Abstract
Previous work (P. Castric, F. J. Cassels, and R. W. Carlson, J. Biol. Chem. 276:26479-26485, 2001) has shown the Pseudomonas aeruginosa 1244 pilin glycan to be covalently bound to a serine residue. N-terminal sequencing of pilin fragments produced from endopeptidase treatment and identified by reaction with a glycan-specific monoclonal antibody indicated that the glycan was present between residue 75 and the pilin carboxy terminus. Further sequencing of these peptides revealed that serine residues 75, 81, 84, 105, 106, and 108 were not modified. Conversion of serine 148, but not serine 118, to alanine by site-directed mutagenesis, resulted in loss of the ability to carry out pilin glycosylation when tested in an in vivo system. These results showed the pilin glycan to be attached to residue 148, the carboxy-terminal amino acid. The carboxy-proximal portion of the pilin disulfide loop, which is adjacent to the pilin glycan, was found to be a major linear B-cell epitope, as determined by peptide epitope mapping analysis. Immunization of mice with pure pili produced antibodies that recognized the pilin glycan. These sera also reacted with P. aeruginosa 1244 lipopolysaccharide as measured by Western blotting and enzyme-linked immunosorbent assay.
The pili of the opportunistic pathogen Pseudomonas aeruginosa are thin protein fibers extending from one or both of the cell poles (42). These processes are important for virulence in that they mediate adhesion to host tissue (16, 35, 45). The pili are also capable of extension and retraction (1, 38), a trait that influences colonization and facilitates twitching, a form of motility important in pathogen dissemination (9, 22).
The pili of P. aeruginosa are assembled from a monomeric subunit, pilin, that has a molecular weight in the range of 15 to 17,000 and has the characteristics associated with the type IV pili (31). One of these characteristics is the presence of a methylated amino-terminal phenylalanine. It has been shown that, in addition to this posttranslational modification, the pilin of P. aeruginosa 1244 is also glycosylated and that this modification requires the presence of a functional pilO gene (4). Recent work (5) has revealed this glycan to be a trisaccharide containing pseudaminic acid, xylose, and N-acetylfucosamine [α-5NβOHC47NFmPse-(2→4)-β-Xyl-(1→3)-β-FucNAc]. This glycan is identical to the O-antigen repeating unit of P. aeruginosa serotype O7 (International Antigen Typing System; 29) lipopolysaccharide (LPS), the structure of which has been determined by Knirel et al. (24, 25). O7 is the LPS serotype to which strain 1244 belongs, suggesting that the glycan and the O-antigen repeating unit have the same origin.
It has also been shown that this trisaccharide is present in a ratio of one glycan per pilin subunit and that this structure is attached to P. aeruginosa 1244 pilin via an O linkage between FucNAc C-1 and the β carbon of a serine residue (5). The glycans of both the Neisseria meningitidis and N. gonorrhoeae pilins have been shown to be O linked to serine 63 (33, 41), a position N proximal to the pilin β-sheet region (33). Work described in the present paper revealed that the glycan of P. aeruginosa 1244 pilin is attached to serine 148, the carboxy-terminal residue of this protein, and is therefore C proximal to the predicted β-sheet region of this protein. The location of the P. aeruginosa pilin glycan is also significant because of its close proximity to the pilin disulfide loop, a structure important in pilus adhesion (20, 27). The work presented in this paper shows that the 1244 pilin glycan, as well as the adjacent portion of this loop region, is an important B-cell epitope. Of particular significance to vaccine design is the finding that the pilin glycan of this organism stimulates antibodies that recognize P. aeruginosa 1244 LPS.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
P. aeruginosa strain 1244 was obtained from J. C. Sadoff, Walter Reed Army Institute of Research, and was originally isolated from human blood by A. T. McManus, U.S. Army Institute of Surgical Research, San Antonio, Tex. Strain 1244N3 was provided by S. Lory, University of Washington, Seattle. All strains were grown aerobically on Luria-Bertani (LB) plates or broth cultures at 37°C. Broth cultures were grown on a rotatory shaker at 275 rpm. Additives to growth media were used at the following concentrations: carbenicillin (CB), 200 μg/ml; tetracycline, 50 μg/ml; isopropyl-β-d-thiogalactopyranoside (IPTG), 5.0 mM.
Isolation and purification of pili.
Strain 1244N3, a mutant that is unable to make pilin (34), could produce glycosylated strain 1244 pilin when carrying pPAC46, a plasmid containing the strain 1244 pilA and pilO genes under the control of a tac promoter (4). Glycosylated pili produced by hyperexpression of the pilA gene of pPAC46 were isolated and purified as described previously (4). This protocol yielded 10 to 20 mg of pili per liter of culture that contained trace amounts of LPS. In order to reduce this contamination, these pili were subjected to gel filtration and chromatofocusing in the presence of the detergent β-octyl glucoside (BOG). A 1.2-mg sample of purified pili was suspended in 700 μl of a solution containing 25 mM Bis-Tris/HCl (pH 7.0), 1% BOG, and 0.02% sodium azide and incubated at 24°C for 30 min. This mixture was microcentrifuged briefly, and the supernatant fluid was applied to a Superose 12 column (1.0 by 30 cm) that had been equilibrated with the same buffer solution. Fractions (250 μl) were taken at a flow rate of 0.5 ml/min. The single major peak observed, as determined by A280, was collected. This material was applied to a Mono P anion-exchange column (0.5 by 10 cm) that had been equilibrated with 25 mM Bis-Tris (pH 6.25) containing 0.02% sodium azide. Pilin monomer was eluted by the addition of a solution containing 10% Polybuffer 74 (pH 4.0), 1% BOG, and 0.02% sodium azide. The column was operated at a flow rate of 1.0 ml/min, and 0.5-ml fractions were taken. The single peak, which was identified by A280, was pooled and dialyzed twice against 1 liter of 5 mM Bis-Tris (pH 7.0), which allowed reaggregation of the pilin subunits. To test for the presence of LPS, approximately 200 μg of reaggregated pilin was first proteolytically digested by the procedure of Hitchcock and Brown (18) and then analyzed by Western blotting with LPS-specific monoclonal antibody 11-14 as the probe. Known amounts of P. aeruginosa 1244 LPS, either analyzed separately or added to aliquots of digested pilin, were used as the standard. LPS contamination, if present, was below the level of the lowest standard used (present at a concentration of 0.5 ng of LPS/μg of pilin).
Endopeptidase digestion of pilin.
As a pretreatment step, 350 μg of pure glycosylated P. aeruginosa 1244 pili was suspended in 125 μl of 0.1% sodium dodecyl sulfate (SDS) and heated at 95°C for 10 min. The digestion mixture was made up of 350 μg of pretreated pilin in a final volume of 800 μl that contained 0.1% SDS, 12.5 μg of GluC (V8 protease; Boehringer Mannheim), and 25 mM Tris/HCl (pH 8.0). This material was incubated for 3 h at 24°C. The reaction was stopped by treatment at 95°C for 10 min, after which it was aliquoted and stored at −20°C. In an alternate digestion, the incubation period was extended to 8 h. The method of Judd (21) was used for polyacrylamide gel electrophoresis (PAGE) and Coomassie brilliant blue (CBB) staining of pilin peptides, while the method of Nesterenko et al. (30) was used for silver staining (7). Peptide sequencing was performed by J. Hempel at the University of Pittsburgh Peptide Sequencing Facility.
Trypsin digestion of pilin also employed a pretreatment step that began with the incubation of 5.2 mg of pure pili overnight in 0.5 ml of 1.0% BOG at 4°C. This material was microcentrifuged, and the supernatant fluid was transferred to a fresh tube, where guanidine HCl was added to a concentration of 8 M in a final volume of 1.5 ml. This material was incubated overnight at 24°C and then dialyzed twice against 1.0 liter of 10.0 mM Tris/HCl (pH 8.0). The digestion mixture contained the pretreated pilin, 25 μg of trypsin (Boehringer Mannheim), 0.6 μg of sodium azide, and 20 mM Tris/HCl (pH 8.0) in a final volume of 2.1 ml. Incubation was at 37°C for 15 h, after which the digestion product was aliquoted and stored at −20°C. A 0.5-ml portion of this material was subjected to gel filtration with a fast protein liquid chromatography system in which a Pharmacia Superdex Peptide column (1.0 by 30 cm) was employed. The elution buffer was 10 mM Tris/HCl (pH 8.0), and 0.5-ml fractions were taken at a flow rate of 0.5 ml/min. Absorbance was monitored at 214 and 280 nm. The column eluate was assayed for sugar with an orcinol spot test (37), in which material in the molecular weight range of 400 to 800 produced a positive reaction. These fractions were pooled, dried, and resuspended in deionized water, after which this material was tested by high-performance thin-layer chromatography with the following solvents: isopropanol-ammonium hydroxide (2:1) (8), chloroform-methanol-water (10:10:3) (3), and butanol-acetic acid-water (3:1:1) (8). Duplicate samples were run, one of which was developed with orcinol spray while the other was treated with ninhydrin (0.3% ninhydrin in 95% ethanol, followed by treatment at 90°C for 30 min). Authentic P. aeruginosa 1244 pilin aminoglycan (5) was used as the standard.
Site-directed mutagenesis.
Site-directed mutagenesis was done with the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, Calif.), which is based on the procedure of Kunkel (26). The template employed for the generation of both mutants was pUC46, a plasmid containing the pilA and pilO genes from pPAC46 (4) ligated between the SphI and HindIII sites of pUC19. The oligonucleotide primers for the mutant in which pilin serine 118 was converted to alanine were 5′-GGCTGTAATCACTGTTGCGCGTAAAAATGACGG-3′ and its complement. The primers used to generate the mutant in which pilin serine 148 was changed to alanine were 5′-GCTAATTGCCCGAAAGCCTAATCGGTTTTTTGAGTTG-3′ and its complement. Confirmation of the single base change caused by each treatment was done by nucleotide sequencing at the University of Pittsburgh Biotechnology Center. The EcoRI/HindIII fragments of the mutant plasmids were ligated with broad-host-range vector pMMB66EH (14) which had been digested with the same enzymes. The resulting plasmids, pS118A and pS148A, were moved into P. aeruginosa 1244N3 to test for pilA gene expression and determine the glycosylation state with Western and isoelectric focusing blots.
Peptide synthesis.
Continuous overlapping peptides were synthesized by a standard protocol (10, 15) with blocks of derivatized pins purchased from Cambridge Research Biochemicals, Inc. (Wilmington, Del.), and equipment and software previously described (2). 9-Fluorenylmethoxy carbonyl amino acid pentafluorophenyl esters were purchased from Peninsula Laboratories (Belmont, Calif.) and used without further treatment or analysis. The activating agent 1-hydroxybenzotriazole monohydrate was purchased from Aldrich Chemical Co. (Milwaukee, Wis.). Solvents were reagent grade and were from Fisher Scientific (Springfield, N.J.). Peptides were synthesized as 12-mers and remained linked to the resin. Peptide composition was confirmed by amino acid analysis of one control pin per plate. Pins were hydrolyzed in 6 N HCl at 110°C for 24 h, and the analysis was performed on a Beckman 9600 amino acid analyzer.
A nontethered peptide, TPTAWKPNYAPANC, was synthesized with an Applied Biosystems 430A peptide synthesizer. In order to conjugate this peptide to ovalbumin, 5.0 mg of this protein dissolved in 0.5 ml of phosphate-buffered saline (PBS) was reacted with 5.0 mg of bromoacetylhydroxysuccinimide ester dissolved in 100 μl of acetonitrile and the solution was adjusted to pH 7.5. This was incubated with stirring for 1 h at room temperature, after which the protein was removed by gel filtration with Sephadex G-25. A 5.0-mg sample of the peptide was dissolved in 2.0 ml of PBS. To this was added 100 μl of tributylphosphine (5% in isopropanol), after which the pH was adjusted to 7.0. The mixture was incubated for 1 h at room temperature with stirring. The bromoacetylated protein was combined with the reduced peptide and allowed to incubate for 1 h at room temperature, after which the solution was thoroughly dialyzed against MilliQ water.
Production of sera.
To obtain the anti-pilus polyclonal antisera used in the tethered-peptide pin test, outbred Swiss mice (16 to 18 g) were injected subcutaneously with 100 μl of a pilus preparation diluted 1:1 with Freund's incomplete adjuvant. Three groups of mice (four animals per group) were immunized with purified P. aeruginosa 1244 pili. The first two groups (referred to as series 2 and 4) received 10-μg doses, while the third group (series 7) received 7.6-μg doses. Boosts with this same protocol were carried out at weeks 1, 2, and 3. Blood was harvested at week 4, and the serum portion was separated and stored at −20°C. Each of the sera produced gave enzyme-linked immunosorbent assay (ELISA) dilution endpoints (negative log10 of the serum dilution that produces a ΔA405 of 0.200 in 30 min at 24°C) in the range of 10−5 to 10−6 when tested with pure undenatured strain 1244 pili as the antigen. An antibody capture ELISA was performed as described previously (5). These sera also unambiguously recognized (Western blotting) pure denatured strain 1244 pili at a dilution of 10−5. Anti-pilin peptide sera were obtained by immunizing one series of four mice with a conjugated peptide with this same protocol, except that individual doses contained 27 μg of antigen.
The procedure for obtaining anti-pilus sera used for determination of glycan immunogenicity was identical to the above-described protocol employing 10-μg pilin doses. Immunization with pure native pili (series 24) used four mice, while immunization with reaggregated pili (series 25) employed five animals. Immunizations with LPS involved three series of two mice each. Here, the same protocol was used, with pure P. aeruginosa 1244 LPS being administered at 0.025 (series 20), 0.05 (series 21), and 0.10 (series 22) μg per dose. For each set of treatments, sham-immunized mice were used to obtain control sera.
Anti-pilus monoclonal antibodies were provided by J. C. Sadoff, Walter Reed Army Institute of Research.
Tethered-peptide pin assay.
An antibody capture ELISA was used to determine the reaction of sera with tethered synthetic peptides. Polystyrene plates were blocked (0.5% casein and 0.5% bovine serum albumin in PBS) for 60 min at room temperature. The tethered peptides were blocked with this reagent in a separate plate. A 50-μl volume of serum, diluted in blocking reagent to 10−3, was added to each well of a blocked plate, after which the tethered-peptide block was introduced and incubated for 2 h at room temperature. After three washes with PBS, the peptide pins were transferred to a blocked plate containing 50 μl of alkaline phosphatase-labeled secondary antibody (goat anti-mouse immunoglobulin G [heavy and light chains]; Kirkegaard & Perry Laboratories, Inc., Gaithersburg, Md.), diluted to 10−3 in blocking reagent, in each well. This mixture was incubated for 2 h at room temperature. After three more washes with PBS, the pins were transferred to a plate containing 75 μl of substrate (10% diethanolamine, 0.05% p-nitrophenyl phosphate [pH 9.8; Sigma Chemical Company, St. Louis, Mo.]), where they were incubated for 60 min at room temperature. At this time, the A405 was measured on a Dynatech MR 5000 plate reader. Before reassay of the pins, bound protein was removed by bath sonication (30 min at 70°C in PBS containing 1% SDS and 0.2% 2-mercaptoethanol), followed by a hot water rinse and boiling-methanol treatment (65°C, 2 min). Interpretation of ELISA results employed strategies described by Carter et al. (2).
Preparation of conjugated pilin glycan.
Pure aminoglycan from P. aeruginosa 1244 pilin was prepared as previously described (5). This procedure involved total proteolysis of pure pili, followed by purification with gel filtration and thin-layer chromatography. Analysis of this material (5) showed it to contain no peptide or polysaccharide contamination. This molecule was attached to ovalbumin by first dissolving 175 nmol of this protein with 25 nmol of aminoglycan in 2.0 ml of PBS. To this mixture was slowly added 2.0 ml of 0.2% glutaraldehyde in PBS at room temperature. After stirring for 1 h at room temperature, the reaction was stopped by dropwise addition of 0.5 ml of 1.0 M glycine in PBS. This material was dialyzed twice against 2.0 liters of deionized water and stored frozen.
Immunoblotting.
For Western blotting, protein or LPS separated by PAGE was electroblotted to nitrocellulose paper and blocked as previously described (7). Alkaline phosphates-labeled anti-mouse secondary antibody (Kirkegaard & Perry Laboratories) was used, and Naphthol AS-MX Phosphate/Fast Red functioned as the substrate. Dot blotting was carried out by spotting proteins to be tested on nitrocellulose paper (0.05-μm pore size), which was then blocked and probed as described for Western blotting (7). Pili to be used for immunoblotting of isoelectric focusing gels were prepared as follows. P. aeruginosa 1244N3 containing either pS118A or pS148A was grown on LB plates containing tetracycline, CB, and IPTG. Nonglycosylated 1244 pilin was prepared from P. aeruginosa PA103(pPAC24) from LB plates containing CB and IPTG. Cells from two or three plates were resuspended with 5 ml of LB and vortexed vigorously to separate pili, after which the cells were removed by centrifugation (15 min, 5,000 rpm, SS34 rotor). The supernatant fluid was made 3% with polyethylene glycol 8000 and 0.1 M with MgCl2 and stored overnight at 4°C. This material was centrifuged (30 min, 9,000 rpm, SS34 rotor), and the pellet was resuspended with 2% BOG. Crude glycosylated pili were prepared from broth cultures of strain 1244N3(pPAC46) as described above, precipitated with polyethylene glycol-MgCl2, and suspended in 1% BOG. PhastGels (pH 3 to 9; Amersham Pharmacia, Piscataway, N.J.) were soaked in a solution containing 2% BOG and Ampholine 3.5-9.5 (50 μl/ml) for 20 min at room temperature. The PhastSystem electrophoresis unit (Amersham Pharmacia) was used for electrofocusing in accordance with the manufacturer's instructions. Focused proteins were transferred to nitrocellulose paper by diffusion blotting. Probing and development of the immunoblot were done as described above.
RESULTS
Pilin location of glycan moiety.
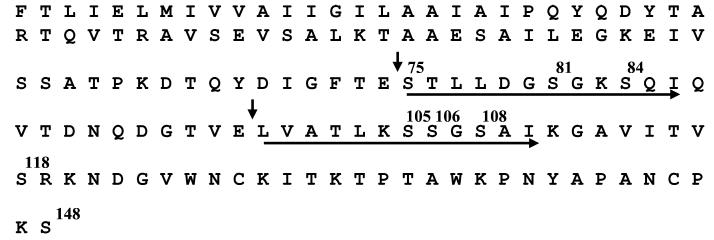
Previous work (5) has shown that the P. aeruginosa 1244 pilin glycan is covalently attached to one of the protein's 13 serine residues. In order to determine the location of the modified serine, a sample of pure P. aeruginosa 1244 pili was digested with endopeptidase GluC (V8 protease). PAGE, followed by silver staining, showed that this treatment produced a protein fragment with a molecular weight of approximately 9,000 (Fig. 1, lane 1). Western blot analysis of the digested pilin with a glycan-specific monoclonal antibody indicated that this fragment contained the pilin glycan (Fig. 1, lane 2). N-terminal sequence analysis of an identical portion of the blot, visualized with CBB but not subjected to blocking, revealed that its amino terminus was pilin residue 75 (Fig. 2). These results suggested that the glycan was attached to one of the eight serines present between this residue and the pilin carboxy terminus. Sequencing through residue 86 of the 9,000-molecular-weight fragment showed that serines 75, 81, and 84 produced normal signals, indicating that these amino acids were not modified (Fig. 2).
FIG. 1.
Electrophoretic analysis of purified P. aeruginosa 1244 pilin digested with GluC (V8 protease). The pilin used in lanes 1 and 2 was digested for 3 h, while the pilin used in lane 3 was digested for 8 h, under the conditions described in Materials and Methods. Each lane represents the digestion product of approximately 2 μg of pilin. Lanes: 1, silver staining; 2, Western blotting with pilin glycan-specific monoclonal antibody 11-14 as the probe; 3, CBB staining. The arrow indicates the position of undigested P. aeruginosa 1244 pilin. The values on the left are relative molecular weights in thousands.
FIG. 2.
Results of N-terminal sequencing of P. aeruginosa 1244 pilin fragments produced by GluC digestion. Vertical arrows indicate the N termini of assayed fragments. Horizontal arrows indicate the portions of the fragment that were sequenced.
The presence of a glutamate residue at position 98 and the production of a smaller reactive GluC fragment (Fig. 1, lane 2) suggested that it should be possible to further dissect this part of the pilin molecule. More extensive pilin digestion with GluC, followed by PAGE and staining with CBB, resulted in the appearance of an approximately 7,000-molecular-weight fragment (Fig. 1, lane 3). Sequencing of this band showed that residue 98 was at its N terminus (Fig. 2). Further sequencing through residue 110 showed that serines 105, 106, and 108 also produced normal high-performance liquid chromatography responses, suggesting that glycan attachment was not at these sites (Fig. 2).
In order to examine this point further, pure pilin samples were subjected to trypsin digestion. Since this procedure would produce fragments too small to be detected by immunoblotting, the products of this treatment were separated by gel filtration with a Superdex Peptide column. Fractions were assayed by an orcinol spot test, by which it was determined that carbohydrate-positive material was present in the 400- to 800-molecular-weight range (results not shown). Analysis of this fraction with high-performance thin-layer chromatography (with either orcinol or ninhydrin development) showed that it contained an orcinol-positive compound that had Rf values identical to those of the aminoglycan (5) produced by total proteolysis of P. aeruginosa 1244 pilin (results not shown). The estimated size range of the material isolated was consistent with the molecular weight of 771 determined for the pilin aminoglycan (5). The proximity of serine 148 to lysine 147 suggests that this is the only pilin serine that could produce the aminoglycan after trypsin digestion.
Due to the inaccessibility of serine 148 to sequence analysis, site-directed mutagenesis was used to determine the association of glycan with this residue. In order to do this, a single base substitution of the cloned pilA gene was carried out that converted serine-148 to alanine, producing mutant S148A. The same strategy was employed to convert serine 118 to alanine, making mutant S118A. These altered pilin genes were subcloned into a broad-host-range vector and expressed, under the control of a tac promoter, in a P. aeruginosa 1244 mutant that was normally unable to produce pilin. Both pS118A and pS148A produced functional pili, as indicated by the ability of this strain to carry out twitching motility and its sensitivity to pilus-specific phage (results not shown).
Pilin produced by these mutant pilA genes was analyzed by Western blotting with a monoclonal antibody previously determined to be specific for pilin protein (6). These results (Fig. 3A) showed that pS118A produced a pilin with a size identical to that of glycosylated pilin, while the mobility of the pS148A pilin matched that of the nonglycosylated form. Separation of the mutant pilins by electrofocusing, followed by blotting to nitrocellulose paper and probing with a pilin protein-specific monoclonal antibody (Fig. 3B), showed that the pS118A pilin had an acidic pI and focused at the same pH as wild-type glycosylated pilin. Pilin produced by pS148A and nonglycosylated pilin both focused at a more neutral pH (Fig. 3B). Probing of an identical blot with pilin glycan-specific monoclonal antibody 11-14 showed that while both the glycosylated pilin reference and the pS118A pilin reacted with this antibody, neither nonglycosylated nor pS148A pilin was recognized (Fig. 3C). Together, these results support the trypsin digestion data and indicate that the pilin glycan is attached to residue 148, the carboxy-terminal amino acid of the pilin molecule.
FIG. 3.
Immunoblots of P. aeruginosa 1244 pilins produced by site-directed mutagenesis. (A) Western blotting with pilin protein-specific monoclonal antibody 6-45 as the probe. Each lane contained approximately 0.5 μg of pilin. Lanes: 1, glycosylated strain 1244 pilin; 2, nonglycosylated strain 1244 pilin; 3, pilin produced by pS118A; 4, pilin produced by pS148A. Arrows indicate the positions of molecular weight standards. The values on the left are relative molecular weights in thousands.(B) Diffusion blot of electrofocusing gels probed with pilin protein-specific monoclonal antibody 6-45. Each lane contained approximately 0.1 μg of pilin. Lanes: 1, glycosylated strain 1244 pilin; 2, nonglycosylated strain 1244 pilin; 3, pilin produced by pS118A; 4, pilin produced by pS148A. Arrows indicate the pH gradient of the focusing gel. (C) Diffusion blot of electrofocusing gels probed with pilin glycan-specific monoclonal antibody 11-14. Each lane contained approximately 0.1 μg of pilin. Lanes: 1, glycosylated strain 1244 pilin; 2, nonglycosylated strain 1244 pilin; 3, pilin produced by pS118A; 4, pilin produced by pS148A. Arrows indicate the pH gradient of the focusing gel.
Proximity of glycan to pilus B-cell epitopes.
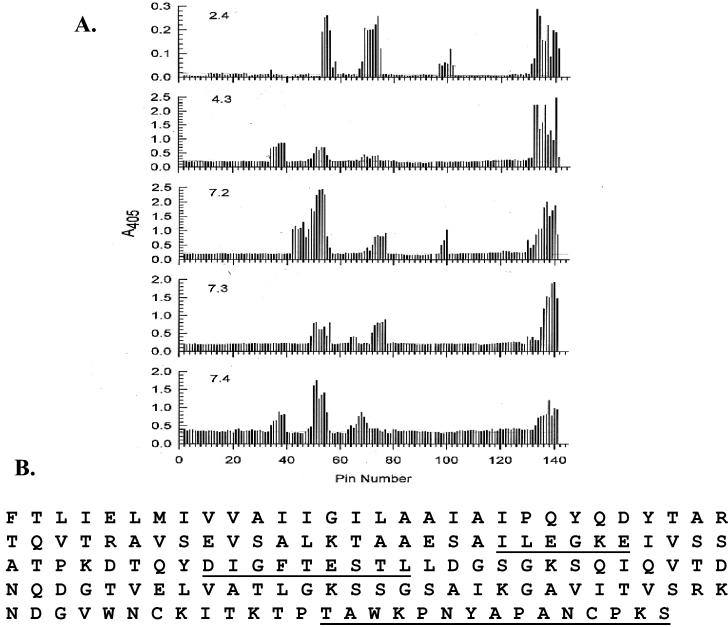
The site of pilin glycosylation is adjacent to the disulfide loop region, a structure that serves as the pilus receptor for host cell glycolipid recognition (20). In addition, this region is an important B-cell epitope for the pili of P. aeruginosa strains PAK and PAO (36, 39). Since P. aeruginosa 1244 pilin has significant structural differences from these strains (6), it was necessary to carry out B-cell epitope mapping to determine the degree and pattern of immunogenicity of the P. aeruginosa 1244 pilin loop region. To do this, sera from mice immunized with pure strain 1244 pili were assayed with the tethered-peptide assay of Geysen et al. (15). The antigens employed were chemically synthesized 12-mer peptides (sequentially overlapping by a single residue) that, together, encompassed the P. aeruginosa 1244 pilin primary structure. Reaction of the sera with these peptides produced distinctive and unambiguous patterns of reactivity, indicating that the antibodies in the sera tested had significant affinity for the tethered peptides (Fig. 4A).
FIG. 4.
Linear B-cell epitopes of P. aeruginosa 1244 pili. (A) Antibody capture ELISA results obtained by testing mouse sera, produced by immunization with pure undenatured P. aeruginosa 1244 pili, against a set of overlapping tethered 12-mer peptides representing the strain 1244 pilin primary structure. Pins 1 and 95 were removed prior to the assay. (B) P. aeruginosa 1244 pilus B-cell epitope map. The underlined portions of the pilin primary structure indicate the epitope regions.
Analysis of the pin recognition pattern of these sera allowed the determination of reactive residues and the assignment of linear epitope regions (Fig. 4B). Although some variation of individual serum response was seen, three consensus epitope regions, based on the pin reaction frequency, were apparent. Region one, which was composed of residues 51 through 57 (ILEGKE), reacted with all five sera. Region two, residues 69 through 77 (DIGFTESTL), reacted with four of the five sera. Region three, which was made up of residues 134 through 148 (TAWKPNYAPANCPKS), reacted with all five sera. Region three contained a portion of the disulfide loop region, as well as the carboxy-terminal domain. Based on the magnitude of the ELISA reactions, region three produced the strongest response. This portion of the 1244 pilin molecule has also been shown to be the epitope for three anti-1244 pilus monoclonal antibodies (6).
A peptide corresponding to a portion of the region 3 epitope (TPTAWKPNYAPANC) was synthesized and covalently coupled to a carrier protein. While the carrier protein was unreactive, the coupled peptide was recognized by sera from all of the mice immunized with strain 1244 pili, as determined by ELISA (results not shown). Mice immunized with this coupled peptide produced antibodies that recognized native pili, also as determined by ELISA (results not shown). These sera also recognized the peptides comprising region 3 of the tethered-peptide pin set (results not shown). Together, these results show that this epitope region, which is the site of glycan attachment, is a major pilin B-cell epitope.
The immunogenicity of the pilin glycan.
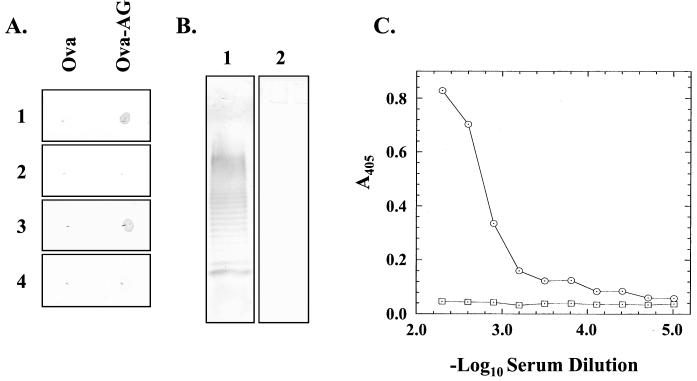
In order to determine whether the pilin glycan is also able to produce a B-cell response, sera from mice were immunized with purified P. aeruginosa 1244 glycosylated pili. These sera, as expected from the results described above, reacted strongly with pili (as determined by ELISA) and pilin (as determined by Western blotting) from strain 1244 (results not shown). A protein conjugate was prepared to test for the presence of glycan-specific antibodies in these sera. Figure 5A shows that this conjugate reacts with monoclonal antibody 11-14, a glycan-specific antibody (5), but not with an antibody (monoclonal antibody 5-44) directed against 1244 pilin protein (6). Figure 5A also shows that one of the sera raised by immunization with 1244 pili, 24-3, recognized the conjugated glycan but not the unconjugated carrier protein, indicating the presence of antibodies specific for the pilin glycan structure. In addition, serum from sham-immunized mice failed to react with the conjugate.
FIG. 5.
Immunogenicity of the native P. aeruginosa 1244 pilus glycan. (A) Dot blot reaction of 2 μg of either ovalbumin (Ova) or ovalbumin-pilin aminoglycan (Ova-AG). Blots were probed with the following sera: 1, anti-pilin glycan monoclonal serum 11-14 (5); 2, anti-pilin protein-specific monoclonal serum 5-44 (7); 3, polyclonal serum 24-3 raised against pure P. aeruginosa 1244 pili; 4, serum from a sham-immunized mouse. The monoclonal sera were tested at a dilution of 10−3, while the polyclonal sera were diluted to 2 × 10−3. (B) Western blotting of approximately 1 μg of pure P. aeruginosa 1244 LPS. Lane 1 was probed with anti-pilus serum 24-3 at a dilution of 5 × 10−3, and lane 2 was probed with serum 20-1 (at a dilution of 10−2), which was obtained from a mouse immunized with 0.1 μg of strain 1244 LPS per dose. (C) ELISA with a plate coated with P. aeruginosa 1244 LPS. The circles represent the reaction of serum 24-3, while the squares are the response of the serum from a sham-immunized mouse.
The antigenic and structural similarities between the pilin glycan and the LPS O-antigen repeating unit of P. aeruginosa 1244 (5) suggested that antibodies directed against the pilin glycan might also recognize LPS from this organism. When serum 24-3 was tested for reactivity with strain 1244 LPS by Western blotting, a strong reaction was seen (Fig. 5B). This was further explored with an ELISA (Fig. 5C), which showed that serum 24-3 reacted strongly with LPS, producing a dilution endpoint of approximately 3.15. The average endpoint of the four sera assayed was 3.25, while control sera produced no anti-LPS response (Fig. 5C). In addition, the other three anti-pilus sera also gave a positive Western blot reaction against strain 1244 LPS (results not shown).
Since LPS is a contaminant of nearly any gram-negative bacterial cell preparation, it had to be determined whether this material was responsible for the results described above. Western blot analysis of proteolytically digested pilin was carried out with known amounts of pure authentic 1244 LPS as the standard. This method indicated that LPS was present at approximately 0.5% (wt/wt) of the amount of the pilin in the preparation (results not shown). Three sets of two mice each were then immunized with purified strain 1244 LPS in amounts equivalent to 200, 100, and 50%, respectively, of the LPS contamination levels present in the pilin doses. No detectable reaction against pure LPS was shown by Western blotting (Fig. 5B) or ELISA (results not shown). These results suggest that the anti-LPS response seen with the pilus immunizations came from B-cell stimulation by the pilin glycan and was not due to LPS contamination.
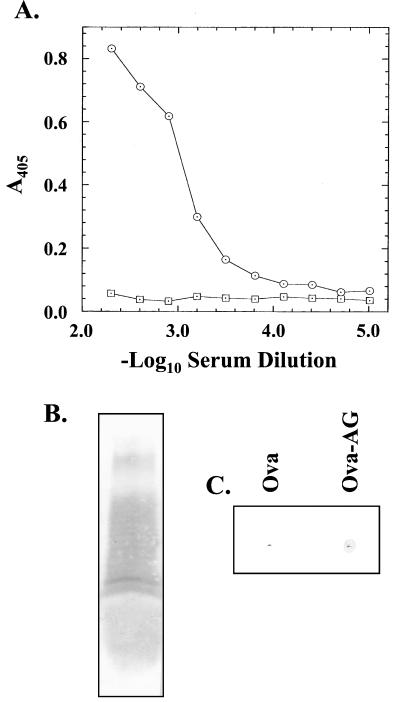
To confirm these results, the residual amounts of LPS in the pilus preparations were reduced to undetectable levels. This was done by gel filtration and chromatofocusing of purified pili treated with BOG, a detergent known to depolymerize P. aeruginosa pili (43). Dialysis of these purified pilin monomers removed the detergent and allowed the reaggregation of this material into a nonnative filament (44). No LPS was detected when 200 μg of the repolymerized pilin was proteolytically digested and subjected to Western blotting with anti-LPS monoclonal antibody 11-14 as the probe (results not shown). Immunization of mice with LPS-free reaggregated pili produced sera that recognized native glycosylated P. aeruginosa 1244 pili, as determined by ELISA, and pilin, as determined by Western blotting (results not shown). Reactivity of one of these sera, 25-5, showed strong recognition of strain 1244 LPS (Fig. 6A), as determined by ELISA, with a dilution endpoint of 3.40. This was a value similar to that obtained with sera from mice immunized with native glycosylated pili. One of the sera in this series did not react with LPS, while the dilution endpoint average of the other three sera was 3.19. Control sera produced no anti-LPS response (Fig. 6A). Serum 25-5 also produced a strong reaction against LPS, as determined by Western blotting (Fig. 6B). In addition, this serum was tested by dot blotting a for reaction against the aminoglycan-ovalbumin conjugate (Fig. 6C), where a positive reaction was seen. Together, these results provide evidence that the pilin glycan is an important B-cell epitope and that the antibodies formed recognize P. aeruginosa 1244 LPS.
FIG. 6.
Immunogenicity of the glycan of reaggregated P. aeruginosa 1244 pilin. (A) ELISA with a plate coated with P. aeruginosa 1244 LPS. The circles represent the reaction of serum 25-5, while the squares are the response of the serum from a sham-immunized mouse. (B) Western blot of approximately 1 μg of pure P. aeruginosa 1244 LPS. Lane 1 was probed with serum 25-5 at a dilution of 5 × 10−3. (C) Dot blot reaction of 2 μg of either ovalbumin (Ova) or ovalbumin-pilin aminoglycan (Ova-AG). The blot was probed with polyclonal serum 25-5, which was raised against reaggregated pure P. aeruginosa 1244 pili. This serum was diluted to 2 × 10−3.
DISCUSSION
Previous work in our laboratory has shown that the P. aeruginosa 1244 pilin glycan is an O-linked trisaccharide that occurs at a ratio of one glycan per pilin subunit (5). Work described in this paper has established that this glycan is attached to serine 148, the carboxy-terminal residue of mature pilin. This location can be compared with the glycosylation sites of the neisserial pilins, where the trisaccharide structure of N. meningitidis and the disaccharide of N. gonorrhoeae are each attached through serine 63 (33, 41), while the glycerol phosphate of N. meningitidis pilin is covalently bound at serine 93 (40). N. gonorrhoeae pilin is also phosphorylated at serine residue 68 (11).
Crystallographic analysis of native pilin monomers from N. gonorrhoeae (12, 33) and two strains of P. aeruginosa (17, 23) has produced a model structure for type IV pilins that shows that these proteins are made up of two major regions. The first is a hydrophobic α-helical segment that corresponds to the amino-proximal constant region and occupies approximately one-third of the pilin primary structure. This part of the pilin monomer is connected to a region composed of curved antiparallel β-sheet segments that fold over the C-terminal portion of the α-helix structure. The sheet region culminates in the carboxy-terminal disulfide loop structure found in all type IV pilins. Current models (12, 17, 23, 33) suggest that intertwining subunit α-helices form the interior of the fiber, with the remaining portion of the pilin subunit forming the surface layers of the pilus. If the subunit model can be applied to P. aeruginosa 1244 pilin, the glycan would be found at the edge of the pilin β-sheet region. While it is not clear exactly how pilin is organized in the pilus fiber, it seems likely that the 1244 pilin glycan would exist at the interface of adjacent subunits, where it could provide pilus stability through subunit-subunit interaction. The neisserial di- and trisaccharide modifications, the phosphorylation site of N. gonorrhoeae pilin phosphate, and the glycerol phosphate of N. meningitidis pilin (11, 33, 40, 41) are also found at the periphery of the β-sheet region (although in relatively different positions). This pattern suggests that pilus modification occurs predominantly at the subunit-subunit junctions. This arrangement would leave the β-sheet pilin facet free to interact with the pilus environment, suggesting a role for these glycans in maintaining the integrity of the pilus fiber through subunit interaction.
It is not obvious from this model whether the glycan is present at the pilus fiber surface or if it is buried within the junction between the subunits. The inhibition of P. aeruginosa 1244 twitching motility, a pilus-mediated form of motility that is important in the virulence of this organism (9, 22), by a pilin glycan-specific monoclonal antibody (5) showed that the pilin glycan is located at the pilus surface. This was confirmed by our present finding that the glycan of the native pilus produced a strong humoral response. However, it is not clear whether this structure is exposed uniformly over the entire fiber or if it is restricted to certain regions. An example of such a situation is the disulfide loop region of the P. aeruginosa PAO and PAK pilins, which is necessary for host receptor binding (19). This structure is only functional at the pilus tip (28), suggesting a conformational difference of the pilin subunit with position in the pilus fiber. The B-cell response to both the glycan and the protein epitopes of 1244 pilin could also be directed to subunits in specific relative positions on the pilus fiber surface. For example, pilin at the tip could, due to the absence of proximal pilins or to a conformational change induced by the lack of these neighboring subunits, be the only subunits in the fiber in which the glycan epitope is presented. Electron microscopic work with labeled epitope-specific antibodies is necessary to clarify this question.
The proximity of the pilin glycan to the disulfide loop region suggests possible roles for this structure in pilus function. For example, the glycan might influence disulfide loop-mediated pilus adhesion by strengthening binding to host receptors or through recognition of alternate host surface structures. Furthermore, enhanced pilus binding would be expected to increase the effectiveness of twitching motility. Strengthening of binding between P. aeruginosa cells through pilus cross-linking could also be enhanced by glycan-glycan interaction, which, in the case of strain 1244, might include the formation of salt bridges between glycan pseudaminic acids. Complexing of pili from separate cells in this manner would also be expected to enhance colonization through biofilm formation.
The location of the pilin glycan in relation to the disulfide loop, the putative receptor-binding region of 1244 pilin, suggests that it might protect this vital pilus component. While the glycan could be expected to shield the pilus from neutralizing antibodies directed against this structure, the strong humoral response seen against this region suggests that this is not the case. A more likely prospect is that it acts to protect the pilin carboxy terminus and the disulfide loop region against proteolytic degradation. This would be particularly important for maintaining the adhesion functionality of pilus tips, the site where binding takes place (38). If this is the case, it would be interesting to determine why certain P. aeruginosa strains (13, 32) do not produce glycosylated pili.
The evidence that twitching motility is inhibited by a pilin glycan-specific monoclonal antibody (5) suggests that this saccharide is a potentially protective epitope and that antibody binding to it could be expected to inhibit colonization and migration. The proximity of the glycan to the disulfide loop region may mean that an anti-glycan antibody inhibits twitching by blocking the pilus tip, the site of pilus attachment in twitching motility (38). These observations suggest that the glycan should be considered in the design of any P. aeruginosa pilus-based vaccine. It is especially relevant that mice immunized with glycosylated pili produced antibodies that reacted with the pilin glycan. This suggests that protection by pilus immunization could operate through glycan-specific, as well as pilin protein-specific, antibodies. Of particular interest is the finding that antibodies produced in response to immunization with P. aeruginosa 1244 glycosylated pili recognized LPS from this organism. These results suggest that immunization with glycosylated pili might produce O-antigen-based, as well as pilus-based, protection. An important feature here would be the lack of LPS toxicity due to the absence of lipid A in the preparations. For this approach to have maximal effectiveness, pilin glycosylation with the O-antigen repeating unit should not be restricted to certain LPS serotypes. Our results have shown that O antigen from International Antigen Typing System serotypes 04, 06, and O11 also provides a substrate for pilin glycosylation (unpublished observations), suggesting that O-antigen distribution in pilin glycosylation is broad. The use of a pilus-based vaccine that would stimulate a response to an LPS target is a novel approach that merits further investigation.
Acknowledgments
This work was supported by grant R15 AI43317 from the National Institute of Allergy and Infectious Diseases and U.S. Army grant DAAL03-88-G-0103.
Editor: V. J. DiRita
REFERENCES
- 1.Bradley, D. E. 1972. Evidence for the retraction of Pseudomonas aeruginosa RNA phage pili. Biochem. Biophys. Res. Commun. 47:142-149. [DOI] [PubMed] [Google Scholar]
- 2.Carter, J. M., S. VanAlbert, J. S. Lee, J. Lyons, and C. Deal. 1992. Shedding light on peptide synthesis. Biotechnology 10:509-513. [DOI] [PubMed] [Google Scholar]
- 3.Cassels, F. J., and J. London. 1989. Isolation of a coaggregation-inhibiting cell wall polysaccharide from Streptococcus sanguis H1. J. Bacteriol. 171:4019-4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castric, P. 1995. pilO, a gene required for glycosylation of Pseudomonas aeruginosa 1244 pilin. Microbiology 141:1247-1254. [DOI] [PubMed] [Google Scholar]
- 5.Castric, P., F. J. Cassels, and R. W. Carlson. 2001. Structural characterization of the Pseudomonas aeruginosa 1244 pilin glycan. J. Biol. Chem. 276:26479-26485. [DOI] [PubMed] [Google Scholar]
- 6.Castric, P. A., and C. D. Deal. 1994. Differentiation of Pseudomonas aeruginosa pili based on sequence and B-cell epitope analyses. Infect. Immun. 62:371-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castric, P. A., H. F. Sidberry, and J. C. Sadoff. 1989. Cloning and sequencing of the Pseudomonas aeruginosa 1244 pilin structural gene. Mol. Gen. Genet. 216:75-80. [DOI] [PubMed] [Google Scholar]
- 8.Castric, P. A., and G. A. Strobel. 1969. Cyanide metabolism by Bacillus megaterium. J. Biol. Chem. 244:4089-4094. [PubMed] [Google Scholar]
- 9.Comolli, J. C., A. R. Hauser, L. Waite, C. B. Whitchurch, J. S. Mattick, and J. N. Engel. 1999. Pseudomonas aeruginosa gene products PilT and PilU are required for cytotoxicity in vitro and virulence in a mouse model of acute pneumonia. Infect. Immun. 67:3625-3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deal, C. D. 1994. Synthesis of peptides immobilized on polypropylene pins, p. 9.7.1-9.7.14. In J. Colligan (ed.), Current protocols in immunology. John Wiley & Sons, Inc., New York, N.Y.
- 11.Forest, K. T., S. E. Dunham, M. Koomey, and J. A. Tainer. 1999. Crystallographic structure reveals phosphorylated pilin from Neisseria: phosphoserine sites modify type IV pilus surface chemistry and fibre morphology. Mol. Microbiol. 31:743-752. [DOI] [PubMed] [Google Scholar]
- 12.Forest, K. T., and J. A. Tainer. 1997. Type-4 pilus-structure: outside to inside and top to bottom—a minireview. Gene 192:165-169. [DOI] [PubMed] [Google Scholar]
- 13.Frost, L. S., and W. Paranchych. 1977. Composition and molecular weight of pili purified from Pseudomonas aeruginosa K. J. Bacteriol. 131:259-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furste, J. P., W. Pansegrau, F. R. Blocker, P. Scholz, M. Bagdasarian, and E. Lanka. 1986. Molecular cloning of the plasmid RP4 primase region in a multi-host-range tacP expression vector. Gene 48:119-131. [DOI] [PubMed] [Google Scholar]
- 15.Geysen, H. M., R. H. Meloen, and S. J. Barteling. 1984. Use of peptide synthesis to probe viral antigens for epitopes to a resolution of a single amino acid. Proc. Natl. Acad. Sci. USA 81:3998-4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hahn, H., P. M. Lane-Bell, L. M. Glasier, J. F. Nomellini, W. H. Bingle, W. Paranchych, and J. Smit. 1997. Pilin-based anti-Pseudomonas vaccines: latest developments and perspectives. Behring Inst. Mitt. 98:315-325. [PubMed] [Google Scholar]
- 17.Hazes, B., P. A. Sastry, K. Hayakawa, R. J. Read, and R. T. Irvin. 2000. Crystal structure of Pseudomonas aeruginosa PAK pilin suggests a main-chain-dominated mode of receptor binding. J. Mol. Biol. 299:1005-1017. [DOI] [PubMed] [Google Scholar]
- 18.Hitchcock, P. J., and T. M. Brown. 1983. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J. Bacteriol. 154:269-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Irvin, R. T. 1993. Attachment and colonization of Pseudomonas aeruginosa: role of the surface structures, p. 19-42. In E. A. Mario Campa (ed.), Pseudomonas aeruginosa as an opportunistic pathogen. Plenum Press, New York, N.Y.
- 20.Irvin, R. T., P. Doig, K. K. Lee, P. A. Sastry, W. Paranchych, T. Todd, and R. S. Hodges. 1989. Characterization of the Pseudomonas aeruginosa pilus adhesin: confirmation that the pilin structural protein subunit contains a human epithelial cell-binding domain. Infect. Immun. 57:3720-3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Judd, R. C. 1994. Basic protein and peptide protocols. Methods Mol. Biol. 32:49-57. [DOI] [PubMed] [Google Scholar]
- 22.Kang, P. J., A. R. Hauser, G. Apodaca, S. M. J. Fleiszig, J. Wiener-Kronish, K. Mostov, and J. N. Engel. 1997. Identification of Pseudomonas aeruginosa genes required for epithelial cell injury. Mol. Microbiol. 24:1249-1262. [DOI] [PubMed] [Google Scholar]
- 23.Keizer, D. W., C. M. Slupsky, M. Kalisiak, A. P. Campbell, M. P. Crump, P. A. Sastry, B. Hazes, R. T. Irvin, and B. D. Sykes. 2001. Structure of a pilin monomer from Pseudomonas aeruginosa: implications for the assembly of pili. J. Biol. Chem. 276:24186-24193. [DOI] [PubMed] [Google Scholar]
- 24.Knirel, Y. A. 1990. Polysaccharide antigens of Pseudomonas aeruginosa. Crit. Rev. Microbiol. 17:273-304. [DOI] [PubMed] [Google Scholar]
- 25.Knirel, Y. A., N. A. Kocharova, A. S. Shashkov, B. A. Dmitriev, N. K. Kochetkov, E. S. Stanislavsky, and G. M. Mashilova. 1987. Somatic antigens of Pseudomonas aeruginosa: the structure of O-specific polysaccharide chains of the lipopolysaccharides from P. aeruginosa O5 (Lanyi) and immunotype 6 (Fisher). Eur. J. Biochem. 163:639-652. [DOI] [PubMed] [Google Scholar]
- 26.Kunkel, T. A. 1985. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc. Natl. Acad. Sci. USA 82:488-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee, K. K., P. Doig, R. T. Irvin, W. Paranchych, and R. S. Hodges. 1989. Mapping the surface regions of Pseudomonas aeruginosa PAK pilin: the importance of the C-terminal region for adherence to human buccal epithelial cells. Mol. Microbiol. 3:1493-1499. [DOI] [PubMed] [Google Scholar]
- 28.Lee, K. K., H. B. Sheth, W. Y. Wong, R. Sherburne, W. Paranchych, R. S. Hodges, C. A. Lingwood, H. Krivan, and R. T. Irvin. 1994. The binding of Pseudomonas aeruginosa pili to glycosphingolipids is a tip-associated event involving the C-terminal region of the structural pilin subunit. Mol. Microbiol. 11:705-713. [DOI] [PubMed] [Google Scholar]
- 29.Liu, P. V., H. Matsumoto, H. Kusama, and T. Bergan. 1983. Survey of heat-stable, major somatic antigens of Pseudomonas aeruginosa. Int. J. Syst. Bacteriol. 33:256-264. [Google Scholar]
- 30.Nesterenko, M. V., M. Tilley, and S. J. Upton. 1994. A simple modification of Blum's silver stain method allows for 30 minute detection of proteins in polyacrylamide gels. Biochem. Biophys. Methods 28:239-242. [DOI] [PubMed] [Google Scholar]
- 31.Paranchych, W., and L. S. Frost. 1988. The physiology and biochemistry of pili. Adv. Microb. Physiol. 29:53-114. [DOI] [PubMed] [Google Scholar]
- 32.Paranchych, W., P. A. Sastry, L. S. Frost, M. Carpenter, G. D. Armstrong, and T. H. Watts. 1979. Biochemical studies on pili isolated from Pseudomonas aeruginosa strain PAO. Can. J. Microbiol. 25:1175-1181. [DOI] [PubMed] [Google Scholar]
- 33.Parge, H. E., K. T. Forest, M. J. Hickey, D. E. Christensen, E. D. Getzoff, and J. A. Tainer. 1995. Structure of the fibre-forming protein pilin at 2.6 A resolution. Nature 378:32-38. [DOI] [PubMed] [Google Scholar]
- 34.Ramphal, R., L. Koo, K. S. Ishimoto, P. A. Totten, J. C. Lara, and S. Lory. 1991. Adhesion of Pseudomonas aeruginosa pilin-deficient mutants to mucin. Infect. Immun. 59:1307-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramphal, R., J. Sadoff, M. Pyle, and J. D. Silipigni. 1984. Role of pili in the adherence of Pseudomonas aeruginosa to injured tracheal epithelium. Infect. Immun. 44:38-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sastry, P. A., J. R. Pearlstone, L. B. Smillie, and W. Paranchych. 1985. Studies on the primary structure and antigenic determinants of pilin isolated from Pseudomonas aeruginosa K. Can. J. Biochem. Cell Biol. 63:284-291. [DOI] [PubMed] [Google Scholar]
- 37.Schnaar, R. L., and L. K. Needham. 1994. Thin layer chromatography of glycosphingolipids. Methods Enzymol. 230:371-389. [DOI] [PubMed] [Google Scholar]
- 38.Skerker, J. M., and H. C. Berg. 2001. Direct observation of extension and retraction of type IV pili. Proc. Natl. Acad. Sci. USA 98:6901-6904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smart, W., P. A. Sastry, W. Paranchych, and B. Singh. 1993. Immune recognition of polar pili from Pseudomonas aeruginosa O. Infect. Immun. 61:3527-3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stimson, E., M. Virji, S. Barker, M. Panico, I. Blench, J. Saunders, G. Payne, E. R. Moxon, A. Dell, and H. R. Morris. 1996. Discovery of a novel protein modification: alpha-glycerophosphate is a substituent of meningococcal pilin. Biochem. J. 316:29-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stimson, E., M. Virji, K. Makepeace, A. Dell, H. R. Morris, G. Payne, J. R. Saunders, M. P. Jennings, S. Barker, M. Panico, I. Blench, and E. R. Moxon. 1995. Meningococcal pilin: a glycoprotein substituted with digalactosyl-2,4-diacetamido-2,4,6-trideoxyhexose. Mol. Microbiol. 17:1201-1214. [DOI] [PubMed] [Google Scholar]
- 42.Strom, M. S., and S. Lory. 1993. Structure-function and biogenesis of the type IV pili. Annu. Rev. Microbiol. 47:565-596. [DOI] [PubMed] [Google Scholar]
- 43.Watts, T. H., C. M. Kay, and W. Paranchych. 1982. Dissociation and characterization of pilin isolated from Pseudomonas aeruginosa strains PAK and PAO. Can. J. Biochem. 60:867-872. [DOI] [PubMed] [Google Scholar]
- 44.Watts, T. H., D. G. Scraba, and W. Paranchych. 1982. Formation of 9-nm filaments from pilin monomers obtained by octyl-glucoside dissociation of Pseudomonas aeruginosa pili. J. Bacteriol. 151:1508-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wood, D. E., D. C. Strauss, W. G. Johanson, V. K. Berry, and J. A. Bass. 1980. Role of pili in adherence of Pseudomonas aeruginosa to mammalian buccal epithelial cells. Infect. Immun. 29:1146-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]