Abstract
Segmented filamentous bacteria (SFB) are found in multiple species and play an important role in the development of mucosal immunity. The mechanism by which the bacteria interact with the immune system has not been well defined. We provide morphologic evidence of direct interaction between SFB and intraepithelial mononuclear cells.
Segmented filamentous bacteria (SFB) are autochthonous bacteria that colonize the small intestines of a wide range of species (4, 8, 17, 18, 20, 21). SFB are generally considered nonpathogenic (13, 18, 20, 21) and host specific (26). The organisms have not been successfully cultured in vitro but have been characterized by 16S rRNA analysis to be closely related to the genus Clostridium (22). As a group, these organisms have been provisionally named Candidatus Arthomitus (23). Intestinal colonization by SFB is influenced by various factors, including diet, weaning, strain, housing, and the immune status of both the mother and the host (10, 12, 13, 15, 16). SFB attach apically to epithelial cells, with only minor disruption of microvilli and localized actin polymerization at the attachment site (4, 9, 20; M. A. Jepson, M. A. Clark, N. L. Simmons, and B. H. Hirst, abstract from the 649th Meeting of the Biochemical Society 1993, Biochem. Soc. Trans. 22: 91S, 1994).
SFB are suggested to function as an important microbial component of the gastrointestinal ecosystem (4). These organisms may play a role in disease prevention by inhibiting colonization by pathogens such as Escherichia coli and Salmonella enterica (5, 7). The SFB are most visible soon after weaning and disappear weeks later. The disappearance of SFB coincides with the time of activation of mucosal immunity, suggesting immune-mediated clearance of the organisms at that time (24). SFB colonization in mice increases the number of immunoglobulin A-secreting cells and the levels of immunoglobulin A in both secretions and serum (14, 25, 28). Colonization of mice by SFB is associated with the activation and increased numbers of intraepithelial lymphocytes (25, 27). Furthermore, in germfree mice, SFB colonization is associated with the expression of major histocompatibility complex class II molecules on intestinal epithelial cells (28). SFB are thought to play a significant role in the stimulation of the mucosal immune system (14, 25).
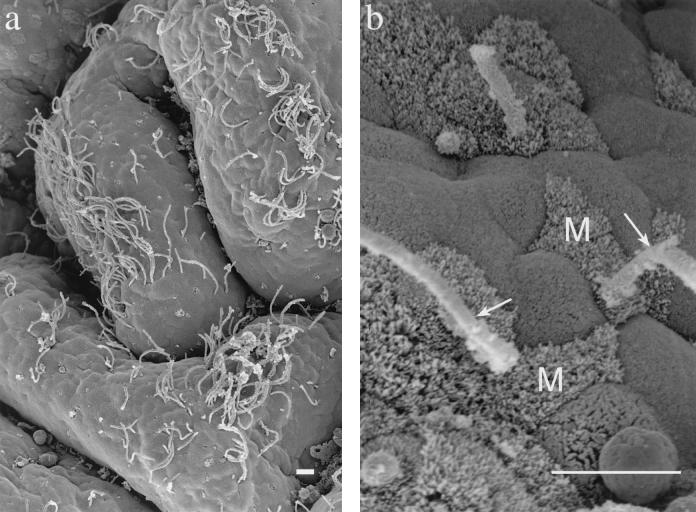
In a separate study of ileal-gut loops prepared from 4- to 5-week-old pigs, SFB colonization in the ileum of one pig was an incidental observation. In this pig, four gut loops had been prepared from the region containing the continuous ileal Peyer's patch. Sample preparation for transmission electron microscopy and scanning electron microscopy was done as previously described (1, 11). Scanning electron micrographs showed SFB distributed over the epithelial tissue of the ileum (Fig. 1a). This colonization was observed in two of the four sequentially prepared loops. The SFB were distributed on both the follicle-associated epithelium and the absorptive epithelium. Most of the SFB were located on the upper one-third of the villi. SFB attached to the apical membrane or along the lateral borders of epithelial cells (Fig. 1b). The cell types involved included enterocytes, goblet cells, and M cells.
FIG. 1.
Scanning electron micrographs of ileal tissue with associated SFB. (a) Villi with SFB. Bar = 10 μm. (b) SFB (arrows) attached near M cells (M) of the follicle-associated epithelium. Bar = 10 μm.
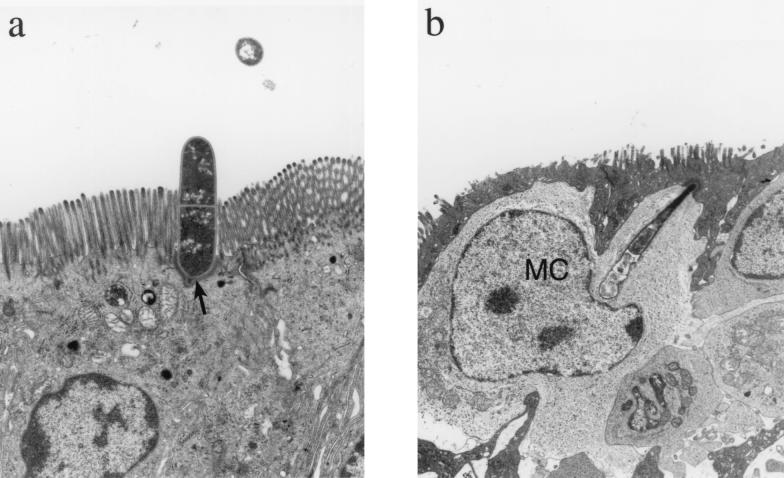
Transmission electron micrographs showed the SFB to be attached to the apical membranes of epithelial cells. The proximal bacterial segment attached to and indented the apical cell membrane (Fig. 2a). In the attachment interface, the proximal bacterial segment possessed a small protuberance at its base. The epithelial cell membrane closely paralleled the shape of the bacteria and contained an adjacent line of intracellular electron-dense material.
FIG. 2.
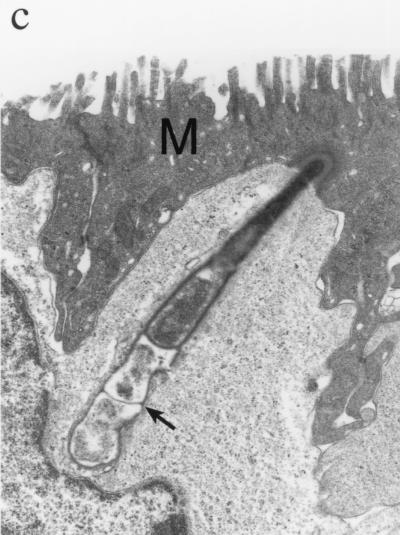
Transmission electron micrographs of SFB attached to the intestinal epithelium. (a) SFB (arrow) attached to the apical portion of the enterocyte. Magnification, ×4,300. (b) SFB extending through an M cell and into intimate association with a mononuclear cell (MC). Magnification, ×4,300. (c) The SFB segments (arrow) which are below the M cell (M) and which are most intimately associated with the mononuclear cell are irregularly shaped and less electron dense. Magnification, ×11,500.
In addition, an SFB was observed to extend from an M cell into intimate association with an intraepithelial mononuclear cell (Fig. 2b and c). In this section, the SFB did not penetrate the mononuclear cell membrane; however, it did extend the membrane deeply into the mononuclear cell cytoplasm, to a point of indentation near the nucleus. The segments of the SFB farthest into the mononuclear cell were more irregular in shape and less electron dense than the apical segments.
The SFB morphology and cellular colonization in the pig ileum were similar to those found in previous work (20). The SFB were seen in two out of the four ileal loops, suggesting limited colonization within the region of the continuous ileal Peyer's patch. In addition, direct interaction between an SFB and an intraepithelial mononuclear cell was observed subjacent to an M cell. The bacterial segments most intimately associated with the mononuclear cell were morphologically degenerate. We speculate that this intimate interaction may represent the early processing of the bacteria by the mononuclear cell.
M cells are specialized antigen-sampling epithelial cells that are found over gut-associated lymphoid tissue such as the continuous ileal Peyer's patch in pigs (3). Macrophages, dendritic cells, and lymphocytes are mononuclear cells that can reside in the basolateral space of the M cell (6, 19). These intraepithelial leukocytes receive and process the sampled antigen or bacteria for the induction of immune response or tolerance (2, 6).
The mechanism of SFB presentation to mucosal immune cells has not been well defined. Enterocyte phagocytosis and processing of attached SFB have been suggested to be mechanisms of immune cell presentation (29). However, no direct interactions of SFB with immune cells have been documented. Our observation of an intimate interaction between SFB and the host's intraepithelial mononuclear cells suggests a direct mechanism of SFB presentation for mucosal immune stimulation.
Editor: B. B. Finlay
REFERENCES
- 1.Ackermann, M. R., N. F. Cheville, and B. L. Deyoe. 1988. Bovine ileal dome lymphoepithelial cells: endocytosis and transport of Brucella abortus strain 19. Vet. Pathol. 25:2835.. [DOI] [PubMed] [Google Scholar]
- 2.Alpan, O., G. Rudomen, and P. Matzinger. 2001. The role of dendritic cells, B cells, and M cells in gut-oriented immune responses. J. Immunol. 166:4843-4852. [DOI] [PubMed] [Google Scholar]
- 3.Beier, R., and A. Gerbert. 1998. Kinetics of particle uptake in the domes of Peyer's patches. Am. J. Physiol. 275:G130-G137. [DOI] [PubMed] [Google Scholar]
- 4.Davis, C. P., and D. C. Savage. 1974. Habitat, succession, attachment, and morphology of segmented, filamentous microbes indigenous to the murine gastrointestinal tract. Infect. Immun. 10:948-956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garland, C. D., A. Lee, and M. R. Dickson. 1982. Segmented filamentous bacteria in the rodent small intestine: colonization of growing animals and possible role in host resistance to Salmonella. Microb. Ecol. 8:181-190. [DOI] [PubMed] [Google Scholar]
- 6.Gebert, A. 1997. The role of M cells in the protection of mucosal membranes. Histochem. Cell Biol. 108:455-470. [DOI] [PubMed] [Google Scholar]
- 7.Heczko, U., A. Abe, and B. B. Finlay. 2000. Segmented filamentous bacteria prevent colonization of enteropathogenic Escherichia coli O103 in rabbits. J. Infect. Dis. 181:1027-1033. [DOI] [PubMed] [Google Scholar]
- 8.Hoskins, J. D., W. G. Henk, and Y. Z. Abdelbaki. 1982. Scanning electron microscopic study of the small intestine of dogs from birth to 337 days of age. Am. J. Vet. Res. 43:1715-1720. [PubMed] [Google Scholar]
- 9.Jepson, M. A., M. A. Clark, N. L. Simmons, and B. H. Hirst. 1993. Actin accumulation at sites of attachment of indigenous apathogenic segmented filamentous bacteria to mouse ileal epithelial cells. Infect. Immun. 61:4001-4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang, H.-Q., N. A. Bos, and J. J. Cebra. 2001. Timing, localization, and persistence of colonization by segmented filamentous bacteria in the neonatal mouse gut depend on immune status of mothers and pups. Infect. Immun. 69:3611-3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelley, R. O., A. F. Dekker, and J. G. Bluemink. 1973. Ligand-mediated osmium binding: its application in coating biological specimens for scanning electron microscopy. J. Ultrastruct. Res. 45:254-258. [DOI] [PubMed] [Google Scholar]
- 12.Klaasen, H. L. B. M., J. P. Koopman, M. E. Van den Brink, M. H. Bakker, and A. C. Beynen. 1992. Influence of a natural-ingredient diet containing Phaseolus vulgaris on the colonization by segmented, filamentous bacteria of the small bowel of mice. Int. J. Vitam. Nutr. Res. 62:334-341. [PubMed] [Google Scholar]
- 13.Klaasen, H. L. B. M., J. P. Koopman, M. E. Van den Brink, M. H. Bakker, F. G. J. Poelma, and A. C. Beynen. 1993. Intestinal, segmented, filamentous bacteria in a wide range of vertebrate species. Lab. Anim. 27:141-150. [DOI] [PubMed] [Google Scholar]
- 14.Klaasen, H. L. B. M., P. J. Van der Heijden, W. Stok, F. G. J. Poelma, J. P. Koopman, M. E. Van den Brink, M. H. Bakker, W. M. C. Eling, and A. C. Beynen. 1993. Apathogenic, intestinal, segmented, filamentous bacteria stimulate the mucosal immune system of mice. Infect. Immun. 61:303-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koopman, J. P., A. M. Stadhouders, H. M. Kennis, and H. De Boer. 1987. The attachment of filamentous segmented microorganisms to the distal ileal wall of the mouse: a scanning and transmission electron microscopy study. Lab. Anim. 21:48-52. [DOI] [PubMed] [Google Scholar]
- 16.Koopman, J. P., M. E. Van den Brink, P. M. Scholten, M. Van der Heyden, F. W. Van Schie, M. P. C. Hectors, and F. Nagengast. 1989. The influence of stress and cheese-whey on intestinal parameters in mice. Vet. Q. 11:24-28. [DOI] [PubMed] [Google Scholar]
- 17.Lowden, S., and T. Heath. 1995. Segmented filamentous bacteria associated with lymphoid tissues in the ileum of horses. Res. Vet. Sci. 59:272-274. [DOI] [PubMed] [Google Scholar]
- 18.Pearson, G. R., M. S. McNulty, R. M. McCracken, and W. Curran. 1982. Scanning electron microscopic observations of segmented filamentous bacteria in the small intestine of domestic fowl. Vet. Rec. 111:366-367. [DOI] [PubMed] [Google Scholar]
- 19.Rescigno, M., M. Urbano, B. Valzasina, M. Francolini, G. Rotta, R. Bonasio, F. Granucci, J. P. Kraehenbuhl, and P. Ricciardi-Castagnoli. 2001. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat. Immunol. 2:361-367. [DOI] [PubMed] [Google Scholar]
- 20.Sanford, S. E. 1991. Light and electron microscopic observations of a segmented filamentous bacterium attached to the mucosa of the terminal ileum of pigs. J. Vet. Diagn. Investig. 3:328-333. [DOI] [PubMed] [Google Scholar]
- 21.Smith, T. M. 1997. Segmented filamentous bacteria in the bovine small intestine. J. Comp. Pathol. 177:185-190. [DOI] [PubMed] [Google Scholar]
- 22.Snel, J., H. J. Blok, H. M. P. Kengen, W. Ludwig, F. G. J. Poelma, J. P. Koopman, and A. D. L. Akkermans. 1994. Phylogenic characterization of Clostridium related segmented filamentous bacteria in mice based on 16S ribosomal RNA analysis. Syst. Appl. Microbiol. 17:172-179. [Google Scholar]
- 23.Snel, J., P. P. Heinen, H. J. Blok, R. J. Carman, A. J. Duncan, P. C. Allen, and M. D. Collins. 1995. Comparison of 16S rRNA sequences of segmented filamentous bacteria isolated from mice, rats, and chickens and proposal of “Candidatus Arthomitus.” Int. J. Syst. Bacteriol. 45:780-782. [DOI] [PubMed] [Google Scholar]
- 24.Snel, J., C. C. Hermsen, H. J. Smits, N. A. Bos, W. M. C. Eling, J. J. Cebra, and P. J. Heidt. 1998. Interactions between gut-associated lymphoid tissue and colonization levels of indigenous, segmented, filamentous bacteria in the small intestine of mice. Can. J. Microbiol. 44:1177-1182. [DOI] [PubMed] [Google Scholar]
- 25.Talham, G. L., H.-Q. Jiang, N. A. Bos, and J. J. Cebra. 1999. Segmented filamentous bacteria are potent stimuli of a physiologically normal state of the murine gut mucosal immune system. Infect. Immun. 67:1992-2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tannock, G. W., J. R. Miller, and D. C. Savage. 1984. Host specificity of filamentous, segmented microorganisms adherent to the small bowel epithelium in mice and rats. Appl. Environ. Microbiol. 47:441-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Umesaki, Y., Y. Okada, S. Matsumoto, A. Imaoka, and H. Setoyama. 1995. Segmented filamentous bacteria are indiginous intestinal bacteria that activate intraepithelial lymphocytes and induce MHC class II molecules and fucosyl asialo GM1 glycolipids on the small intestinal epithelial cells in the ex-germ-free mouse. Microbiol. Immunol. 39:555-562. [DOI] [PubMed] [Google Scholar]
- 28.Umesaki, Y., H. Setoyama, S. Matsumoto, A. Imaoka, and K. Itoh. 1999. Differential roles of segmented filamentous bacteria and clostridia in development of the intestinal immune system. Infect. Immun. 67:3504-3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamauchi, K.-E., and J. Snel. 2000. Transmission electron microscopic demonstration of phagocytosis and intracellular processing of segmented filamentous bacteria by intestinal epithelial cells of the chick ileum. Infect. Immun. 68:6496-6504. [DOI] [PMC free article] [PubMed] [Google Scholar]