that may be important in triggering atherogenesis (27, 46). Both clinical and experimental data support considering atherosclerosis as a chronic inflammatory disorder associated with endothelial and cholesterol balance dysfunction (10, 14). A number of inflammatory diseases, including lupus erythermatosis, arthritis, Crohn's disease, and others, have been reported to be accompanied by the premature development of atherosclerosis (25, 26, 41).
NF-κB is a central mediator of gene expression induced by proinflammatory and proatherogenic stimuli, including inflammatory cytokines, oxidative stress, LPS, and bacterial products (35). Toll-like and interleukin receptor families deliver signals from a wide spectrum of ligands (58) through downstream signaling to activate NF-κB translocation, inducing the coordinated expression of specific genes (35, 52). The NF-κB-responsive genes (35) encoding leukocyte adhesion molecules (such as vascular cell adhesion molecular-1 and intracellular adhesion molecule-1), chemotactic factors (such as monocyte chemoattractant protein-1), and growth factors (such as macrophage colony-stimulating factor) have been demonstrated to be involved in the early development of atherosclerotic lesions (16, 20, 32, 44). However, these observations have provided little insight so far into understanding of the abnormal cholesterol metabolism in lesion sites.
The interaction of high-density lipoproteins (HDL) or lipid-poor apolipoproteins with specific surface receptors has been reported to activate cholesterol translocation to the cell surface (59) and its diffusion onto appropriate particles against a cholesterol concentration gradient (45). Cholesterol efflux allows peripheral cells to eliminate cholesterol excess, which is transported to the liver for further secretion into the bile or conversion into bile acids (34, 38). It has been suggested that the intracellular cholesterol pool, which is readily available for both esterification and transportation to the plasma membrane, plays a central role in macrophage transformation into foam cells. Consequently, mechanisms mediating cholesterol efflux are critical for maintaining cholesterol homeostasis in the macrophage (16).
Earlier studies have demonstrated that LPS, as a potent NF-κB activator, is able to alter lipid metabolism in macrophages (50). A severalfold increase of triglyceride content and the cholesterol esterification rate has been reported to transform macrophages into foam-like cells (16). However, the question of whether this observation involved expression of multiple HDL receptors has not been addressed. In the present study we investigated the effect of LPS on the expression of the two primary HDL receptors, scavenger receptor B1 (SR-B1) and ATP binding cassette A1 (ABCA1), in the mouse monocyte-macrophage RAW cell line. These data suggest the involvement of NF-κB activation in the LPS-induced decrease of SR-B1 and ABCA1 transporter expression.
MATERIALS AND METHODS
Cell culture and treatment.
RAW 264.7 mouse monocyte-macrophages (ATCC TIB 71) were grown in 12-well plates in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum, penicillin (100 U/ml), and streptomycin (100 μg/ml) in a humidified atmosphere containing 5% CO2 and 95% air at 37°C. The experiments were carried out on the confluent monolayers in serum-free DMEM. Cells were treated with the following LPS preparations at 10 ng/ml for 24 h: full-length LPS from Escherichia coli serotype 0111:B4 (Sigma Chemical Co., St. Louis, Mo.) or Re595 mutant LPS, diphosphoryl lipid A (DPLA), or monophosphoryl lipid A (MPLA) (from Salmonella enterica serovar Minnesota; Sigma Chemical Co.). The serine protease inhibitor tosylphenyl chloromethyl ketone (TPCK) or tosyllysyl chloromethyl ketone (TLCK) (Sigma Chemical Co.) was added to the cells at 20 mM 2 h before the LPS treatment and was present in the experimental medium simultaneously with LPS for the next 22 h.
Western immunoblot analysis.
At the end of the incubation the cells were harvested, washed with phosphate-buffered saline (PBS) (pH 7.4) containing 5 mM EDTA and 1 mM phenylmethylsulfonyl fluoride, and incubated in the same buffer containing 2% Triton X-100 for 15 min at 4°C. Following lysis, cell debris was removed by the centrifugation (12,000 × g, 4°C, 10 min). The supernatants were delipidated by addition of a mixture of methanol and chloroform (4:1) and consequent centrifugation at 12,000 × g for 10 min at 4°C. The pellets were dissolved in the sample buffer and heated to 82°C (15 min). The aliquots of samples were then applied to 4 to 20% precast gels (Invitrogen, Carlsbad, Calif.) and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Proteins were electrophoretically transferred to nitrocellulose, and the membranes were incubated with TBS (200 mM Tris-HCl, 150 mM NaCl, 5% nonfat dry milk) blocking solution for 1 h at room temperature. Membranes were incubated with rabbit polyclonal anti-SR-B1 antibodies (diluted 1:1,000) (Novus, Littleton, Colo.) and mouse monoclonal anti-β-actin antibodies (1:10,000) (Sigma Chemical Co.) overnight, washed three times with TBS containing 0.1% Tween 20, and then incubated with goat anti-rabbit or anti-mouse antiserum (1:10,000) conjugated to alkaline phosphatase for 1 h at room temperature. Quantitative comparison of the bands was performed by densitometry.
RNA isolation and cDNA preparation.
The total RNA of cultured cells was isolated and purified using Trizol reagent (Gibco BRL, Grand Island, N.Y.) according to the manufacturer's protocol. The concentration and quality of RNA were determined by UV absorbance at 260 and 280 nm. To prepare cDNA, total RNA (3 μg) was added to a mixture containing the following: 6 μl of 5× first-strand buffer (75 mM KCl, 50 mM Tris-HCl [pH 8.3], 3 mM MgCl2), 1.5μl of deoxynucleoside triphosphates (10 mM [each] dATP, dCTP, dTTP, and dGTP), 1.5 μl of 0.0156 U (0.4 μg) of random hexamers, and 0.2 U of Moloney murine leukemia virus reverse transcriptase (all from Gibco BRL, Gaithersburg, Md.); 0.004 U of RNasin (Promega, Madison, Wis.); and RNase-free water to a final volume of 30 μl per 3μg of cDNA. Samples were incubated at 37°C for 60 min. Preliminary experiments were undertaken to achieve optimal conditions for amplifying mRNA for each of the gene products.
Reverse transcription-PCR (RT-PCR).
A mixture of 37.2 μl of RNase-free water, 5 μl of 10× reaction buffer, and 0.5 μl (5 U) of AmpliTaq Gold DNA polymerase (Applied Biosystems, Roche Molecular Systems, Inc., Branchburg, N.J.), sense and antisense primers (1 μl each), and 0.3μl (3 μCi) of [α-32P]dCTP (Amersham Pharmacia Biotech, San Diego, Calif.) was vortexed, and 46 μl was aliquoted into each tube, containing 4 μl of cDNA, and overlaid with 50 μl of mineral oil (Sigma Chemical Co.). cDNA was amplified in a Perkin-Elmer (Norwalk, Conn.) System 2400 DNA thermal cycler, with denaturation for 1 min at 94°C, annealing for 1 min at 50°C, and extension for 2 min at 72°C for GADPH (glyceraldehyde-3-phosphate dehydrogenase) (19 cycles), ABCA1 (28 cycles), SR-B1, and interleukin-1β (IL-1β) (27 cycles). The primers used in these analyses were as follows: GADPH, 5′-GTC TTC ACC ACC ATG GAG AAG-3′ and 5′-GCT TCA CCA CCT TCT TGA TGT CAT C-3′; SR-B1, 5′-CCA CCC AAC GAA GGC TTC TGC-3′ and 5′-CTG AAT GGC CTC CTT ATC C-3′; ABCA1, 5′-CAA CTA CAA AGC CCT CTT TG-3′ and 5′-CTT GGC TGT TCT CCA TGA AG-3′; and IL-1β, 5′-CTG AAA GCT CTC CAC CTC-3′ and 5′-GTG CTG ATG TAC CAG TTG-3′.
For the densitometry analysis, the intensities of the bands were measured with the Gel-Pro Analyzer 3.0 computer program and normalized with GADPH intensity.
125I-HDL binding assay.
125I-HDL binding experiments were performed as previously described (3). RAW cells cultured in 12-well plates were incubated with 1 μg of LPS per ml for 24 h. After three subsequent washes with PBS, the cells were chilled on ice and binding of 125I-HDL (5 μg/ml) was determined in the absence (total binding) or presence (nonspecific binding) of a 50-fold excess of unlabeled ligand. Radioactivity was counted in an LKB-Wallac Ultragamma counter. The protein contents of samples were determined after hydrolysis in 0.1 N NaOH followed by neutralization with 0.1 N HCl by the method of Bradford (4).
Cholesterol efflux studies.
The cholesterol efflux assay was performed essentially according to the protocol described in detail elsewhere (31). At 70 to 80% confluence, cholesterol-loaded cells grown on 24-well plates were incubated with DMEM containing 1 μCi of [1,2-3H]cholesterol (50 Ci/mmol; Amersham Pharmacia Biotech, Piscataway, N.J.) per ml for 48 h. Cells were washed three times with PBS containing 1 mg of bovine serum albumin per ml (PBS-BSA), and cellular cholesterol pools were allowed to equilibrate for another 24 h in DMEM containing 1 mg of BSA per ml (DMEM-BSA) and increasing doses (0, 10, 30, 100, 300, and 1,000 ng/ml) of LPS. After intensive washing of cells with PBS-BSA, efflux studies (24 h) were carried out using 100 μg of HDL (Calbiochem, San Diego, Calif.) per ml prepared in DMEM-BSA as the cholesterol acceptor. After the efflux period, medium was collected and centrifuged (10,000× g for 5 min), and radioactivity was counted by liquid scintillation counting. The residual radioactivity in the cell fraction was determined after an overnight extraction with hexane-isopropanol (3:2). The percent efflux was calculated by dividing the radioactive counts in the efflux medium by the sum of the radioactive counts in the medium and the cell fraction. DMEM-BSA was used as the blank, the radioactive counts in which were subtracted from the counts obtained in the presence of a cholesterol acceptor.
Statistical analysis.
All results were reproduced in at least two independent experiments. The results are presented as the means of triplicate determinations ± standard deviations. Comparisons between groups of data were performed by a Student's t test. P values of less than 0.05 were considered statistically significant.
RESULTS
Time courses of ABCA1, SR-B1, and IL-1β mRNA biosynthesis and SR-B1 protein expression in response to LPS exposure.
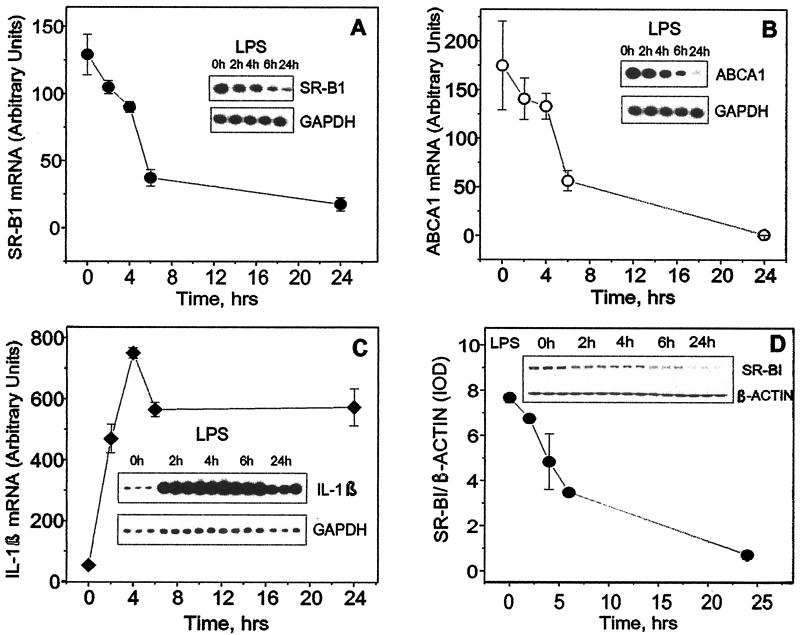
To study the kinetics of LPS effects upon the mRNA levels of SR-B1, ABCA1, and IL-1β (IL-1β was used as a well-established LPS-up-regulated cytokine), RAW cells were exposed to LPS (1 μg/ml) for increasing periods of time (Fig. 1). The decrease in both SR-B1 and ABCA1 gene expression was detectable as early as 4 h after exposure to LPS and reached its maximum after 24 h. The LPS-induced increase of IL-1β gene expression had more rapid kinetics and reached its maximum after 4 h of LPS exposure, remaining still significantly elevated versus the control level at 24 h. SR-B1 protein production was decreased by 50% after 6 h (Fig. 1D). This demonstrates that in addition to the widely known ability of LPS to induce the expression of proinflammatory cytokine genes, LPS is a powerful inhibitor of SR-B1 and ABCA1 expression in murine monocytes.
FIG. 1.
Effect of LPS on the time course of SR-B1 (A), ABCA1 (B), and IL-1β (C) mRNA and SR-B1 protein (D) expression. (A, B, and C) Total mRNA was isolated from RAW cells after 0, 2, 4, 6, and 24 h of incubation with LPS (1 μg/ml) in serum-free medium. Levels of mRNA expression for the indicated genes were tested by RT-PCR analyses. Corresponding samples were analyzed for GAPDH mRNA as controls. Levels of mRNA were quantitated by scanning densitometry and corrected relative to the levels of housekeeping gene mRNA. Data are presented as the ratio of integral optical density of the indicated gene to that of the GADPH gene multiplied by 100. (D) Cultured cells were treated with LPS for the indicated periods of time, and SR-B1 protein expression was estimated by Western blot analysis. Simultaneously, β-actin levels in the corresponding samples were determined to confirm equal protein loading. Nitrocellulose membranes were scanned, and the integral optical density (IOD) values of the protein bands were estimated with the GelPro computer program. Data are expressed as the ratio of the SR-B1 band integral optical density to the corresponding β-actin band integral optical density. Error bars indicate standard deviations.
Dose-dependent response of LPS-sensitive genes to LPS.
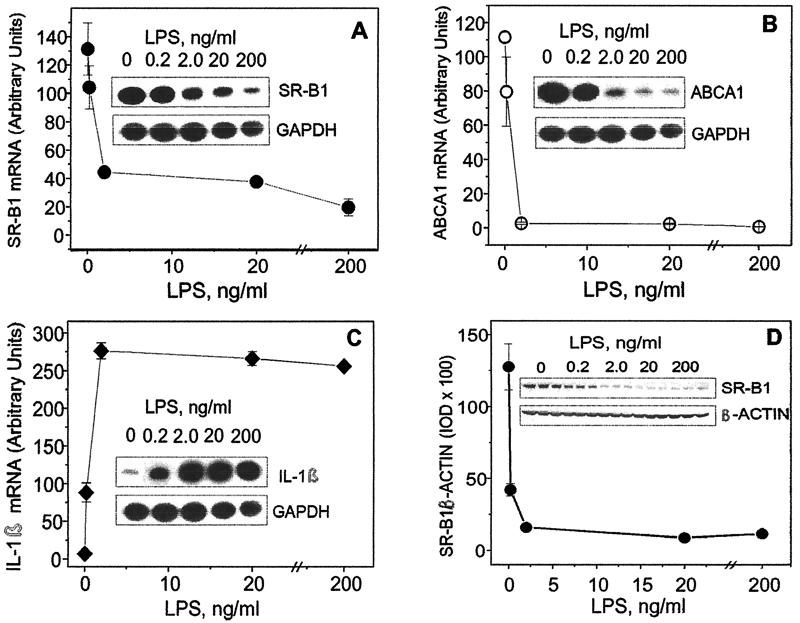
To study the dose dependence of the LPS effect upon the SR-B1, ABCA1, and IL-1β mRNAs as well as on SR-B1 protein expression, RAW cells were exposed to the increasing concentrations of LPS (0.2 to 200 ng/ml) for 24 h. As shown in Fig. 2A to C, LPS induced a dose-dependent increase of IL-1β mRNA or decrease of ABCA1 and SR-B1 mRNA with very similar patterns. SR-B1 gene expression was significantly reduced, but less so than ABCA1 expression. SR-B1 protein expression demonstrates a pattern of LPS inhibition (Fig. 2D) similar to that for the mRNA.
FIG. 2.
Dose-dependent effect of LPS on the expression of ABCA1 (A), SR-B1 (B), and IL-1β (C) genes and SR-B1 protein (D). Cultured cells were exposed to the increasing concentrations (0, 0.2, 2, 20, and 200 ng/ml) of LPS for 24 h. Levels of mRNA expression for the indicated genes were tested by RT-PCR analyses, and SR-B1 protein expression was estimated by Western blot analysis. For further steps, see the Fig. 1 legend. The results shown represent one of two experiments that yielded similar results. IOD, integrated optical density. Error bars indicate standard deviations.
LPS-mediated suppression of 125I-HDL binding and cholesterol efflux to HDL.
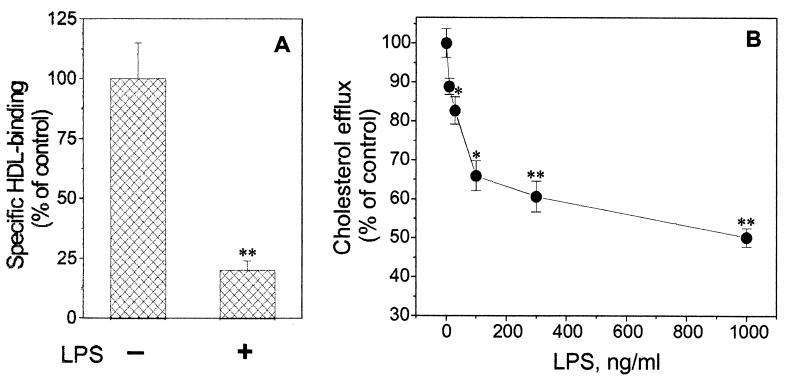
In order to determine if there is any correlation between LPS-mediated down regulation of both HDL binding protein mRNA and their physiological function, we performed 125I-HDL binding assay after the incubation of cells with LPS (1 μg/ml) for 24 h. As a result of pretreatment with LPS, we observed significant decrease of the specific 125I-HDL binding (Fig. 3A), to 20% of the control level. Next we performed cholesterol efflux experiments, using HDL (100 μg/ml) as the cholesterol acceptor. Following preincubation with increasing LPS concentrations (0 to 1,000 ng/ml), we were able to detect a dose-dependent inhibitory effect upon the HDL-mediated [3H]cholesterol efflux in cultured RAW cells (Fig. 3B). The highest dose of the LPS (1 μg/ml) resulted in an approximately 50% decrease of [3H]cholesterol efflux to HDL compared to that in untreated cells.
FIG. 3.
Down regulation of specific HDL binding and HDL-mediated cholesterol efflux by LPS. (A) Effect of LPS on 125I-HDL specific binding in RAW cells. The cells were incubated with 1 μg of LPS per ml for 24 h. Following three PBS washes, the specific binding of 125I-HDL (5 μg/ml) was determined at 4°C as the difference between the total and nonspecific binding (in the absence or presence of a 50-fold excess of unlabeled HDL). (B) Dose-dependent response of HDL-mediated [3H]cholesterol efflux to LPS stimulation. RAW cells preloaded with cholesterol were labeled with 1 μCi of [1,2-3H]cholesterol (50 Ci/mmol) per ml. Before the cholesterol efflux determination, the cells were pretreated with the increasing concentrations (0 to 1,000 ng/ml) of LPS for 24 h. After HDL (100 μg/ml) as the cholesterol acceptor was added, the [3H]cholesterol efflux assay was performed after an additional 24 h. Cholesterol efflux was calculated as the amount of radioactivity present in the medium divided by the total radioactivity (medium plus cell) in each well. The data shown represent one of two independent experiments that yielded similar results. ∗, P < 0.05; ∗∗, P < 0.01 (compared to untreated control samples). Error bars indicate standard deviations.
Comparison of the abilities of different LPS preparations to modulate the expression of LPS-responsive genes.
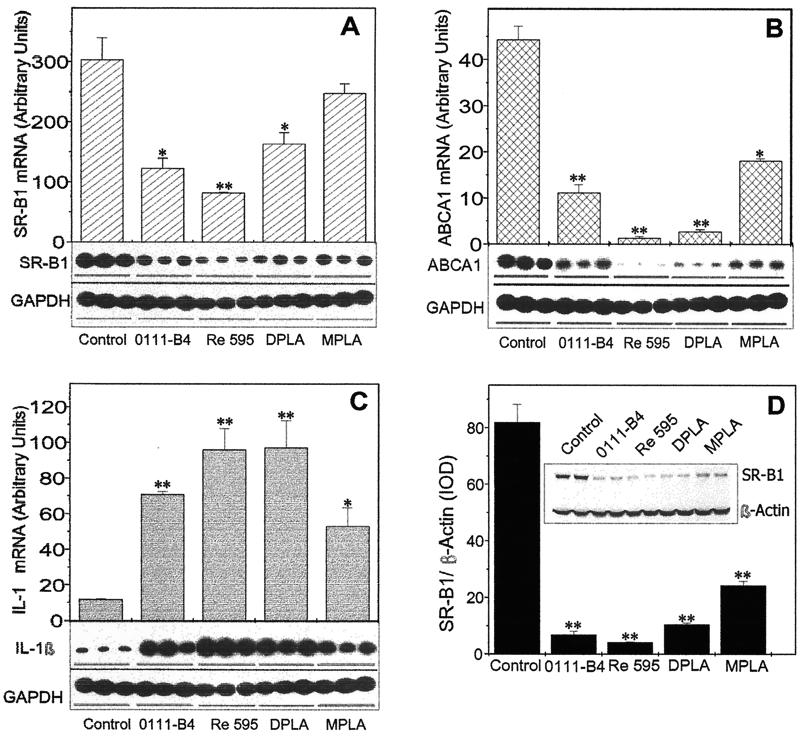
Although the lipid A component is proposed as being the active portion for LPS bioactivities, a variety of lipid A partial structures and analogues are reported to have different properties. The complete form of LPS from E. coli (serotype 0111:B4), LPS of the Re595 mutant of Salmonella enterica serovar Minnesota (lacking O antigen and outer core polysaccharide), Re595 DPLA (lacking O antigen and outer and inner core polysaccharides), and Re595 MPLA (lacking O antigen, outer and inner polysaccharides, and one phosphoryl group) were evaluated for their ability to affect IL-1β, ABCA1, and SR-B1 mRNA expression. As shown in Fig. 4, MPLA had decreased activity compared to the other LPS derivatives, which markedly (7- to 10-fold) elevated the IL-1β mRNA level and significantly inhibited ABCA1 (by 75 to 80%) and SR-B1 (50%) mRNA biosynthesis at a concentration of 10 ng/ml after 24 h. Under the same conditions, SR-B1 protein expression demonstrated very similar relative responses to the LPS analogues (Fig. 4D). However, when a concentration of 1 μg/ml was used, no significant differences were observed between the LPS derivatives in their ability to suppress SR-B1 production (data not shown). These results indicate that for the suppressive effect of the LPS preparations on SR-B1 and ABCA1 expression as well as on SR-B1 protein expression, the relative activities are LPS (Re595) > LPS (0111:B4) > DPLA > MPLA. For IL-1β mRNA up regulation, a statistically significant difference could be observed only for MPLA, which demonstrated approximately one-half the potency for IL-1β gene induction of Re595 LPS or DPLA.
FIG. 4.
Comparison of Re595, 0011:B4, DPLA, and MPLA potency to modulate LPS-sensitive gene expression and to suppress SR-B1 protein expression. RAW cells were exposed to different LPS preparations (10 ng/ml) for 24 h in serum-free medium. (A, B, and C) Total mRNA was isolated and treated as described in the Fig. 1 legend. The levels of mRNA expression for the indicated genes were tested by RT-PCR analyses. (D) The level of SR-B1 protein expression was determined by Western blot analysis. The results represent one of two separate experiments that yielded similar results. ∗, P < 0.05; ∗∗, P < 0.01 (compared to control untreated samples). IOD, integrated optical density. Error bars indicate standard deviations.
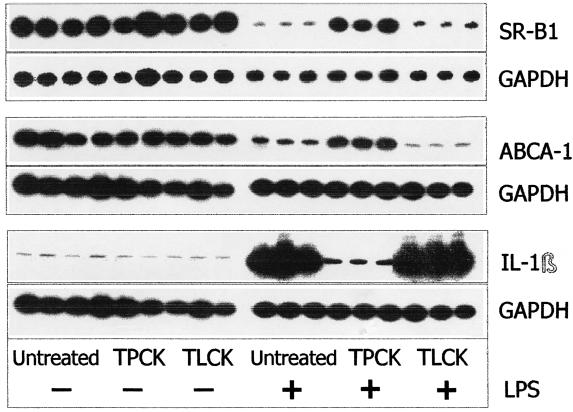
Effect of NF-κB inhibitors on LPS-modulated gene expression.
NF-κB is a major transcription factor that up regulates proinflammatory cytokine expression (2, 31). To examine whether the suppressive effect of LPS on SR-B1 and ABCA1 expression was mediated through of NF-κB, RAW cells were incubated with TPCK (chymotrypsin-like serine protease inhibitor, a potent inhibitor of NF-κB activation) or TLCK (a structural analog of the TPCK that lacks NF-κB inhibitor activity) in the presence of LPS (10 ng/ml) for 24 h. TPCK treatment effectively prevented the LPS-induced SR-B1 and ABCA1 mRNA level decrease as well as the increase of IL-1β (Fig. 5). At the same time, the negative control, TLCK, was not able to reverse these LPS-mediated effects. These data demonstrate that inhibition of NF-κB is sufficient to block LPS-induced suppression of both the ABCA1 and SR-B1 genes, indicating the involvement of NF-κB activation as the mechanism in this process.
FIG. 5.
Effect of protease inhibitor TPCK and its structural analogue TLCK on LPS-induced changes in IL-1β, SR-B1, and ABCA1 mRNA expression. The cells were incubated with TPCK or TLCK (negative control) alone for 2 h prior to LPS addition and then for 22 h in the presence of LPS (10 ng/ml). After the incubation, total mRNA was isolated and the samples were analyzed by RT-PCR. The results shown represent one of two separate experiments that yielded similar results.
DISCUSSION
In atherosclerosis, both clinical and biochemical evidence strongly suggests that lesion development can be accelerated by local actions of inflammatory cytokines on endothelial cells (18, 30). There are now several reports linking atherosclerosis with infection and inflammation. For example, a higher incidence of coronary artery heart disease occurs in patients with Chlamydia pneumoniae and cytomegalovirus infections, and these microorganisms have been detected in atherosclerosis plaques (8, 33). In addition, LPS can induce oxidative modification of low-density lipoproteins (7), a major mediator in atherogenesis (27). Recent data obtained by Kalayoglu and Byrne (22) indicate that chlamydial LPS induces macrophage foam cell formation and suggest that infected macrophages chronically exposed to chlamydial LPS may accumulate excess cholesterol, contributing to atheroma development.
No previous data suggest a modulating role of low-dose LPS exposure on the expression of two key HDL binding proteins, ABCA1 and SR-B1. In our study we have demonstrated that LPS is able to negatively regulate the SR-B1 and ABCA1 mRNA levels in RAW cells. The expression of the SR-B1 protein was similarly suppressed. In our study we did not investigate the LPS effect upon ABCA1 protein expression by the immunoblotting assay, as we were not able to detect ABCA1 in our samples under standard conditions. The choice of murine macrophages for our investigation was determined by the fact that the macrophage-like RAW 264.7 cell line is a well-characterized model system in terms of LPS-induced macrophage activation resulting in the proinflammatory cell response (13, 49). In addition, this cell line is a model widely used in numerous macrophage regulatory studies of cholesterol efflux (48, 51). At the same time, we acknowledge that this cell line (like other models) has potential limitations, as the regulatory pathways of gene expression may reflect differences of species or transformation. Our findings concerning the inhibitory effect of LPS on SR-B1 gene expression as well as on protein expression are consistent with the data of Buechler et al. (5), who first provided evidence that the expression of CLA-1, the human homologue of SR-B1, is suppressed by high doses of LPS in human monocytes and macrophages. In that study, LPS markedly inhibited CLA-1 mRNA and protein expression at a concentration of 1 μg/ml. In our experiments, LPS was an inhibitor of SR-B1 and ABCA1 mRNA expression as well as SR-B1 protein expression at 0.2 ng/ml, a concentration that can be pathophysiologically relevant.
SR-B1 and its human homologue CLA-1, both membrane proteins, are highly expressed in the liver, adrenal gland, and ovary (23) as well as in atherosclerotic lesions of ApoE-deficient mice (20). SR-B1 binds HDL with high affinity and mediates uptake of esterified and free cholesterol from HDL in liver and steroidogenic tissues (1, 36). In addition to its role in cholesterol delivery to peripheral tissues, recent experimental data strongly suggest a possible physiological role of SR-B1 in the cellular efflux of both cholesterol from intracellular pools and plasma membrane sterols to HDL. According to recently reported data, SR-B1-mediated bidirectional cholesterol flux is not a result of the tethering of the donor or acceptor particle to the cell surface receptor but is rather due to the lipid organization changes within the plasma membrane lipid bilayer (11, 45). Thus, assuming the ability of SR-B1 to induce changes in the lipid domain organization within the plasma membrane, the mechanism of its participation in the exchange of cholesterol between the cell plasma membranes and phospholipid-containing acceptors can be regarded as gradient diffusion. The direction of the cholesterol movement is considered to be determined by its differential concentration gradient across the membrane as well as by the type of lipoprotein particle and cell type (11, 45). Consistent with this concept, it seems reasonable to suggest that in macrophages, SR-B1 is more likely to be involved in the promotion of cholesterol efflux rather than in sterol transfer into the cells. Therefore, functional SR-B1, which is apparently responsible for the passive constituent of HDL-mediated cholesterol efflux, should be of particular importance, since macrophages are the predecessors of foam cells. From this point of view, our findings, revealing a suppressive effect of LPS on SR-B1 expression in mouse macrophages indicate that the diffusive component of cholesterol efflux, which is likely mediated by SR-B1, might be markedly impaired upon the exposure of cells to endotoxin.
In addition to the LPS inhibitory effect on SR-B1 mRNA and protein expression, we have demonstrated LPS's ability to down regulate ABCA1 expression. This transporter encodes a membrane protein that plays a critical role in ApoA-I-dependent cholesterol and phospholipid efflux from cells (39). ABCA1 has been found to have key functions in regulating HDL plasma concentrations and the balance of cholesterol within the cell (54). The crucial step towards understanding the physiological role of this protein was the identification of ABCA1 as the defective gene in Tangier disease, a rare disorder characterized by very low plasma HDL and the inability of cells to efflux intracellular cholesterol to lipid-poor ApoA-I, the primary HDL precursor (39). Thus, mutations within the ABCA1 gene result in the impairment of the first stage in reverse cholesterol transport, cholesterol transfer from intracellular compartments to the plasma membrane. Ineffective cholesterol efflux permits formation of foam cells, the progenitors of arterial lesions. Immunocytochemical studies have demonstrated that ABCA1 is presumably localized on the plasma membrane (24, 55), and according to more recent observations, it is also present in the cytosol and Golgi compartment of unstimulated fibroblasts (47) as well as on intracellular vesicles of stably and transiently transfected HeLa cells (37). These last observations provide new insights into its important role in intracellular cholesterol trafficking.
With the known important role of ABCA1 as the mediator of cholesterol efflux, our data demonstrating that extremely low LPS concentrations cause almost complete suppression of ABCA1 expression suggest another intriguing possibility: the combined inhibitory effects of LPS on the expression of the SR-B1 and ABCA1 genes may severely impair both components of the efflux process. This includes gradient diffusion facilitated by SR-B1, as well as the ABCA1-mediated component, including intracellular trafficking of lipids with their subsequent delivery onto the outer lipid bilayer leaflet of the plasma membrane.
Additional experimental evidence of LPS's possible role as a potent proatherogenic stimulus is provided by our data demonstrating its ability to down regulate specific HDL binding as well as HDL-mediated cholesterol efflux. According to the results obtained in our study, LPS dramatically inhibited (up to 20% of control level) specific HDL binding and moderately decreased (50% inhibition of control level) cholesterol efflux to HDL in cultured RAW cells. In our study the effective dose of LPS able to elicit 50% inhibition of the HDL-mediated cholesterol efflux turned out to be essentially higher than the dose required for 50% decreases of SR-B1 and ABCA1 mRNA expression. Different experimental conditions could possibly be the cause of the observed differences. Unlike RT-PCR and Western blotting analyses, the cholesterol efflux estimation was performed 24 h after the withdrawal of LPS from cultured cells. As a result, there is a reasonable expectation that within the subsequent 24 h (the duration of the cholesterol efflux experiment) following LPS removal from cells, its suppressive effect upon HDL receptor gene expression could be partially reversed.
Lipopolysaccharides are composed of the O antigen and the core part (21). The latter includes lipid A, the biologically active portion (60), which also anchors LPS in the outer bacterial wall (12). It is generally assumed that the lipid A component is responsible for the endotoxic properties of LPS (12, 53). Some experimental evidence indicates that the LPS polysaccharide chain and phosphoryl groups may contribute to the some of the LPS effects on macrophages. In our study we compared various modified LPS preparations in terms of their ability to induce suppression of the ABCA1 and SR-B1 genes, as well as to stimulate IL-1β mRNA expression. It was found that the Re595 mutant LPS, which has been used in all of our experiments, as well as Re595 DPLA displayed the same activity as the complete form of LPS from E. coli. Re595 MPLA appeared to be a less potent modulator of SR-B1, ABCA1, and IL-1β gene expression. With a higher LPS concentration, the various LPS preparations demonstrated equal inhibitory effects upon the SR-B1 protein expression. These results indicate that the phosphorylated lipid A portion of LPS is required for maximal LPS effects on SR-B1 and ABCA1 gene expression. Apparently, LPS modifications that do not affect the negative charge of its glycolipid interface (lipid A) do not markedly alter these endotoxin activities.
While investigating the LPS-mediated effects on SR-B1 and ABCA1 gene expression in RAW cells, we conducted parallel studies of IL-1β mRNA changes. Our results demonstrated a dose and time dependence of IL-1β mRNA expression similar to that for the negatively regulated LPS-responsive genes. These data suggest the involvement of a similar, if not the same, signaling cascade for the LPS effects on ABCA1, SR-B1, and IL-1β gene expression. However, to date there are no reports confirming this suggestion. Numerous studies have demonstrated that the LPS activation of monocytes and macrophages is associated with NF-κB activation (17, 29, 35). It has been demonstrated that transcription factor NF-κB is critical for the expression of multiple genes involved in inflammatory responses (2, 9, 17). Our studies evaluating the inhibitory effect of LPS on ABCA1 and SR-B1 gene expression by utilizing the NF-κB inhibitor TPCK clearly demonstrated the involvement of NF-κB activation with the observed LPS effect. However, it remains to be determined if NF-κB can directly interact with the promoter binding sequences blocking SR-B1 and ABCA1 gene transcription, an effect never reported for NF-κB, or if this is through an unknown intermediate messenger(s) that is able to down regulate gene transcription. According to the recent data of Panousis and Zuckerman (40), the proinflammatory lymphokine gamma interferon is capable to induce down regulation of ABCA1 gene expression in macrophage-derived foam cells. Another recent report provides experimental evidence that gamma interferon is able to modulate intracellular signaling responses of the LPS-initiated NF-κB pathway. It has been demonstrated that priming macrophages with gamma interferon strongly enhanced IκB-α degradation in response to LPS, resulting in a significant increase in NF-κB DNA binding activity (19). Alternatively, the pathway of the observed LPS suppressive effect on HDL receptor gene expression could possibly involve an apoptotic mechanism, since LPS itself as well as LPS-induced cytokines, including TNF-α, IL-1β, and gamma interferon, are known to be potent apoptotic factors (15, 57). In our experiments we did not observe any morphological changes typical for apoptosis or any apparent evidence of cytotoxicity, although we acknowledge that in order to exclude or confirm this possibility, further investigation directed at evaluating the apoptosis markers in cultured cells exposed to LPS is required.
In conclusion, this study provides new insights into the possible role of LPS. Previous studies have shown LPS to be proatherogenic, able to induce chronic inflammation and subsequent foam cell formation, which is the hallmark of early lesions in atherosclerosis (43). Our data demonstrating that LPS down regulated the gene expression of two key HDL binding proteins involved in cholesterol efflux provide a potential mechanism for LPS contributing to atherogenesis: the serious impairment of pathways that are primarily responsible for HDL production and normal excretion of cholesterol. Our results clearly suggest that the mechanism of the LPS inhibitory effect on both genes is NF-κB dependent but may involve an unknown intermediate factor(s), which remains to be identified. Considering the critical roles of SR-B1 and the ABCA1 transporter in the regulation of HDL metabolism and cholesterol homeostasis, further investigation of the mechanisms underlying the suppressive effect of LPS is important for a better understanding of HDL metabolism and atherogenesis.
Editor: B. B. Finlay
REFERENCES
- 1.Acton, S. L., A. Rigotti, K. T. Landschulz, S. Xu, H. H. Hobbs, and M. Krieger. 1996. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science 271:518-520. [DOI] [PubMed] [Google Scholar]
- 2.Baeuerle, P. A., and V. R. Baichwal. 1997. NF-kappa B as a frequent target for immunosuppressive and anti-inflammatory molecules. Adv. Immunol. 65:111-137. [PubMed] [Google Scholar]
- 3.Bocharov, A. V., T. G. Vishnyakova, I. N. Baranova, A. P. Patterson, and T. L. Eggerman. 2001. Characterization of a 95 kDa high affinity human high density lipoprotein-binding protein. Biochemistry 140:4407-4416. [DOI] [PubMed] [Google Scholar]
- 4.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 5.Buechler, C., M. Ritter, C. D. Quoc, A. Agildere, and G. Schmitz. 1999. Lipopolysaccharide inhibits the expression of the scavenger receptor Cla-1 in human monocytes and macrophages. Biochem. Biophys. Res. Commun. 262:251-254. [DOI] [PubMed] [Google Scholar]
- 6.Byrne, G. I., and M. V. Kalayoglu. 1999. Chlamydia pneumoniae and atherosclerosis: links to the disease process. Am. Heart J. 138:S488-490. [DOI] [PubMed] [Google Scholar]
- 7.Cathcart, M. K., A. K. McNally, D. W. Morel, and G. M. Chisolm. 1989. Superoxide anion participation in human monocyte-mediated oxidation of low-density lipoprotein and conversion of low-density lipoprotein to a cytotoxin. J. Immunol. 142:1963-1969. [PubMed] [Google Scholar]
- 8.Chiu, B., E. Viira, W. Tucker, and I. W. Fong. 1997. Chlamydia pneumoniae, cytomegalovirus, and herpes simplex virus in atherosclerosis of carotid artery. Circulation 96:2144-2148. [DOI] [PubMed] [Google Scholar]
- 9.Cogswell, J. P., M. M. Godlevski, G. B. Wisely, W. C. Clay, L. M. Leesnitzer, J. P. Ways, and J. G. Gray. 1994. NF-kappa B regulates IL-1 transcription through a consensus NF-kappa B binding site and a nonconsensus CRE-like site. J. Immunol. 153:712-723. [PubMed] [Google Scholar]
- 10.Danesh, J., R. Collins, and R. Peto. 1997. Chronic infections and coronary heart disease: is there a link? Lancet 350:430-436. [DOI] [PubMed] [Google Scholar]
- 11.de la Llera-Moya, M., G. H. Rothblat, M. A. Connelly, G. Kellner-Weibel, S. W. Sakr, M. C. Phillips, and D. L. Williams. 1999. Scavenger receptor BI (SR-BI) mediates free cholesterol flux independently of HDL tethering to the cell surface. J. Lipid Res. 40:575-580. [PubMed] [Google Scholar]
- 12.Galanos, C., O. Lüderitz, E. T. Rietschel, O. Westphal, H. Brade, L. Brade, M. Freudenberg, F. U. Schade, M. Imoto, S. Yoshimura, S. Kusumoto, and T. Shiba. 1985. Synthetic and natural Escherichia coli free lipid A express identical endotoxic activities. Eur. J. Biochem. 148:1-5. [DOI] [PubMed] [Google Scholar]
- 13.Gao, J. J., Q. Xue, C. J. Papasian, and D. C. Morrison. 2001. Bacterial DNA and lipopolysaccharide induce synergistic production of TNF-alpha through a post-transcriptional mechanism. J. Immunol. 166:6855-6860. [DOI] [PubMed] [Google Scholar]
- 14.Gaydos, C. A., J. T. Summersgill, N. N. Sahney, J. A. Ramirez, and T. C. Quinn. 1996. Replication of Chlamydia pneumoniae in vitro in human macrophages, endothelial cells, and aortic artery smooth muscle cells. Infect. Immun. 64:1614-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geng, Y. J., Q. Wu, M. Muszynski, G. K. Hansson, and P. Libby. 1996. Apoptosis of vascular smooth muscle cells induced by in vitro stimulation with interferon-gamma, tumor necrosis factor-alpha, and interleukin-1 beta. Arterioscler. Thromb. Vasc. Biol. 16:19-27. [DOI] [PubMed] [Google Scholar]
- 16.Glass, C. K., and J. L. Witztum. 2001. Atherosclerosis: the road ahead. Cell 104:503-516. [DOI] [PubMed] [Google Scholar]
- 17.Guha, M., and N. Mackman. 2001. LPS induction of gene expression in human monocytes. Cell. Signal. 13:85-94. [DOI] [PubMed] [Google Scholar]
- 18.Hansson, G. K. 1997. Cell-mediated immunity in atherosclerosis. Curr. Opin. Lipidol. 8:301-311. [DOI] [PubMed] [Google Scholar]
- 19.Held, T. K., X. Weihua, L. Yuan, D. V. Kalvakolanu, and A. S. Cross. 1999. Gamma interferon augments macrophage activation by lipopolysaccharide by two distinct mechanisms, at the signal transduction level and via an autocrine mechanism involving tumor necrosis factor alpha and interleukin-1. Infect. Immun. 67:206-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iiyama, K., L. Hajra, M. Iiyama, H. Li, M. DiChiara, B. D. Medoff, and M. I. Cybulsky. 1999. Patterns of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 expression in rabbit and mouse atherosclerotic lesions and at sites predisposed to lesion formation. Circ. Res. 85:199-207. [DOI] [PubMed] [Google Scholar]
- 21.Jansson, P.-E. 1999. The chemistry of O-polysaccharide chains in bacterial lipopolysaccharides, p. 155-178. In H. Brade, D. C. Morrison, S. Opal, and S. Vogel, (ed.), Endotoxin in health and disease. Marcel Dekker, Inc., New York, N.Y.
- 22.Kalayoglu, M. V., and G. I. Byrne. 1998. A Chlamydia pneumoniae component that induces macrophage foam cell formation is chlamydial lipopolysaccharide. Infect. Immun. 66:5067-5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Landschulz, K. T., R. K. Pathak, A. Rigotti, M. Krieger, and H. H. Hobbs. 1996. Regulation of scavenger receptor, class B, type I, a high density lipoprotein receptor, in liver and steroidogenic tissues of the rat. J. Clin. Invest. 98:984-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lawn, R. M., D. P. Wade, M. R. Garvin, X. Wang, K. Schwartz, J. G. Porter, J. J. Seilhamer, A. M. Vaughan, and J. F. Oram. 1999. The Tangier disease gene product ABC1 controls the cellular apolipoprotein-mediated lipid removal pathway. J. Clin. Investig. 104:R25-R31. [DOI] [PMC free article] [PubMed]
- 25.Lenardo, M. J., and D. Baltimore. 1989. NF-kB: a pleiotropic mediator of inducible and tissue-specific gene control. Cell 58:227-229. [DOI] [PubMed] [Google Scholar]
- 26.Liao, W. 1999. Endotoxin: possible roles in initiation and development of atherosclerosis. J. Lab. Clin. Med. 128:452-460. [DOI] [PubMed] [Google Scholar]
- 27.Lopes-Virella, M. F. 1993. Interactions between bacterial lipopolysaccharides and serum lipoproteins and their possible role in coronary heart disease. Eur. Heart J. 14:118-124. [PubMed] [Google Scholar]
- 28.Lynn, W. A., and J. Cohen. 1995. Adjunctive therapy for septic shock: a review of experimental approaches. Clin. Infect. Dis. 20:143-158. [DOI] [PubMed] [Google Scholar]
- 29.Manna, S. K., and B. B. Aggarwal. 2000. Wortmannin inhibits activation of nuclear transcription factors NF-kappaB and activated protein-1 induced by lipopolysaccharide and phorbol ester. FEBS Lett. 473:1-126. [DOI] [PubMed] [Google Scholar]
- 30.Mantovani, A., S. Sozzani, A. Vecchi, M. Introna, and P. Allavena. 1997. Cytokineactivation of endothelial cells: new molecules for an old paradigm. Thromb. Haemost. 78:406-414. [PubMed] [Google Scholar]
- 31.Marcil, M., Y. Lu, L. Krimbou, B. Boucher, J. Oram, J. S. Cohn, and J. Genest. 1999. Cellular cholesterol transport and efflux from fibroblasts are abnormal in subjects with familial HDL deficiency. Arterioscler. Thromb. Vasc. Biol. 19:159-169. [DOI] [PubMed] [Google Scholar]
- 32.Matsushita, H., R. Morishita, T. Nata, M. Aoki, H. Nakagami, Y. Taniyama, K. Yamamoto, J. Higaki, K. Yasufumi, and T. Ogihara. 2000. Hypoxia-induced endothelialapoptosis through nuclear factor-kappaB (NF-kappaB)-mediated bcl-2 suppression: in vivo evidence of the importance of NF-kappaB in endothelial cell regulation. Circ. Res. 86:974-981. [DOI] [PubMed] [Google Scholar]
- 33.Mendall, M. A., D. Carrington, D. Strachan, P. Patel, N. Molineaux, J. Levy, T. Tossey, A. J. Camm, and T. C. Northfield. 1995. Chlamydia pneumoniae: risk factors for seropositivity and association with coronary heart disease. J. Infect. 30:121-128. [DOI] [PubMed] [Google Scholar]
- 34.Mendez, A. J. 1997. Cholesterol efflux mediated by apolipoproteins is an active cellular process distinct from efflux mediated by passive diffusion. J. Lipid Res. 38:1807-1821. [PubMed] [Google Scholar]
- 35.Muller, J. M., H. W. L. Ziegler-Heitbrock, and P. A. Baeuerle. 1993. Nuclear factor kappa B, a mediator of lipopolysaccharide effects. Immunobiology 187:233-256. [DOI] [PubMed] [Google Scholar]
- 36.Murao, K., V. Terpstra, S. R. Green, N. Kondratenko, D. Steinberg, and O. Quehenberger. 1997. Characterization of CLA-1, a human homologue of rodent scavenger receptor BI, as a receptor for high density lipoprotein and apoptotic thymocytes. J. Biol. Chem. 27 2:17551-17557. [DOI] [PubMed] [Google Scholar]
- 37.Neufeld, E. B., A. T. Remaley, S. J. Demosky, J. A. Stonik, A. M. Cooney, M. Comly, N. K. Dwyer, M. Zhang, J. Blanchette-Mackie, S. Santamarina-Fojo, and H. B. Brewer. 2001. Cellular localization and trafficking of the human ABCA1 transporter. J. Biol. Chem. 276:27584-27590. [DOI] [PubMed]
- 38.Oram, J. F., and S. Yokoyama. 1996. Apolipoprotein-mediated removal of cellular cholesterol and phospholipids. J. Lipid Res. 37:2473-2491. [PubMed] [Google Scholar]
- 39.Oram, J. F. 2000. Tangier disease and ABCA1. Biochim. Biophys. Acta 1529:321-330. [DOI] [PubMed] [Google Scholar]
- 40.Panousis, C. G., and S. H. Zuckerman. 2000. Interferon-gamma induces downregulation of Tangier disease gene (ATP-binding-cassette transporter 1) in macrophage-derived foam cells. Arterioscler. Thromb. Vasc. Biol. 20:1565-1571. [DOI] [PubMed] [Google Scholar]
- 41.Petri, M., S. Perez-Gutthann, D. Spence, and M. C. Hochberg. 1992. Risk factors for coronary artery disease in patients with systemic lupus erythematosus. Am. J. Med. 93:513-519. [DOI] [PubMed] [Google Scholar]
- 42.Rietschel, E. T., and H. Brade. 1992. Bacterial endotoxins. Sci. Am. 267:54-61. [DOI] [PubMed] [Google Scholar]
- 43.Ross, R. 1993. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 362:801-809. [DOI] [PubMed] [Google Scholar]
- 44.Ross, R. 1999. Atherosclerosis--an inflammatory disease. N. Engl. J. Med. 340:115-126. [DOI] [PubMed] [Google Scholar]
- 45.Rothblat, G. H., M. de la Llera-Moya, V. Atger, G. Kellner-Weibel, D. L. Williams, and M. C. Phillips. 1999. Cell cholesterol efflux: integration of old and new observations provides new insights. J. Lipid Res. 40:781-796. [PubMed] [Google Scholar]
- 46.Saikku, P., M. Leinonen, K. Mattila, M. R. Ekman, M. S. Nieminen, P. H. Makela, J. K. Huttunen, and V. Valtonen. 1988. Serological evidence of an association of a novel Chlamydia, TWAR, with chronic coronary heart disease and acute myocardial infarction. Lancet ii:983-986. [DOI] [PubMed]
- 47.Schmitz, G., and W. E. Kaminski. 2001. ABC transporters and cholesterol metabolism. Front. Biosci. 6:D505-D514. [DOI] [PubMed]
- 48.Schwartz, K., R. M. Lawn, and D. P. Wade. 2000. ABC1 gene expression and ApoA-I-mediated cholesterol efflux are regulated by LXR. Biochem. Biophys. Res. Commun. 274:794-802. [DOI] [PubMed] [Google Scholar]
- 49.Scott, M. G., C. M. Rosenberger, M. R. Gold, B. B. Finlay, and R. E. Hancock. 2000. An alpha-helical cationic antimicrobial peptide selectively modulates macrophage responses to lipopolysaccharide and directly alters macrophage gene expression. J. Immunol. 165:3358-3365. [DOI] [PubMed] [Google Scholar]
- 50.Spinelle-Jaegle, S., P. Devillier, S. Doucet, S. Millet, C. Banissi, A. Diu-Hercend, and E. Ruuth. 2001. Inflammatory cytokine production in interferon-γ-primed mice, challenged with lipopolysaccharide. Inhibition by SK&F 86002 interleukin-1b-converting enzyme inhibitor. Eur. Cytokine Netw. 2:280.. [PubMed] [Google Scholar]
- 51.Suzuki, S., S. Abe-Dohmae, T. Fukutomi, S. Ito, M. Itoh, and S. Yokoyama. 2000. Enhancement of the cAMP-induced apolipoprotein-mediated cellular lipid release by calmodulin inhibitors W7 and W5 from RAW 264 mouse macrophage cell line cells. J. Cardiovasc. Pharmacol. 36:609-616. [DOI] [PubMed] [Google Scholar]
- 52.Sweet, M. J., and D. A. Hume. 1996. Endotoxin signal transduction in macrophages. J. Leukoc. Biol. 60:8-26. [DOI] [PubMed] [Google Scholar]
- 53.Takayama, K., N. Qureshi, C. R. Raetz, E. Ribi, J. Peterson, J. L. Cantrell, F. C. Pearson, J. Wiggins, and A. G. Johnson. 1984. Influence of fine structure of lipid A on Limulus amebocyte lysate clotting and toxic activities. Infect. Immun. 2:350-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wade, D. P., and J. S. Owen. 2001. Regulation of the cholesterol efflux gene, ABCA1. Lancet 357:161-163. [DOI] [PubMed] [Google Scholar]
- 55.Wang, N., D. L. Silver, P. Costet, and A. R. Tall. 2000. Specific binding of ApoA-I, enhanced cholesterol efflux, and altered plasma membrane morphology in cells expressing ABC1. J. Biol. Chem. 275:33053-33058. [DOI] [PubMed] [Google Scholar]
- 56.Williams, K. J., and I. Tabas. 1998. The response-to-retention hypothesis of atherogenesis reinforced. Curr. Opin. Lipidol. 9:471-474. [DOI] [PubMed] [Google Scholar]
- 57.Xaus, J., M. Comalada, A. F. Valledor, J. Lloberas, F. Lopez-Soriano, J. M. Argiles, C. Bogdan, and A. Celada. 2000. LPS induces apoptosis in macrophages mostly through the autocrine production of TNF-alpha. Blood 95:3823-3831. [PubMed] [Google Scholar]
- 58.Yang, R. B., M. R. Mark, A. Gray, A. Huang, M. H. Xie, M. Zhang, A. Goddard, W. I. Wood, A. L. Gurney, and P. J. Godowski. 1998. Toll-like receptor-2 mediates lipopolysaccharide-induced cellular signaling. Nature 395:284.. [DOI] [PubMed] [Google Scholar]
- 59.Yokoyama, S. 1998. Apolipoprotein-mediated cellular cholesterol efflux. Biochim. Biophys. Acta 1392:1-15. [DOI] [PubMed] [Google Scholar]
- 60.Zähringer, U., B. Lindner, and E. T. Rietschel. 1999. Chemical structure of lipid A: recent advances in structural analysis of biologically active molecules, p. 93-115. In H. Brade, D. C. Morrison, S. Opal, and S. Vogel, (ed.), Endotoxin in health and disease. Marcel Dekker, Inc., New York, N.Y.