Abstract
Administration of alpha/beta interferon (IFN-α/β) to mice infected with Mycobacterium tuberculosis has been shown to increase mycobacterial growth. Because IFN-α/β has direct pleiotropic effects on the differentiation and functional activities of macrophages, we evaluated the effect of IFN-α/β on mycobacterial growth in human monocytes/macrophages in vitro. Monocytes cultured at optimal cell density could control the growth of M. bovis BCG, as assessed both by measurement of luciferase activity expressed by a mycobacterial reporter strain and by counting of CFU. In contrast, unrestrained mycobacterial growth was observed when monocytes were treated with alpha interferon (IFN-α) 3 days prior to or concomitant with infection. This striking loss of mycobacteriostatic activity was observed with IFN-α and IFN-β and was induced in both freshly isolated monocytes and culture-derived macrophages. Pretreatment of monocytes with IFN-α modified cellular morphology and reduced viability following culture, but neither was observed for culture-derived macrophages, indicating that the effects of IFN-α on mycobacteriostatic activity and cell differentiation and death could be dissociated. These results are compatible with the possibility that the secretion of IFN-α/β could directly promote mycobacterial growth in patients harboring these organisms.
Following infection with Mycobacterium tuberculosis, most immunocompetent adults are able to develop an immune and inflammatory response that controls mycobacterial proliferation, and although these individuals continue to harbor small numbers of viable mycobacteria, clinical signs of tuberculosis do not develop (36, 37). In a minority of individuals, a variety of factors, including aging, stress, malnutrition, alcoholism, intercurrent infections, and cancer, can subsequently blunt this acquired resistance, resulting in the reactivation of dormant mycobacteria and the development of overt disease.
The changes in the immune and inflammatory response that predispose individuals to the reactivation of mycobacterial infection are not completely defined (35). Considerable evidence indicates that antigen-specific T-cell-mediated immunity is required for the control of infection by pathogenic mycobacteria (14, 35). The development of anergy and reductions in the numbers of CD4 T cells in patients with certain viral diseases (e.g., infection with human immunodeficiency virus [HIV] and measles) undoubtedly contribute to the susceptibility of these individuals to tuberculosis (15, 20, 26, 29, 37). It is also recognized that numerous cytokines produced by host immune and inflammatory cells can modify the effector functions of macrophages, and changes in production of these mediators could have deleterious effects on the control of mycobacterial infections (42). In this regard, Manca et al. (28) recently showed that infection of mice with a highly virulent clinical isolate of M. tuberculosis resulted in higher levels of mRNA for alpha interferon (IFN-α) in the lungs of these animals than those observed after infection with a less virulent strain and that intranasal instillation of IFN-α/β in infected mice increased mycobacterial growth and impaired survival. IFN-α/β can modulate interleukin 12 (IL-12)-dependent IFN-γ production (9, 21, 31) and impair Th1 responses in mice infected with M. tuberculosis (28). Although these actions could explain the deleterious effect of this cytokine on control of mycobacterial growth, the possible direct effects of IFN-α/β on macrophages were not evaluated. In this study we demonstrate that exposure to IFN-α/β strongly enhances mycobacterial replication in human macrophages, suggesting another pathway through which increased production of IFN-α/β could contribute to increased susceptibility to tuberculosis.
MATERIALS AND METHODS
Purification and culture of human monocytes.
Monocytes were isolated by centrifugal elutriation from leukapheresis concentrates obtained from healthy volunteers as previously described (6, 41). Monocytes had a viability of >95% and a purity of at least 92% in all experiments. For some experiments, monocytes that had been frozen at −80°C in 10% dimethyl sulfoxide were used.
To initiate experiments, monocytes were resuspended in complete medium (Iscove's modified Dulbecco's medium [Sigma, St. Louis, Mo.] supplemented with 2 mM l-glutamine [Life Technologies, Rockville, Md.], 200 U of penicillin G per ml, 1 μg of kanamycin [Sigma] per ml, and 20% human AB serum [Institut Jacques Boy, Reims, France]). Unless otherwise indicated, cells were cultured in 96-well flat-bottom plates with opaque sides and transparent bottoms (EGG Wallac, Turku, Finland) at 2 × 105 cells/well in a final volume of 200 μl of medium. In most experiments, cells were maintained at 37°C in 95% air-5% CO2 for 3 days prior to infection. In some experiments, cells were plated at 5 × 104 cells/well and cultured for 10 days prior to infection, in which case medium was replaced after 7 days of culture.
To test the effect of IFNs on mycobacteriostatic activity, cells were cultured in the presence of 0.0005 to 10 ng (25 fM to 0.5 nM) of IFN-α (3.0 × 108 U/mg; Peprotech, Inc., Rocky Hill, N.J.) per ml or 0.005 to 100 ng (0.25 pM to 5 nM) of IFN-β (6.3 × 107 U/mg; Biosource, Camarillo, Calif.) per ml. In some cases, 10 ng of granulocyte-macrophage colony-stimulating factor (GM-CSF) or 10 ng of IL-3 (Boehringer Mannheim, Mannheim, Germany) per ml was added to the cultures. In most experiments, monocytes were pretreated with cytokines for 3 days prior to infection. In some cases, cells were not pretreated with cytokines, and IFN was added on the day of infection. To produce conditioned medium, monocytes were pretreated with 3,000 U of IFN-α per ml for 3 days, washed, and cultured at 2 × 105 cells/well for 7 days. Medium was removed, passed through a 0.45-μm-pore-size filter, and stored at −20°C until use.
Infection of monocytes.
The construction of the mycobacterial reporter strain expressing luciferase activity, the preparation of mycobacterial suspensions used for infection, and the infection of monocytes have been described (6). Most mycobacteria in the suspension were individual organisms, although a small proportion of aggregates containing fewer than five mycobacteria were also present. Essentially 100% of the organisms were viable as assessed by using a live/dead BacLight viability stain (Molecular Probes, Eugene, Oreg.). To infect cells, culture medium was removed and 25 μl of a mycobacterial suspension containing 2 × 103 to 6 × 103 mycobacteria/μl was added, followed by fresh complete medium with or without other additions as indicated above to a final volume of 200 μl (multiplicity of infection = 0.3 to 1).
Evaluation of mycobacterial growth.
The techniques used for the assessment of mycobacterial number based on luciferase activity, expressed as relative light units (RLU), and the enumeration of CFU have been described (6). When mycobacterial number was evaluated in parallel 24 h after infection based on CFU and luciferase activity, a CFU/RLU ratio of 16.3 ± 9.4 (mean ± standard error of the mean [SEM], n = 6) was observed; this ratio was not different for cultures containing IFN-α-pretreated and unpretreated cells. Cell viability was evaluated by trypan blue exclusion. DNA content of cultures was determined by the method of Labarca and Paigen (24).
When unpretreated monocytes were infected and the cells were washed 24 h later, 73% ± 3% of mycobacteria remained associated with cells, whereas all mycobacteria were removed from wells not containing monocytes. Because IFN-α-pretreated monocytes are removed from the wells by washing, analogous experiments could not be performed, but IFN-α pretreatment could have resulted in, at most, a 25% increase in mycobacterial uptake.
Statistical methods.
All results are expressed as means ± standard deviations (SD) unless otherwise indicated. Comparisons between groups were made by analysis of variance (ANOVA) (unpaired data) or repeat measures ANOVA (paired data). Posttest comparisons (performed only if P was <0.05) were made using the Newman-Keuls test. Comparisons between two groups were performed using Student's t test.
RESULTS
Effect of IFN-α/β on replication of M. bovis BCG in human monocytes.
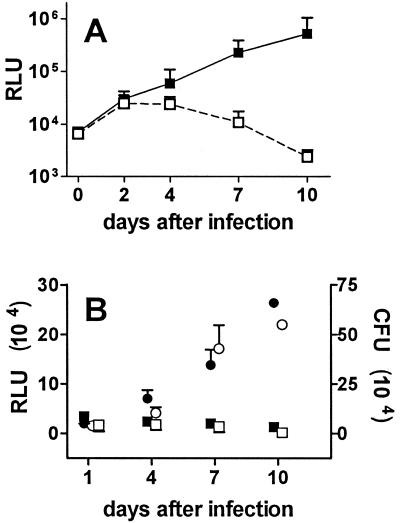
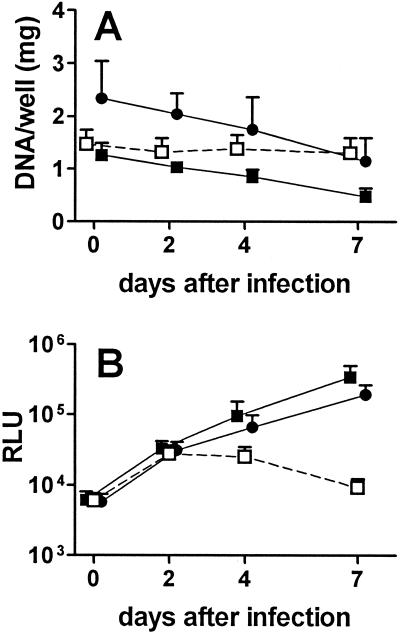
Monocytes were cultured at 2 × 105 cells/well and infected 3 days later with M. bovis BCG, and mycobacterial number was assessed based on the measurement of luciferase activity expressed by the mycobacterial reporter strain. Following infection of unpretreated monocytes, modest mycobacterial growth was observed over the first 4 days, after which luciferase activity decreased to levels below that originally present (Fig. 1A). In contrast, when monocytes were pretreated for 3 days with 3,000 U of IFN-α per ml prior to infection, striking and sustained mycobacterial growth was observed. IFN-α pretreatment increased mycobacterial growth in cells from all individuals, and luciferase activity was at least 10-fold higher at 7 days in IFN-α-pretreated than in unpretreated macrophages from 10 of 12 donors. To confirm the striking effect of IFN-α pretreatment on mycobacteriostatic activity, mycobacterial growth in IFN-α-pretreated and unpretreated macrophages was assessed by the measurement of CFU. As shown in Fig. 1B, the results were comparable to those obtained with the luciferase assay. Thus, mycobacterial number in IFN-α-pretreated macrophages increased 7-fold (CFU) and 11-fold (RLU) between days 1 and 7, whereas mycobacterial number in unpretreated macrophages decreased by 40% (CFU) and 20% (RLU) over the same interval.
FIG. 1.
Effect of pretreatment of human macrophages with IFN-α on intracellular growth of M. bovis BCG. (A) Human monocytes were cultured at 2 × 105 cells/well in the presence of 3,000 U of recombinant human IFN-α per ml (▪) or culture medium only (□) for 3 days and infected with M. bovis BCG. At the indicated times after infection, culture medium was removed and the luciferase activity (RLU) expressed by the mycobacterial reporter strain was measured by luminometry. Results are means ± SD for 12 experiments using monocytes from different individuals. Numbers of RLU for unpretreated and IFN-α-pretreated monocytes were significantly different at 4, 7, and 10 days (P < 0.01). (B) Human monocytes were cultured at 2 × 105 cells/well in the presence of 3,000 U of IFN-α (• and ○) per ml or culture medium only (▪ and □) for 3 days and infected with the M. bovis BCG reporter strain. After the indicated times in culture, the number of mycobacteria present was assessed by measuring luciferase activity (RLU; ○ and □) and CFU (• and ▪). Results are means ± SD for three independent experiments (days 1, 4, and 7) using cells from different individuals. Data for cells cultured for 10 days were obtained in only one of the experiments. RLU for unpretreated and IFN-α-pretreated monocytes were significantly different at 4 and 7 days (P < 0.01 for both comparisons). Numbers of CFU for unpretreated and IFN-α-pretreated monocytes were significantly different at 4 and 7 days (P < 0.05 and P < 0.01, respectively).
When mycobacteria were cultured in the absence of macrophages, mycobacteria showed little or no growth using both techniques (data not shown). When monocytes were pretreated with IFN-α for 3 days and then sonicated prior to infection, mycobacterial growth was not different from that of BCG grown in the absence of monocytes. Thus, progressive mycobacterial growth was strictly dependent on the presence of viable IFN-α pretreated cells, and the release of factors from dead cells did not stimulate the growth of extracellular organisms (data not shown).
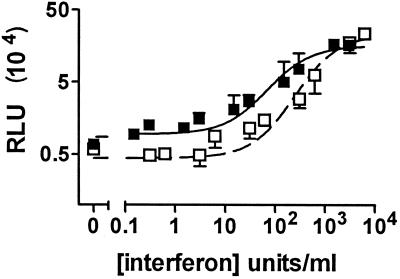
Pretreatment of monocytes with either IFN-α and IFN-β increased mycobacterial growth in a dose-dependent fashion (Fig. 2). The maximal effect of these interferons was similar in magnitude, but the 50% effective concentrations for IFN-α and IFN-β were 70 and 248 U/ml, respectively. Growth of mycobacteria in monocytes pretreated with IFN-α in the presence of either 10 ng of IL-3 or 10 ng of GM-CSF per ml was not significantly different from that in monocytes pretreated with IFN-α alone (P > 0.2, n = 3). When unpretreated macrophages were maintained in the presence of medium conditioned for 7 days by IFN-α-pretreated macrophages, mycobacterial growth 10 days after infection was increased only 1.5-fold compared to that in unpretreated macrophages maintained in fresh medium (data not shown).
FIG. 2.
Effect of pretreating monocytes with various doses of IFN-α and IFN-β on their ability to control mycobacterial growth. Monocytes were cultured at 2 × 105 cells/well in the presence of the indicated concentrations of recombinant human IFN-α (▪) or IFN-β (□) for 3 days and infected with the M. bovis BCG reporter strain. Seven days after infection, culture medium was removed, and the luciferase activity (RLU) was determined. The results are means ± SD for three independent experiments using monocytes from different individuals. The data for each IFN were fitted to a sigmoidal dose response curve to determine the 50% effective concentration (70 and 248 U/ml, respectively, for IFN-α and IFN-β). R2 values were 0.88 and 0.92, respectively.
The effect of IFN-α on cell morphology and survival.
Following culture, unpretreated macrophages were firmly adherent, and the cells progressively increased in size. Seven days after infection, measurement of DNA content demonstrated that 92% ± 4% (mean ± SEM, n = 4) of unpretreated cells remained attached to the surface of the wells, and total cell viability was 94% ± 3%. Pretreatment with IFN-α modified the evolution of the cultures in several important ways. First, the cells remained smaller, and formed clusters containing 20 or more cells. Seven days after infection, measurement of DNA content indicated that only 64% ± 10% of cells remained attached to the surface of the wells, and total cell viability had decreased to 62% ± 9% (P < 0.01 and P < 0.03, respectively, comparing unpretreated and IFN-α-pretreated cells). As previously reported (6), fusion of unpretreated macrophages was frequent after 48 h of infection, resulting in the formation of numerous giant cells, but giant-cell formation was rarely seen in IFN-α-pretreated cells (data not shown).
The effect of IFN-α on mycobacterial growth is independent of its effects on cell viability.
We have recently shown that culture of monocytes at high density improves their ability to control mycobacterial growth (6), raising the possibility that the decrease in cell number occurring in IFN-α-pretreated cultures might account for the strikingly higher mycobacterial proliferation observed. Several observations, however, indicated that cell loss induced by IFN-α did not explain this phenomenon. First, when the number of IFN-α-pretreated cells was increased from 2 × 105 to 4 × 105 cells/well, the number of cells remaining after 7 days of infection was comparable to that for cultures initiated with 2 × 105 unpretreated cells (Fig. 3A). Nevertheless, the exponential growth of mycobacteria observed in cultures containing 2 × 105 monocytes/well pretreated with IFN-α was still observed in cultures seeded at 4 × 105 cells/well (Fig. 3B). Similarly, when the number of mycobacteria used to infect the cells was increased or decreased as much as fourfold, mycobacterial growth in IFN-α-pretreated cells was always at least 10-fold greater than that seen for unpretreated cultures, indicating that a small increase in the multiplicity of infection resulting from cell loss induced by IFN-α could not account for the strong proliferation of mycobacteria seen following pretreatment with IFN-α (data not shown). Finally, the effects of IFN-α on cell viability and mycobacterial growth could be dissociated when more mature macrophages were studied (see below).
FIG. 3.
Cell loss in cultures containing IFN-α-pretreated monocytes does not account for the reduced mycobactericidal activity of these cells. Human monocytes were cultured in the presence of 3,000 U of IFN-α per ml at 4 × 105 (•) or 2 × 105 (▪) cells/well or in culture medium only at 2 × 105 cells/well (□) for 3 days and infected with the M. bovis BCG reporter strain. At the indicated times after infection, culture medium was removed, and the DNA content of adherent cells (A) and luciferase activity (B) was determined. The results are means ± SEM of four independent experiments using cells from different individuals. RLU for unpretreated macrophages were significantly lower than those of IFN-α-pretreated cells at 4 days (P < 0.01 for both comparisons) and 7 days (P < 0.001 for both comparisons) by repeated-measures ANOVA. RLU for IFN-α-pretreated macrophages cultured at 2 × 105 and 4 × 105 cells/well were not significantly different at any time point (P > 0.05).
Conversely, we found that pretreatment of monocytes with 10 ng of IL-10 per ml for 3 days led to a 31% ± 10% decrease in cell survival compared to that of unpretreated cells (mean ± SEM, n = 7, P < 0.02). Despite this significant impairment in viability, it has been shown that IL-10 pretreatment has no significant effect on mycobacterial growth as evaluated by using the techniques employed in this study (7).
The effect of IFN-α on mycobacterial growth in macrophages after different times of maturation in vitro.
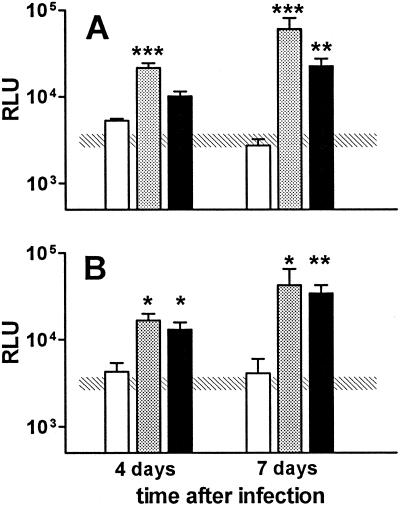
In experiments presented to this point, monocytes were pretreated with IFN-α during the first 3 days of culture, prior to being infected. When macrophages were exposed to IFN-α at later times in culture, this cytokine continued to have a deleterious effect on mycobactericidal activity. When unpretreated monocytes were exposed to 3,000 U of IFN-α per ml on the day of infection, mycobacterial growth was still substantially higher than that observed in cells not treated with IFN-α, although it was less than that observed in macrophages pretreated with IFN-α for 3 days prior to infection (Fig. 4A). We have previously shown that when monocytes are cultured at 5 × 104 cells for 10 days prior to infection, these cells are able to control mycobacterial growth (6). Similar findings were observed in these studies (Fig. 4B). When these cultured macrophages were treated with IFN-α either 3 days prior to infection or at the time of infection, however, their capacity to control mycobacterial growth was severely impaired (Fig. 4B), as observed for freshly cultured monocytes.
FIG. 4.
IFN-α exposure prior to or concomitant with infection impairs the mycobacteriostatic activity of fresh monocytes and cultured macrophages. Human monocytes were cultured at 2 × 105 cells/well for 3 days (A) or at 5 × 104 cells/well for 10 days (B) prior to infection with the M. bovis BCG reporter strain. Cultures were pretreated with 3,000 U of IFN-α per ml for 3 days prior to infection (gray bars), cultured in the presence of 3,000 U of IFN-α per ml starting on the day of infection (black bars), or not exposed to IFN-α (white bars). At the indicated times after infection, culture medium was removed, and the number of mycobacteria present was assessed by measuring luciferase activity (RLU). The results are means ± SEM for four independent experiments, except for cells pretreated with IFN-α in panel B (n = 2). The hatched rectangle represents the mean ± 1 SEM for cells not exposed to IFN-α and evaluated 1 day after infection. ∗, ∗∗, and ∗∗∗, P < 0.05, P < 0.01, and P < 0.001, respectively, compared to unpretreated macrophages.
Although macrophages differentiated in vitro continued to be sensitive to the effect of IFN-α on mycobactericidal activity, the effects of this cytokine on cell morphology and viability were no longer observed. After culture at 5 × 104 cells/well for 10 days, monocytes had increased in size and formed a confluent monolayer of flattened cells with macrophage-like morphology, and following IFN-α treatment either prior to or concomitant with infection, no striking differences in morphology were observed compared to cells not receiving IFN-α (data not shown). Similarly, when cells were cultured for 10 days prior to infection, the DNA content of the cultures 7 days after infection (as a percent of DNA content 1 day after infection) was 93% ± 7% and 94% ± 8%, respectively (n = 2) for cultures pretreated with IFN-α and those receiving IFN-α on the day of infection, and the viability of both populations was >95%.
DISCUSSION
This study demonstrates that when human monocytes are cultured under conditions in which they are otherwise able to control the replication of M. bovis BCG, the exposure of these cells to IFN-α either prior to or concomitant with infection results in exponential intracellular mycobacterial growth. This striking loss of mycobacteriostatic activity was observed with both IFN-α and IFN-β, was induced in freshly isolated monocytes and culture-derived macrophages, and could be separated from the effect of IFN-α on cell viability. These findings suggest that the induction of IFN-α/β in vivo may have a deleterious effect on the control of mycobacterial growth.
We have recently shown that depending on the density at which both monocytes and culture-matured macrophages are maintained, these cells can either exhibit strong mycobacteriostatic activity or be quite permissive for mycobacterial growth (6). Here, the use of high-density cultures, which are otherwise able to inhibit mycobacterial growth, may have been important in demonstrating the strong negative effect of IFN-α on macrophage mycobacteriostatic activity. In this regard, others have reported that treatment of macrophages with IFN-α/β did not increase intracellular mycobacterial growth (5, 47). In these cases, however, the macrophages were cultured under conditions in which they were already permissive for mycobacterial growth. Compatible with our findings, Blanchard et al. reported that monocytes, which otherwise inhibited the growth of Mycobacterium avium-Mycobacterium intracellulare, were sensitive to the inhibitory effect of IFN-α on mycobactericidal activity (5).
IFN-α/β can modulate the secretion of other cytokines by macrophages. IFN-α pretreatment has been found to prime macrophages for increased production of IL-10, a cytokine that has been reported to impair mycobacteriostatic activity (2, 21). We previously found, however, that the addition of several “inhibitory” cytokines (including IL-4, IL-10, IL-13, and transforming growth factor β) did not prevent the control of mycobacterial growth by macrophages cultured at high density, although only a single, relatively high concentration was tested (7). In addition, culture of unpretreated macrophages in the presence of medium conditioned by IFN-α-pretreated macrophages did not result in the extensive mycobacterial growth observed in IFN-α-pretreated macrophages. IFN-α pretreatment can also decrease the production of cytokines that have been reported to improve mycobacteriostatic activity of macrophages (3, 21). Previous work from our group has shown that autocrine stimulation through the release of cytokines does not entirely explain the ability of macrophages cultured at high density (but not low density) to inhibit mycobacterial replication (6). We also found that culture in the presence of IL-3 and GM-CSF (the two cytokines found to improve mycobacteriostatic activity in our system [7]) did not reestablish mycobacteriostatic activity of IFN-α-treated macrophages. Nevertheless, these negative findings do not exclude a possible role for changes in cytokine production as a mechanism explaining the effects of IFN-α/β on mycobacteriostatic activity.
IFN-α/β is also known to have myriad effects on the differentiation and functional capacities of macrophages. IFN-α has been shown to modulate macrophage maturation (4, 25). Consistent with these reports, we found that IFN-α had striking effects on the morphological features of monocytes when added at the initiation of culture. When added to culture-matured macrophages, however, IFN-α still inhibited mycobacteriostatic activity in the absence of important morphological changes, indicating that the effects of this cytokine on mycobacterial growth were not entirely due to early engagement into a distinct differentiation pathway. IFN-α has also be found to increase the sensitivity of monocytes to apoptosis induced by several pathways (1, 34, 44). Although we confirmed the sensitivity of monocytes to IFN-α-induced cell death, similar sensitivity was not observed for monocyte-derived macrophages, suggesting that the sensitivity of macrophages to apoptosis and impaired control of mycobacterial growth were not necessarily linked. In this regard, pretreatment with IL-10 was also found to impair the viability of monocytes, as previously described (12), but striking mycobacterial growth in IL-10-pretreated monocytes was not observed (7). Several studies have indicated that IFN-α can inhibit the generation of O2− and nitrogen free radicals by macrophages (8, 27). We previously found that the ability of monocytes to control mycobacterial growth was not impaired by the addition of an inhibitor of inducible nitric oxide synthase (6), indicating that a reduction in nitrogen free radical formation is unlikely to explain the effects of IFN-α/β on mycobacteriostatic activity. The cellular activities required for the control of mycobacterial growth by macrophages remain to be clearly identified. Further studies comparing unpretreated and IFN-α-pretreated macrophages may be useful in defining these pathways. In addition, it will be important to demonstrate that the observations reported here for M. bovis BCG can be extended to pathogenic mycobacterial species, including M. tuberculosis.
Viral infections are a potent stimulus for the release of IFN-α (13, 39). Our results are consistent with the possibility that increased IFN-α production induced by certain viral infections could contribute to the increased susceptibility of the patients to tuberculosis. Several authors have reported the increased growth of mycobacteria in HIV type 1 (HIV-1)-infected macrophages (23, 38, 45), although the potential role of viral-induced IFN-α in this effect has not been directly studied. In turn, transcription from the long terminal repeat of HIV-1 is strongly enhanced in macrophages recovered from HIV-1-infected individuals with active mycobacterial infections, resulting in increased viral production (10, 11, 22, 30, 32, 46). Thus, in patients infected with both pathogens, a vicious circle could be established that promotes the replication of both HIV-1 and mycobacteria.
Activation of mycobacterial infection is not a recognized adverse effect associated with the therapeutic use of recombinant IFN-α/β. Furthermore, the administration of aerosolized IFN-α to patients receiving antimicrobial treatment for pulmonary tuberculosis was reported to lead to a more rapid decrease in the number of bacilli identified in sputum and earlier resolution of fever and some radiographic abnormalities (16, 17). Immunomodulatory activities of IFN-α may be responsible for this salutary effect. The net effect of IFN-α/β on immune and inflammatory responses in humans and in murine models has, however, been quite variable in different situations (9, 18, 21), and the administration of IFN-α/β to mice infected with M. tuberculosis appears to impair the development of Th1 responses (28), thought to be necessary for protection against mycobacterial infection. There are also some indications that IFN-α/β may exert negative effects on mycobacteriostatic activity. Several case reports describing the appearance of mycobacterial infections in patients receiving IFN-α have been published (19, 33, 40, 43), and the administration of IFN-α/β to mice infected with M. tuberculosis has been shown to enhance mycobacterial growth (28). Rapidly proliferating mycobacteria are more sensitive to antimicrobials. Thus, enhanced mycobacterial replication induced by IFN-α could lead to the earlier response seen in patients with tuberculosis treated with IFN-α. The extent to which increases in IFN-α production influence mycobacterial replication in vivo will require further study. Our in vitro findings suggest that some caution should be exercised in using IFN-α therapy in patients with active mycobacterial disease not receiving simultaneous treatment with antibiotics.
Acknowledgments
This work was supported in part by a grant from Sidaction/Fondation pour la Recherche Médicale. N.B. was supported by a fellowship from the Brazilian Council of Research (CNPq).
We thank Martine Grandsaigne, Martin Vokurka, and Alain Grodet for their help in the initial stages of this project. The help of Xavier Sitthy (Hôpital St. Louis, Paris, France) in obtaining normal human leukocytes is gratefully acknowledged.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Adler, B., H. Adler, T. W. Jungi, and E. Peterhans. 1995. Interferon-α primes macrophages for lipopolysaccharide-induced apoptosis. Biochem. Biophys. Res. Commun. 215:921-927. [DOI] [PubMed] [Google Scholar]
- 2.Aman, M. J., T. Tretter, I. Eisenbeis, G. Bug, T. Decker, W. E. Aulitzky, H. Tilg, C. Huber, and C. Peschel. 1996. Interferon-α stimulates production of interleukin-10 in activated CD4+ T-cells and monocytes. Blood 87:4731-4736. [PubMed] [Google Scholar]
- 3.Aman, M. J., U. Keller, G. Derigs, M. Mohamadzadeh, C. Huber, and C. Peschel. 1994. Regulation of cytokine expression by IFN-α in human bone marrow stromal cells: inhibition of hematopoietic growth factors and induction of interleukin-1 receptor antagonist. Blood 84:4142-4150. [PubMed] [Google Scholar]
- 4.Becker, S. 1984. Influence of interferon on human monocyte to macrophage development. Cell. Immunol. 84:145-153. [DOI] [PubMed] [Google Scholar]
- 5.Blanchard, D. K., M. B. Michelini-Norris, and J. Y. Djeu. 1991. Interferon decreases the growth inhibition of Mycobacterium avium-intracellulare complex by fresh human monocytes but not be culture-derived macrophages. J. Infect. Dis. 164:152-157. [DOI] [PubMed] [Google Scholar]
- 6.Boechat, N., F. Bouchonnet, M. Bonay, A. Grodet, V. Pelicic, B. Gicquel, and A. J. Hance. 2001. Culture at high density improves the ability of human macrophages to control mycobacterial growth. J. Immunol. 166:6203-6211. [DOI] [PubMed] [Google Scholar]
- 7.Bonay, M., F. Bouchonnet, V. Pelicic, B. Lagier, M Grandsaigne, D. Lecossier, A. Grodet, M. Vokurka, B. Gicquel, and A. J. Hance. 1999. Effect of stimulation of human macrophages on intracellular survival of Mycobacterium bovis bacillus Calmette-Guerin. Evaluation with a mycobacterial reporter strain. Am. J. Respir. Crit. Care Med. 159:1629-1637. [DOI] [PubMed] [Google Scholar]
- 8.Conde, M., J. Andrade, F. J. Bedoya, and F. Sobrino. 1994. Inhibitory effect of interferon-α on respiratory burst and glucose metabolism in phagocytic cells. J. Interferon Res. 14:11-16. [DOI] [PubMed] [Google Scholar]
- 9.Cousens, L. P., R. Peterson, S. Hsu, A. Dorner, J. D. Altman, R. Ahmed, and C. A. Biron. 1999. Two roads diverged: interferon α/β- and interleukin 12-mediated pathways in promoting T cell interferon γ responses during viral infection. J. Exp. Med. 189:1315-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denis, C., and E. Ghadirian. 1994. Interaction between Mycobacterium avium and human immunodeficiency virus type 1 (HIV-1) in bronchoalveolar macrophages of normal and HIV-1-infected subjects. Am. J. Respir. Cell Mol. Biol. 11:487-495. [DOI] [PubMed] [Google Scholar]
- 11.Doherty, T. M., C. Chougnet, M. Schito, B. K. Patterson, C. Fox, G. M. Shearer, G. Englund, and A. Sher. 1999. Infection of HIV-1 transgenic mice with Mycobacterium avium induces the expression of infectious virus selectively from a Mac-1-positive host cell population. J. Immunol. 163:1506-1515. [PubMed] [Google Scholar]
- 12.Estaquier, J., and J. C. Ameisen. 1997. A role for T-helper type-1 and type-2 cytokines in the regulation of human monocyte apoptosis. Blood 90:1618-1625. [PubMed] [Google Scholar]
- 13.Ferbas, J. J., J. F. Toso, A. J. Logar, J. S. Navratil, and C. R. Rinaldo, Jr. 1994. CD4+ blood dendritic cells are potent producers of IFN-α in response to in vitro HIV-1 infection. J. Immunol. 152:46-49. [PubMed] [Google Scholar]
- 14.Flynn, J. L., and J. Chan. 2001. Immunology of tuberculosis. Annu. Rev. Immunol. 19:93-129. [DOI] [PubMed] [Google Scholar]
- 15.Fugier-Vivier, I., C. Servet-Delprat, P. Rivailler, M. C. Rissoan, Y. J. Liu, and C. Rabourdin-Combe. 1997. Measles virus suppresses cell-mediated immunity by interfering with the survival and functions of dendritic cells. J. Exp. Med. 186:813-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giosuè, S., M. Casarini, L. Alemanno, G. Galluccio, P. Mattia, G. Pedicelli, L. Rebek, A. Bisetti, and F. Ameglio. 1998. Effects of aerosolized interferon-α in patients with pulmonary tuberculosis. Am. J. Respir. Crit. Care Med. 158:1156-1162. [DOI] [PubMed] [Google Scholar]
- 17.Giosuè, S., M. Casarini, F. Ameglio, P. Zangrilli, M. Palla, A. M. Altieri, and A. Bisetti. 2000. Aerosolized interferon-alpha treatment in patients with multi-drug-resistant pulmonary tuberculosis. Eur. Cytokine Netw. 11:99-103. [PubMed] [Google Scholar]
- 18.Gutterman, J. U. 1994. Cytokine therapeutics: lessons from interferon α. Proc. Natl. Acad. Sci. USA 91:1198-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Habermann, T. M., J. W. Andersen, P. A. Cassileth, J. M. Bennett, and M. M. Oken. 1992. Sequential administration of recombinant interferon alpha and deoxycoformycin in the treatment of hairy cell leukaemia. Br. J. Haematol. 80:466-471. [DOI] [PubMed] [Google Scholar]
- 20.Havlir, D. V., and P. F. Barnes. 1999. Tuberculosis in patients with human immunodeficiency virus infection. N. Engl. J. Med. 340:367-373. [DOI] [PubMed] [Google Scholar]
- 21.Hermann, P., M. Rubio, T. Nakajima, G. Delespesse, and M. Sarfati. 1998. IFN-α priming of human monocytes differentially regulates gram-positive and gram-negative bacteria-induced IL-10 release and selectively enhances IL-12 p70, CD80, and MHC class I expression. J. Immunol. 161:2011-2018. [PubMed] [Google Scholar]
- 22.Honda, Y., L. Rogers, K. Nakata, B. Y. Zhao, R. Pine, Y. Nakai, K. Kurosu, W. N. Rom, and M. Weiden. 1998. Type 1 interferon induces inhibitory 16-kD CCAAT/enhancer binding protein (C/EBP)β, repressing the HIV-1 long terminal repeat in macrophages: pulmonary tuberculosis alters C/EBP expression, enhancing HIV-1 replication. J. Exp. Med. 188:1255-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Imperiali, F. G., A. Zaninoni, L. La Maestra, P. Tarsia, F. Blasi, and W. Barcellini. 2001. Increased Mycobacterium tuberculosis growth in HIV-1-infected human macrophages: role of tumour necrosis factor-alpha. Clin. Exp. Immunol. 123:435-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Labarca, C., and K. Paigen. 1980. A simple, rapid, and sensitive DNA assay procedure. Anal. Biochem. 102:344-352. [DOI] [PubMed] [Google Scholar]
- 25.Lake, F. R., P. W. Noble, P. M. Henson, and D. W. Riches. 1994. Functional switching of macrophage responses to tumor necrosis factor-α (TNFα) by interferons. Implications for the pleiotropic activities of TNFα. J. Clin. Investig. 93:1661-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lisse, I., B. Samb, H. Whittle, H. Jensen, M. Soumare, F. Simondon, and P. Aaby. 1998. Acute and long-term changes in T-lymphocyte subsets in response to clinical and subclinical measles. A community study from rural Senegal. Scand. J. Infect. Dis. 30:17-21. [DOI] [PubMed] [Google Scholar]
- 27.López-Collazo, E., S. Hortelano, A. Rojas, and L. Boscá. 1998. Triggering of peritoneal macrophages with IFN-α/β attenuates the expression of inducible nitric oxide synthase through a decrease in NF-κB activation. J. Immunol. 160:2889-2895. [PubMed] [Google Scholar]
- 28.Manca, C., L. Tsenova, A. Bergtold, S. Freeman, M. Tovey, J. M. Musser, C. E. Barry III, V. H. Freedman, and G. Kaplan. 2001. Virulence of a Mycobacterium tuberculosis clinical isolate in mice is determined by failure to induce Th1 type immunity and is associated with induction of IFN-α/β. Proc. Natl. Acad. Sci. USA 98:5752-5757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mukadi, Y., J. H. Perriens, M. E. St Louis, et al. 1993. Spectrum of immunodeficiency in HIV-1-infected patients with pulmonary tuberculosis in Zaire. Lancet 342:143-146. [DOI] [PubMed] [Google Scholar]
- 30.Newman, G. W., T. G. Kelley, H. Gan, P. Kandil, M. J. Newman, P. Pinkston, R. M. Rose, and H. G. Remold. 1993. Concurrent infection of human macrophages with HIV-1 and Mycobacterium avium results in decreased cell viability, increased M. avium multiplication and altered cytokine production. J. Immunol. 151:2261-2272. [PubMed] [Google Scholar]
- 31.Nguyen, K. B., L. P. Cousens, L. A. Doughty, G. C. Pien, J. E. Durbin, and C. A. Biron. 2000. Interferon α/β-mediated inhibition and promotion of interferon gamma: STAT1 resolves a paradox. Nat. Immunol. 1:70-76. [DOI] [PubMed] [Google Scholar]
- 32.Orenstein, J. M., C. Fox, and S. M. Wahl. 1997. Macrophages as a source of HIV during opportunistic infections. Science 276:1857-1861. [DOI] [PubMed] [Google Scholar]
- 33.Philippot, V., F. Yassir, B. Balme, and H. Perrot. 1996. Abcès sous-cutané à Mycobacterium avium-intracellulare après injections d'interféron alpha chez une malade traitée pour lymphome. Ann. Dermatol. Venereol. 123:103-105. [PubMed] [Google Scholar]
- 34.Quignon, F., F. De Bels, M. Koken, J. Feunteun, J. C. Ameisen, and H. de Thé. 1998. PML induces a novel caspase-independent death process. Nat. Genet. 20:259-265. [DOI] [PubMed] [Google Scholar]
- 35.Raupach, B., and S. H. E. Kaufmann. 2001. Immune responses to intracellular bacteria. Curr. Opin. Immunol. 13:417-428. [DOI] [PubMed] [Google Scholar]
- 36.Rook, G. A. W., and R. Hernandez-Pando. 1996. The pathogenesis of tuberculosis. Annu. Rev. Microbiol. 50:259-284. [DOI] [PubMed] [Google Scholar]
- 37.Schluger, N. W., and W. N. Rom. 1998. The host immune response to tuberculosis. Am. J. Respir. Crit. Care Med. 157:679-691. [DOI] [PubMed] [Google Scholar]
- 38.Shiratsuchi, H., J. L. Johnson, Z. Toossi, and J. J. Ellner. 1994. Modulation of the effector function of human monocytes for Mycobacterium avium by human immunodeficiency virus-1 envelope glycoprotein gp120. J. Clin. Investig. 93:885-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siegal, F. P., N. Kadowaki, M. Shodell, P. A. Fitzgerald-Bocarsly, K Shah, S. Ho, S. Antonenko, and Y. J. Liu. 1999. The nature of the principal type 1 interferon-producing cells in human blood. Science 284:1835-1837. [DOI] [PubMed] [Google Scholar]
- 40.Toren, A., A. Ackerstein, D. Gazit, R. Or, D. Raveh, U. Kupolovicz, D. Engelhard, and A. Naglar. 1996. Oral tuberculosis following autologous bone marrow transplantation for Hodgkin's disease with interleukin-2 and alpha-interferon immunotherapy. Bone Marrow Transplant. 18:209-210. [PubMed] [Google Scholar]
- 41.Wahl, L. M., I. L. Katona, R. L. Wilder, C. C. Winter, B. Haroui, I. Seher, and S. M. Wahl. 1984. Isolation of human mononuclear cell subsets by counterflow centrifugal elutriation (CEE). I. Characterization of B-lymphocyte, T-lymphocyte and monocyte-enriched fractions by flow cytometric analysis. Cell. Immunol. 85:373-383. [DOI] [PubMed] [Google Scholar]
- 42.Wallis, R. S., and J. J. Ellner. 1994. Cytokines and tuberculosis. J. Leukoc. Biol. 55:676-681. [DOI] [PubMed] [Google Scholar]
- 43.Yermiahu, T., and D. Benharroch. 1994. Coomb's-positive hemolytic anemia and tuberculosis in Kaposi's sarcoma treated with interferon. Harefuah 127:445-448. (In Hebrew.) [PubMed]
- 44.Zella, D., O. Barabitskaja, L. Casareto, F. Romerio, P. Secchiero, M. S. Reitz Jr., R. C. Gallo, and F. F. Weichold. 1999. Recombinant IFN-α (2b) increases the expression of apoptosis receptor CD95 and chemokine receptors CCR1 and CCR3 in monocytoid cells. J. Immunol. 163:3169-3175. [PubMed] [Google Scholar]
- 45.Zerlauth, G., E. Maier, H. Chehadeh, K. Zimmermann, M. M. Eibl, and J. W. Mannhalter. 1995. Interaction with human immunodeficiency virus type 1 modulates innate effector functions of human monocytes. J. Infect. Dis. 172:1598-1601. [DOI] [PubMed] [Google Scholar]
- 46.Zhang, Y., K. Nakata, M. Weiden, and W. N. Rom. 1995. Mycobacterium tuberculosis enhances human immunodeficiency virus-1 replication by transcriptional activation at the long terminal repeat. J. Clin. Investig. 95:2324-2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zurbrick, B. G., D. M. Follett, and C. J. Czuprynski. 1988. Cytokine regulation of the intracellular growth of Mycobacterium paratuberculosis in bovine monocytes. Infect. Immun. 56:1692-1697. [DOI] [PMC free article] [PubMed] [Google Scholar]