ABSTRACT
Purpose
To determine changes in the central endothelium and thickness of grafted corneas, and the cumulative probability of developing glaucoma, graft rejection, and graft failure 15 years after penetrating keratoplasty.
Methods
In a longitudinal cohort study of 500 consecutive penetrating keratoplasties by one surgeon, regrafted eyes, fellow eyes of bilateral cases, and patients not granting research authorization were excluded, leaving 388 grafts for analysis. At intervals after surgery, we photographed the endothelium and measured corneal thickness by using specular microscopy. The presence of glaucoma, graft rejection, and graft failure was recorded.
Results
The 67 patients examined at 15 years represented 30% of the available clear grafts (107 patients had died, 76 grafts had failed). Endothelial cell loss from preoperative donor levels was 71 ± 12% (mean ± SD, n = 67), endothelial cell density was 872 ± 348 cells/mm2, and corneal thickness was 0.59 ± 0.06 mm. Endothelial cell density was unchanged between 10 and 15 years (minimum detectable difference was 96 cells/mm2, α = .05, β = .20, n =54), whereas corneal thickness increased (P = .001, n = 55). The mean annual rate of endothelial cell loss from 10 to 15 years after surgery was 0.2 ± 5.7% (n = 54). The cumulative probability of developing glaucoma, graft rejection, or graft failure was 20%, 23%, and 28%, respectively, and six of the eight graft failures after 10 years resulted from late endothelial failure.
Conclusions
From 10 to 15 years after penetrating keratoplasty, the annual rate of endothelial cell loss was similar to that of normal corneas, corneal thickness increased, and late endothelial failure was the major cause of graft failure.
INTRODUCTION
After the importance of the corneal endothelium in penetrating keratoplasty was established in the 1970s,1 we reported 5- and 10-year data on a cohort of patients who had undergone this procedure.2,3 The annual rate of endothelial cell loss from 3 to 5 years after penetrating keratoplasty was 7.8% per year,2 and from 5 to 10 years, was 4.2% per year.3 The 10-year cumulative risk of developing glaucoma, graft rejection, and graft failure was 21%, 21%, and 22%, respectively.3
We report here data for the same cohort of patients, which has now been observed for 15 years after surgery, thus updating the status of the corneal endothelium, central corneal thickness, and the cumulative probability of developing glaucoma, graft rejection, and graft failure.
METHODS
The cohort consists of 500 consecutive patients who had penetrating keratoplasty performed by one surgeon (W.M.B.) between 1976 and 1986. Thirty-six repeated grafts and 70 fellow eyes were excluded from the study, leaving 394 grafts in 394 patients (ie, 394 independent observations) available for analysis to 10 years after surgery. For the 15-year data, six patients had withdrawn research authorization, leaving 388 grafts available for analysis. There were 141 males (36%) and 247 females (64%), and age at keratoplasty was 62 ± 20 years (mean ± SD; range, 3 to 93 years).
The surgical technique has been described in detail previously.2–4 Donor buttons, with mean diameter of 7.9 mm (range, 6.5 to 10.5 mm), were cut from the endothelial side and sutured into the recipient by using a double-running technique, except for high-risk grafts, in which interrupted sutures were used. Postoperatively, prednisolone acetate 1% was administered topically by the patient from the time of epithelial healing to 3 to 6 months, but rarely more than once per day after the second month.
The central donor corneal endothelium was photographed by using a specular microscope before storage in either McCarey-Kaufman medium at 4°C, organ culture at 34°C, or K-Sol medium at 4°C. Follow-up examinations were scheduled for 2 months and for 1, 3, 5, 10, and 15 years after keratoplasty. At each visit, central corneal endothelium was photographed and corneal thickness was measured by contact specular microscopy. Patients were examined to detect complications, including glaucoma, graft rejection, and graft failure.
The outlines or apices of at least 50 endothelial cells were digitized from specular micrographs. We calculated mean endothelial cell area, mean endothelial cell density, coefficient of variation (SD/mean) of endothelial cell area, and the percentage of cells that were hexagonal. Endothelial cell loss was the decrease in cell density between the preoperative examination and the 15-year examination, expressed as a percentage of the preoperative cell density. The annual rate of endothelial cell loss was calculated by assuming that the endothelial cell loss between 10 and 15 years was exponential (first order):
| (1) |
where ECD is endothelial cell density, the subscripts 10 and 15 indicate the postoperative year, r is the annual rate of endothelial cell loss, and t is time in years. On the interval from 10 to 15 years, t = 5 years.
Endothelial cell density through 15 years was also fitted to a biexponential model to combine the rapid, early endothelial cell loss after keratoplasty, and the slow, chronic endothelial cell loss. This model was described by Armitage and associates:5
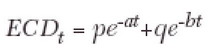 |
(2) |
where ECDt is endothelial cell density at time t, p and q are constants, the sum of which is equal to the initial endothelial cell density, and a and b are the fast and slow exponential rate constants, respectively. The half-lives of the fast and slow components of this model are 0.693/a and 0.693/b, respectively.
Patients were classified as having glaucoma if they had required a surgical procedure to lower intraocular pressure or had used ocular hypotensive agents long-term (for 3 months or longer). Graft rejection was defined as the occurrence of epithelial or endothelial rejection lines, subepithelial infiltrates in the graft only, or a substantial number of new keratic precipitates usually accompanied by segmental ciliary injection, a mild anterior chamber reaction, and an increase in stromal thickness. Only initial rejection episodes were included in the database for computing statistics. Graft failure was defined as an irreversible loss of central graft clarity. Primary endothelial failure was defined as irreversible graft swelling without apparent cause and was classified as either primary donor failure or late endothelial failure. Primary donor failure was defined as unexplained failure of the graft to become thinner and clearer in the first few postoperative weeks. Late endothelial failure was defined as gradual graft decompensation without apparent cause, unresponsive to corticosteroids, and with no recent history of a rejection episode.2
Differences in endothelial cell density, coefficient of variation of cell area, percentage of hexagonal cells, and corneal thickness were compared between groups by using a paired t test when data were normally distributed, or a Wilcoxon signed-rank test when the data were not distributed normally. Differences among diagnoses were tested by using the Kruskal-Wallis test for nonnormal data, and significant differences were investigated by using the Student-Newman-Keuls procedure. Correlations between continuous variables were examined by calculating Pearson’s correlation coefficient (rp) for normal data and Spearman’s rank correlation coefficient (rs) for nonnormal data. The cumulative probability of initial rejection episodes, graft failure, or glaucoma was estimated by using the Kaplan-Meier6 method. Log-rank tests and Cox proportional hazards models were used to evaluate possible risk factors for graft failure. Multivariate models were fit for the overall graft failure end point to determine if the effect of donor age could be explained by other potential risk factors. These models were fit using the Cox proportional hazards models. A two-tailed probability of 5% or less was considered statistically significant.
RESULTS
Of the 394 patients from the original analysis, 332 (84%) of the grafts were for Fuchs’ dystrophy, keratoconus, or corneal edema from aphakia or pseudophakia (Table 1). Sixty-seven patients, 30% of the clear grafts available for follow-up, returned for their 15-year postoperative examination. At 15 years, 76 grafts were known to have failed and 107 patients were known to have died, reducing the overall cohort by 164 because 19 graft failures and deaths occurred in the same patients (Table 2). A further six patients withdrew research authorization during the 10- to 15-year interval, leaving 224 patients who were presumed to be alive and without graft failure (Table 2).
Table 1.
Preoperative recipient diagnoses for the entire cohort of patients
| Diagnosis | No. (%) |
|---|---|
| Fuchs’ dystrophy | 108 (27) |
| Keratoconus | 83 (21) |
| Pseudophakic corneal edema | 73 (19) |
| Aphakic corneal edema | 68 (17) |
| Herpes simplex virus | 13 (3) |
| Other | 49 (12) |
| Total | 394 (100) |
Table 2.
Number of graft failures, deaths, and folow-up rates during each examination interval*
| Examination | No. known to have failed during previous interval | No. known to have died during previous interval | No. presumed alive without failure | No. examined (% of no. presumed alive without failure) |
|---|---|---|---|---|
| Preoperative | – | – | 394 | 394 (100) |
| Postoperative | ||||
| 2 mo | 19 | 2 | 373 | 355 (95) |
| 1 yr | 10 | 8† | 357 | 329 (92) |
| 3 yr | 17 | 10‡ | 331 | 231 (70) |
| 5 yr | 11 | 11† | 311 | 187 (60) |
| 10 yr | 11 | 51§¶ | 257 | 119 (46) |
| 15 yr | 8 | 25¶ | 224# | 67 (30) |
This table has been modified from that published at 10 years after surgery3 because patients with failed grafts who subsequently died had inadvertently been counted twice.
Two deaths were also graft failures in a previous interval.
One patient with graft failure also died in the same interval.
Two patients with graft failure also died in the same interval.
Six deaths were also failures in a previous interval.
Six patients withdrew research authorization.
There were 157 patients who were presumed to be alive and without graft failure who were not examined at 15 years. Of these, there were 58 males (37%) and 99 females (63%), age at keratoplasty was 61 ± 21 years (mean ± SD; range, 3 to 93 years), and 85% of these grafts were for Fuchs’ dystrophy, keratoconus, or corneal edema from aphakia or pseudophakia.
Endothelial Cell Morphometry and Corneal Thickness
The results from all visits for all patients who were seen at 15 years (n = 67) are shown in Table 3. Endothelial cell loss from preoperative donor levels was 71 ± 12%. Endothelial cell density at 15 years after keratoplasty was 872 ± 348 cells/mm2, which did not differ from cell density at 10 years (960 ± 470 cells/mm2, P = .6). The minimum detectable difference was 96 cells/mm2 (α = .05, β = .80, n = 54). From 10 to 15 years, there were no differences in endothelial cell loss, coefficient of variation of cell area, or hexagonal cells, whereas central corneal thickness increased from 0.58 ± 0.06 mm to 0.59 ± 0.06 mm (P = .001, Table 3). The mean rate of endothelial cell loss between 10 and 15 years after surgery was 0.2 ± 5.7% (n = 54).
Table 3.
Corneal endothelium and thickness for all subjects after penetrating keratoplasty (mean ± SD)
| Examination | n | Endothelial cell density (cells/mm2) | Endothelial cell loss (%) | Coefficient of variation of cell area | Hexagonal cells (%) | Central corneal thickness (mm) |
|---|---|---|---|---|---|---|
| Preoperative | 393* | 2973 ± 550 | – | 0.26 ± 0.06 | 68 ± 11 | – |
| Postoperative | ||||||
| 2 mo | 355 | 2467 ± 675 | 17 ± 19 | 0.25 ± 0.06 | 60 ± 10 | 0.54 ± 0.05 |
| 1 yr | 329† | 1958 ± 718 | 34 ± 22 | 0.26 ± 0.06 | 62 ± 9 | 0.54 ± 0.05 |
| 3 yr | 231 | 1376 ± 586 | 53 ± 19 | 0.26 ± 0.07 | 64 ± 11 | 0.56 ± 0.05 |
| 5 yr | 187 | 1191 ± 523 | 59 ± 17 | 0.29 ± 0.09 | 61 ± 13 | 0.57 ± 0.05 |
| 10 yr | 119 | 960 ± 470 | 67 ± 17 | 0.33 ± 0.11 | 56 ± 12 | 0.58 ± 0.06 |
| 15 yr | 67 | 872 ± 348‡ | 71 ± 12‡ | 0.34 ± 0.10‡ | 55 ± 11‡ | 0.59 ± 0.06§ |
n = 393 because the endothelial images from one preoperative donor cornea were not sufficiently clear for analysis.
n = 328 for cell data because endothelial images from one cornea were not sufficiently clear for analysis.
n = 66 for cell data because endothelial images from one cornea were not sufficiently clear for analysis. There was no significant difference between 10 and 15 years after surgery for those patients who had both 10- and 15-year follow-up (n = 54). Minimum detectable differences (α = 0.05, β = 0.80, n = 54) were: endothelial cell density, 96 cells/mm2; endothelial cell loss, 3.4%; coefficient of variation of cell area, 0.044; hexagonal cells, 5.6%.
Central graft thickness was higher at 15 years after surgery than at 10 years for those patients who had both 10- and 15-year follow-up (P = .001, n = 55).
Twenty-seven patients attended all follow-up visits through 15 years and had no episodes of graft rejection or reoperations that might have affected the corneal endothelium (Table 4). Endothelial cell loss from preoperative donor levels was 72 ± 9%. Endothelial cell density at 15 years after keratoplasty was 876 ± 232 cells/mm2, which did not differ from cell density at 10 years (844 ± 261 cells/mm2, P = .42). The minimum detectable difference was 112 cells/mm2 (α = .05, β = .80, n = 27). From 10 to 15 years after keratoplasty, there were no differences in endothelial cell loss, coefficient of variation of cell area, or hexagonal cells, whereas central corneal thickness increased from 0.58 ± 0.05 mm to 0.60 ± 0.06 mm (P = .003, Table 4). The mean rate of endothelial cell loss from 10 to 15 years after surgery was −1.0 ± 5.4% (n = 27), consistent with a small increase in cell density.
Table 4.
Corneal endothelium and thickness for all subjects who returned for all examinations, and who had no rejection episodes or reoperations that might have affected the endothelium (mean ± SD)
| Examination | n | Endothelial cell density (cells/mm2) | Endothelial cell loss (%) | Coefficient of variation of cell area | Hexagonal cells (%) | Central corneal thickness (mm) |
|---|---|---|---|---|---|---|
| Preoperative | 27 | 3196 ± 490 | – | 0.25 ± 0.05 | 70 ± 11 | – |
| Postoperative | ||||||
| 2 mo | 27 | 2556 ± 913 | 20 ± 25 | 0.26 ± 0.07 | 59 ± 11 | 0.53 ± 0.04 |
| 1 yr | 27 | 2177 ± 698 | 31 ± 21 | 0.26 ± 0.05 | 62 ± 9 | 0.55 ± 0.05 |
| 3 yr | 27 | 1481 ± 599 | 54 ± 17 | 0.26 ± 0.05 | 62 ± 12 | 0.56 ± 0.04 |
| 5 yr | 27 | 1192 ± 455 | 62 ± 13 | 0.29 ± 0.08 | 64 ± 9 | 0.56 ± 0.05 |
| 10 yr | 27 | 844 ± 261 | 73 ± 10 | 0.34 ± 0.09 | 54 ± 10 | 0.58 ± 0.05 |
| 15 yr | 27 | 876 ± 232* | 72 ± 9* | 0.37 ± 0.10* | 53 ± 9* | 0.60 ± 0.06† |
There was no significant difference between 10 and 15 years after surgery (paired t tests). Minimum detectable differences (α = 0.05, β = 0.80, n = 27) were: endothelial cell density, 112 cells/mm2; endothelial cell loss, 3.5%; coefficient of variation of cell area, 0.047; hexagonal cells, 6.9%.
Central graft thickness was higher at 15 years after surgery than at 10 years (P = .003).
Endothelial cell loss at 15 years compared to preoperative donor levels was 73 ± 7% for keratoconus (n = 36), 71 ± 8% for Fuchs’ dystrophy (n = 12), 67 ± 14% for pseudophakic corneal edema (n = 8), and 65 ± 24% for aphakic corneal edema (n = 2). The differences in cell loss at 15 years between these four most common preoperative diagnostic groups were not significant (P = .61, Kruskal-Wallis test). The minimum detectable difference between the keratoconus and Fuchs’ dystrophy groups was 9% (α = .05/6, β = .80).
Higher endothelial cell loss was strongly associated with higher preoperative donor endothelial cell density (rs = 0.43, P < .001, n = 66), whereas it was more weakly associated with younger donor age (rs = −0.28, P = .02, n = 66). Endothelial cell loss was not associated with recipient age (rs = −0.19, P = .13, n = 66).
Biexponential Model of Endothelial Cell Loss
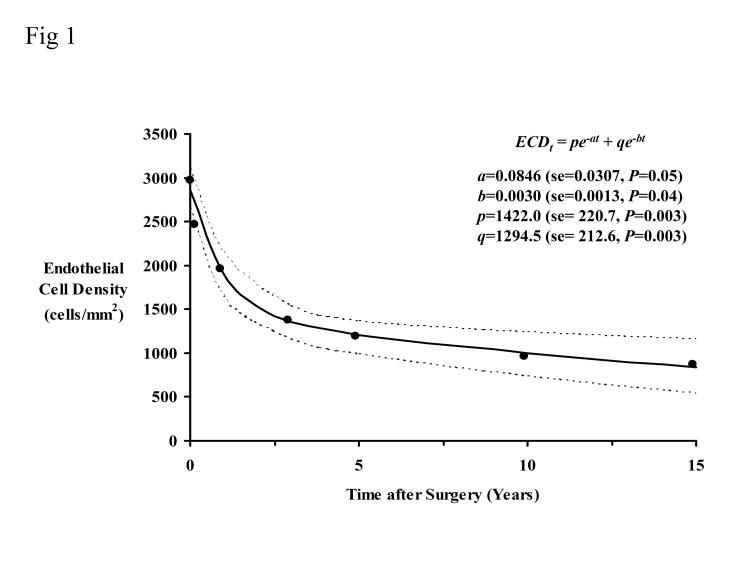
Figure 1 shows the mean endothelial cell densities in Table 3 (all subjects) fitted to the biexponential model (equation 2). The fast half-life was 8.2 months, and the slow half-life was 229.5 months. The residual standard deviation was 123.9 cells/mm2. The rate of cell loss represented by the slow exponential was 3.6% per year.
Figure 1.
Endothelial cell data fitted to a biexponential decay model for all available data through 15 years. The dotted lines represent the 95% confidence interval. The coefficients are shown with their standard error (se) and corresponding P value. The fast half-life was 8.2 months, and the slow half-life was 229.5 months. The residual standard deviation was 123.9 cells/mm2. The rate of cell loss represented by the slow exponential was 3.6% per year.
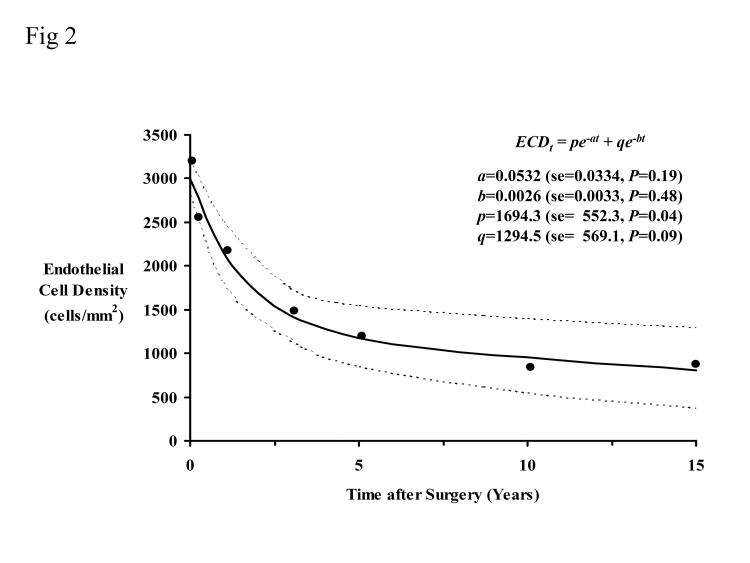
The mean endothelial cell densities in Table 4 (the 27 patients who attended all examinations and had no graft rejection or surgery that could affect the endothelium) can also be fitted to equation 2 (Figure 2). The fast half-life was 13.0 months, and the slow half-life was 266.5 months. The residual standard deviation was 206.0 cells/mm2. The rate of cell loss represented by the slow exponential was 3.1% per year.
Figure 2.
Endothelial cell data fitted to a biexponential decay model for patients who attended every follow-up examination and who had no episode of rejection or surgery that could affect the endothelium (n = 27). The dotted lines represent the 95% confidence interval. The coefficients are shown with their standard error (se) and corresponding P value. The fast half-life was 13.0 months, and the slow half-life was 266.5 months. The residual standard deviation was 206.0 cells/mm2. The rate of cell loss represented by the slow exponential was 3.1% per year.
Glaucoma
Of the 67 patients who returned for the 15-year examination, five developed glaucoma. The onset of glaucoma was within 2 months for one patient (grafted for pseudophakic bullous keratopathy), between 1 and 3 years after surgery for two patients (both were grafted for Fuchs’ dystrophy), between 3 and 5 years after surgery for one patient (grafted for a corneal scar), and between 5 and 10 years after surgery for one patient (grafted for pseudophakic bullous keratopathy). No patient developed glaucoma between 10 and 15 years after surgery.
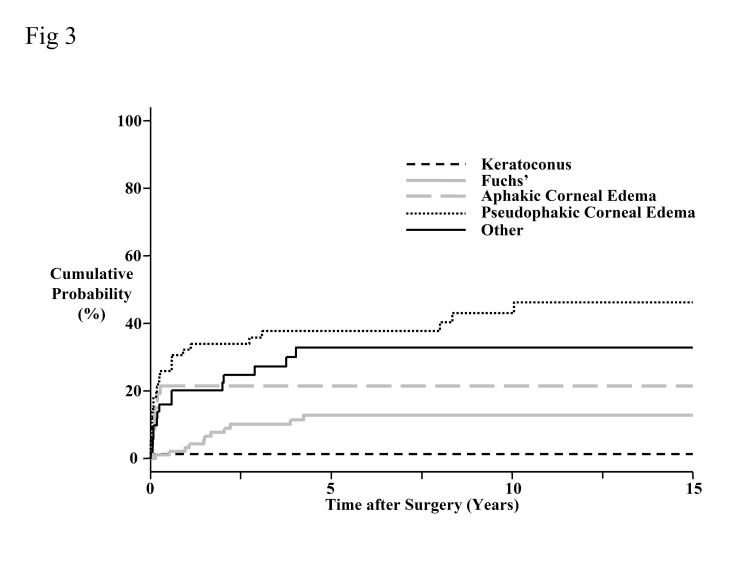
The 15-year cumulative probability for developing glaucoma was 20% (Figure 3). Corneas transplanted for a preoperative diagnosis of keratoconus had a significantly lower cumulative probability of developing glaucoma than corneas transplanted for aphakic or pseudophakic corneal edema (P < .001, Bonferroni-adjusted for 10 comparisons). Similarly, corneas transplanted for Fuchs’ dystrophy had a lower cumulative probability of developing glaucoma than corneas transplanted for pseudophakic corneal edema (P < .001, Bonferroni-adjusted for 10 comparisons).
Figure 3.
Cumulative probability of developing glaucoma by preoperative diagnosis. The overall probability of developing glaucoma at 15 years was 20%. Corneas transplanted for a preoperative diagnosis of keratoconus had a significantly lower cumulative probability of developing glaucoma than corneas transplanted for aphakic or pseudophakic corneal edema (P < .001, Bonferroni-adjusted for 10 comparisons), and corneas transplanted for Fuchs’ dystrophy had a lower cumulative probability of developing glaucoma than corneas transplanted for pseudophakic corneal edema (P < .001, Bonferroni-adjusted for 10 comparisons).
Graft Rejection
Eighteen patients who returned for the 15-year examination had at least one episode of graft rejection. The initial rejection episode was within 1 year of surgery in eight patients, between 1 and 3 years after surgery in five patients, and between 5 and 10 years after surgery in three patients. Only two patients had an initial episode of graft rejection between 10 and 15 years after surgery, and both were endothelial rejection. Of the 18 patients, eight had a preoperative diagnosis of keratoconus, six had Fuchs’ dystrophy, two had pseudophakic bullous keratopathy, and two had corneal scars (one was herpetic).
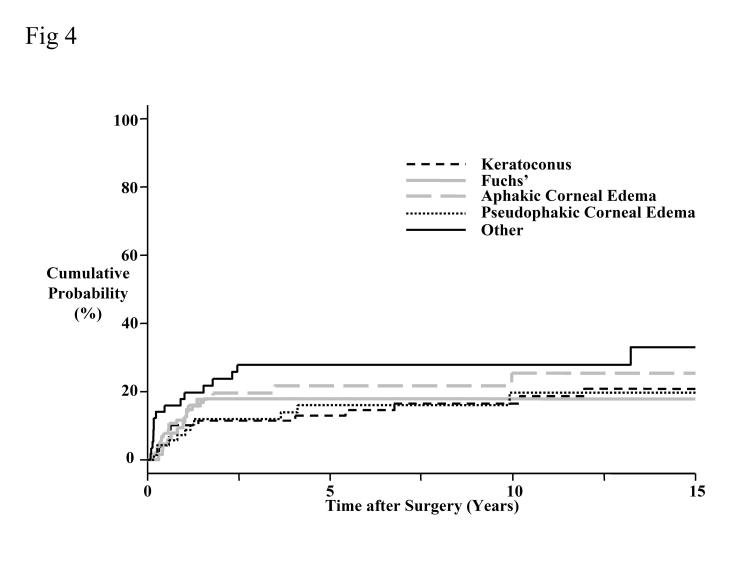
The 15-year cumulative probability for developing graft rejection was 23% (Figure 4). The cumulative probability of developing graft rejection did not differ among preoperative diagnoses (P > .05, Bonferroni-adjusted for 10 comparisons).
Figure 4.
Cumulative probability of developing graft rejection by preoperative diagnosis. The overall probability of developing graft rejection at 15 years was 23%. The cumulative probability of developing graft rejection did not differ among preoperative diagnoses (P > .05, Bonferroni-adjusted for 10 comparisons).
Graft Failure
Eight patients developed graft failure between 10 and 15 years after keratoplasty. Six had late endothelial failure (preoperative diagnosis was Fuchs’ dystrophy in four patients, keratoconus in one, and Chandler’s syndrome in one), one failed from superficial scarring (grafted for keratoconus), and one failed because of glaucoma (grafted for aphakic bullous keratopathy). Late endothelial failure was the leading cause of graft failure by 15 years after keratoplasty, responsible for 29% of all known failures (Table 5).
Table 5.
Reasons for graft failure in the first 15 years after penetrating keratoplasty
| Reason | No. (%) |
|---|---|
| Late endothelial failure* | 22† (29) |
| Irreversible rejection | 19 (25) |
| Primary donor failure | 11 (14) |
| Infection | 8 (11) |
| Epithelial downgrowth | 2 (3) |
| Pupillary block | 2 (3) |
| Herpes simplex virus | 1 (1) |
| Others | 11 (14) |
| Total | 76‡ (100) |
Late endothelial failure was defined as a gradual decrease in central graft clarity of unknown etiology, unresponsive to corticosteroid therapy, and not associated with a recent episode of graft rejection.
Ten of 11 graft failures between 5 and 10 years after surgery, and six of eight graft failures between 10 and 15 years after surgery, were caused by late endothelial failure.
The total number of known graft failures from the entire series over the first 15 years.
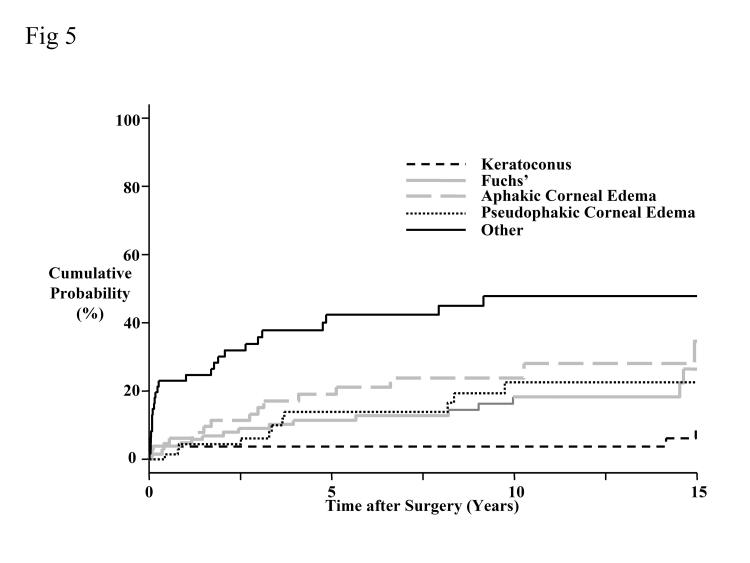
The 15-year cumulative probability for developing overall graft failure was 28% (Figure 5). Corneas transplanted for keratoconus had a lower cumulative probability of failing compared with corneas transplanted for Fuchs’ dystrophy (P = .05, Bonferroni-adjusted for 10 comparisons) or for aphakic corneal edema (P = .006, Bonferroni-adjusted for 10 comparisons). Overall graft failure was not related to preoperative donor endothelial cell density (P = .82) or to recipient age (P = .09), but donor age was related to overall graft failure (P = .03, univariate analysis). Donor age was still related to overall graft failure after using multivariate models to adjust for the effect of preoperative donor endothelial cell density (P = .01) or for recipient age (P = .04). A 10-year increase in donor age increased the risk of failure by 1.2, after adjusting for either preoperative donor endothelial cell density (95% confidence interval [CI], 1.1 to 1.4) or for recipient age (95% CI, 1.0 to 1.3).
Figure 5.
Cumulative probability of developing overall graft failure by preoperative diagnosis. The overall probability of developing graft failure at 15 years was 28%. Corneas transplanted for keratoconus had a lower cumulative probability of failing compared to corneas transplanted for Fuchs’ dystrophy (P = .05, Bonferroni-adjusted for 10 comparisons) or for aphakic corneal edema (P = .006, Bonferroni-adjusted for 10 comparisons).
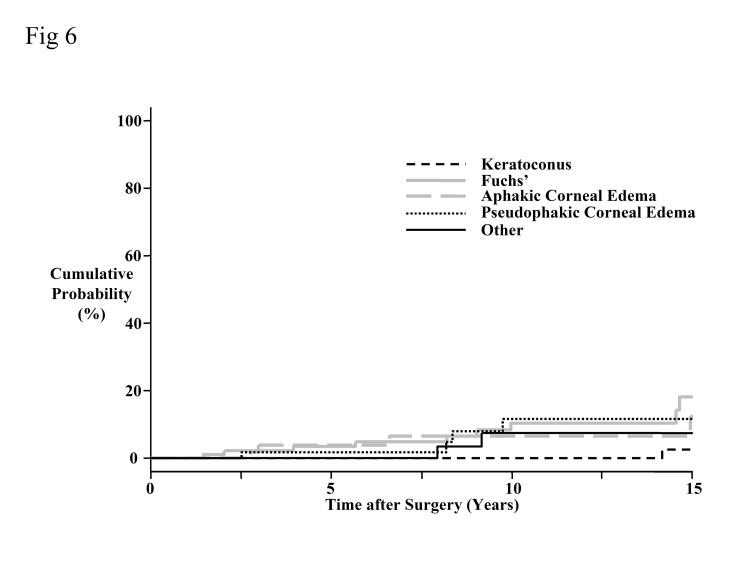
The 15-year cumulative probability for developing late endothelial failure was 12% (Figure 6). Corneas transplanted for keratoconus had a lower cumulative probability of developing late endothelial failure compared to corneas transplanted for Fuchs’ dystrophy (P = .03, Bonferroni-adjusted for 10 comparisons). However, a multivariate analysis showed that this difference was not significant after adjusting for the recipient age (P = .15). Late endothelial failure was not related to donor age (P = .20). Preoperative donor endothelial cell density was related to late endothelial failure (P = .03), with a decrease in preoperative donor endothelial cell density of 500 cells/mm2 increasing the risk of late endothelial failure by 1.6 (95% CI, 1.0 to 2.4). Recipient age was also related to late endothelial failure (P = .02), with a 10-year increase in recipient age increasing the risk of late endothelial failure by 1.4 (95% CI, 1.1 to 1.9).
Figure 6.
Cumulative probability of developing late endothelial failure by preoperative diagnosis. The overall probability of developing late endothelial failure at 15 years was 12%. Corneas transplanted for keratoconus had a lower cumulative probability of developing late endothelial failure compared to corneas transplanted for Fuchs’ dystrophy (P = .03, Bonferroni-adjusted for 10 comparisons).
DISCUSSION
From the original 394 patients in this cohort who underwent penetrating keratoplasty by the same surgeon, 67 attended for a 15-year follow-up examination. Although the 67 patients represent only 30% of the cohort presumed alive and without graft failure, these data are valuable in the long-term prospective evaluation of the corneal endothelium and outcomes after penetrating keratoplasty, and they represent the largest such cohort in the literature. Although the patients who did not return at 15 years had similar preoperative characteristics to the original cohort, this loss to follow-up may have introduced bias, and because our results and conclusions are based on only 30% of the surviving grafts, our data should be interpreted with caution. The complicated follow-up data published at 10 years after surgery3 (equivalent to Table 2 in the present study) were found to contain small inaccuracies because patients with graft failure who subsequently died were often counted twice instead of once when reducing the number of grafts available for analysis from the original cohort. These small inaccuracies were insignificant and have been corrected.
We did not detect differences in any of the endothelial cell variables between 10 and 15 years after surgery, suggesting relative stability of the corneal endothelium compared to the first 10 years after surgery. However, the wide variation in parameters between grafts and our limited sample size at 15 years after surgery are likely to prevent us from detecting small changes in these parameters.
Despite our endothelial data suggesting relative stability between 10 and 15 years after surgery, we were able to detect a continuing increase in central graft thickness. The increase in corneal thickness between 5 and 10 years after surgery can be attributed to the decrease in endothelial cell density and the decreased ability of the endothelium to dehydrate the corneal stroma.3 Although a small, undetected decrease in endothelial cell density could be responsible for the increase in corneal thickness between 10 and 15 years, other mechanisms could also contribute. For example, a loss of the number or function of pump sites in each endothelial cell or chronic changes in the glycosaminoglycan composition of the stroma could increase the hydration of the cornea. This increase in corneal thickness is unlikely to be related to endothelial permeability, which decreases in long-term grafts and would tend to thin the cornea.7,8
Uncomplicated grafts lost endothelial cells at a rate of −1.0 ± 5.4% per year (n = 27) from 10 to 15 years after keratoplasty, similar to the rate for all grafts of 0.2 ± 5.7% per year (n = 54). This compares to a rate of 7.8% per year from 3 to 5 years after keratoplasty2 and 4.2% per year from 5 to 10 years after keratoplasty.3 Although the present study suggests that the rate of endothelial cell loss from 10 to 15 years after surgery is similar to that of normal adult corneas that have not undergone surgery (0.6 ± 0.5% per year9), this may not be a valid conclusion given the wide variation in the rate of cell loss between 10 and 15 years after surgery. Zacks and coworkers studied grafts between 15 and 45 years after surgery, and endothelial cell loss rate was 2.6% per year beyond 15 years.3,10 The large standard deviation in our data demonstrates that individual grafts vary widely from the mean.
High rates of endothelial cell loss have been demonstrated early after penetrating keratoplasty2 and cataract extraction.11 However, the rate of endothelial cell loss after cataract extraction plateaus at 2.5% per year within a year after surgery,11 whereas there is a continued higher rate of cell loss to 10 years after penetrating keratoplasty.3 Armitage and associates5 demonstrated that the changes in endothelial cell density after cataract surgery and penetrating keratoplasty were reasonably approximated by a biexponential model that yielded half-lives and cell loss rates of the rapid and slow components. The penetrating keratoplasty data in Armitage’s study was from the same cohort as the present study, but the 15- and 20-year data were incomplete because not all patients had reached that length of follow-up. We present the biexponential decay model fitted to all the data available from this cohort through 15 years, and the half-lives are similar to those calculated by Armitage and associates. We applied the same model to the data from the 27 patients who attended all examinations to 15 years and had no rejection episodes or further surgery affecting the endothelium. The cell loss rate of 2.4% per year for the late, slow component was similar to the 2.5% per year found after cataract surgery, supporting similar mechanisms of chronic endothelial cell loss after cataract surgery and penetrating keratoplasty.5
In an earlier study, we proposed that the chronic cell loss rate of 2.6% per year found by Zacks and coworkers10 in long-term corneal transplants may represent a stable rate attained years after intraocular surgery.2,3 Although the slow component of the biexponential model (2.4% per year) supports this hypothesis, the actual findings indicate that the cell loss rate in corneas transplanted more than 10 years ago is considerably lower and may be similar to that of normal corneas. In the 54 patients with examinations at both 10 and 15 years, the annual cell loss rate for the interval was 0.2 ± 5.7% (95% CI, −1.3% to 1.7%). For the 27 grafts with no rejection episodes or further surgery affecting the endothelium, the annual cell loss rate from 10 to 15 years postoperative was −1.0 ± 5.4% (95% CI, −3.1% to 1.1%). The chronic annual cell loss rate of 2.4% to 2.6% hypothesized above is well outside the confidence intervals of our findings, whereas the normal cell loss rate of 0.6% per year9 is not.
Endothelial cell loss at 15 years appeared lower in the grafts transplanted for aphakic corneal edema than in those transplanted for keratoconus, Fuchs’ dystrophy, or pseudophakic corneal edema, when compared to preoperative levels. With our limited sample size, however, we were unable to demonstrate a significant difference at 15 years between diagnoses, although we did demonstrate that endothelial cell loss was lowest in the aphakic corneal edema group at 10 years after keratoplasty.3 The highest endothelial cell losses in our study were in grafts for keratoconus. This contradicts other studies,12,13 which have found that endothelial cell loss is least after penetrating keratoplasty for keratoconus, and higher for grafts for Fuchs’ dystrophy or corneal edema. These studies suggest that central endothelial cell density decreases because of peripheral migration of endothelial cells to areas of abnormal host endothelium.12,13 Our results do not support this hypothesis. We have previously shown that graft rejection was lowest for the keratoconus group,3 and thus the high endothelial cell loss for grafts in keratoconus patients cannot be explained by classic graft rejection. Chronic subclinical innate processes between the donor endothelium and the healthy host endothelium of young recipients might account for higher endothelial cell loss in keratoconus patients.
The 15-year cumulative rate of new glaucoma after penetrating keratoplasty was 20%, which is similar to other published rates of 15% to 18%.14,15 None of the patients returning for a 15-year evaluation had developed glaucoma between 10 and 15 years after keratoplasty. The highest risk of developing glaucoma after keratoplasty is within the first 5 years.2,3
The cumulative probability of graft rejection at 15 years after keratoplasty was 23% in our study and did not differ between preoperative diagnoses. Although most episodes of rejection were within the first few years, two patients had an initial episode of graft rejection between 10 and 15 years after keratoplasty. Our cumulative probability is lower than other published rates16,17 and may reflect the low proportion of high-risk grafts in our cohort.
The graft failure rate at 15 years after keratoplasty was 28% and was lowest for corneas transplanted for keratoconus. A multivariate analysis of our data at 15 years continues to show that increased donor age increases the risk of graft failure overall. Eight grafts failed between 10 and 15 years after surgery, and six of these were because of late endothelial failure. Late endothelial failure became the predominant cause of graft failure (16/19) after 5 postoperative years. Although late endothelial failure was not related to donor age, it was associated with lower preoperative donor endothelial cell densities and higher recipient ages. Grafts destined to develop late endothelial failure begin with a lower preoperative endothelial cell density and lose more endothelial cells during preservation and transplantation than grafts that do not develop late endothelial failure.18
In summary, we have followed a cohort of patients to 15 years after penetrating keratoplasty and shown that the rate of endothelial cell loss from 10 to 15 years after surgery may be similar to that of normal corneas, although individual grafts vary widely from the mean. The major clinical complication between 10 and 15 years after keratoplasty is graft failure, which is predominantly caused by late endothelial failure.
DISCUSSION
Dr John D. Gottsch
From 1976 to 1986, Dr Bourne performed 500 consecutive corneal grafts and followed them for endothelial cell density, corneal pachymetry, graft rejection, graft failure and the development of glaucoma. Patients were evaluated at two months and at one, three, five, 10, and now remarkably 15 years later. A wealth of information on long-term graft survival has been obtained and a series of important papers have been published. We are much indebted to Dr Bourne for his foresight in initiating this study and his perseverance in shepherding this research of an important anterior segment procedure that has remained highly relevant through these many years.
The overall picture of endothelial cell density over the entire 15 years is one of a rapid rate of cell loss for the first five years, then slowing to about half that from five to 10 years. At 15 years, the rate of endothelial cell loss appears to slow further and approximates the normal rate of cell loss in eyes without surgery. The mathematical model proposed by Dr Bourne to best fit the 15-year data is a biexponential decay formula that describes a rapid initial rate of cell loss followed by a slower component that persists for years. It is assumed by this model that the slow component can be extrapolated and with the further decrease in the rate of endothelial cell density loss, there hopefully would be a better prognosis for graft survival over the next five years. Could these older grafts at last be stabilizing and are likely to last out the life expectancy of the recipient?
A look at another parameter used quantitatively to measure these grafts during the course of the study suggests that there are ominous storm clouds on the horizon. Pachymetry is an important measure of the health of a corneal graft. As elegantly described by Maurice, in normal eyes the ordered array of collagen fibrils provides for the passage of light rays through the cornea without backscatter. Thus there is corneal clarity and the potential for good vision. However, swelling disrupts the fibril lattice, and then increased light scatter will result, limiting vision. Dr Bourne reports that there was a significant increase in pachymetry measurements from 10 to 15 years. If pachymetry over the 15 years is graphed, corneal thickness does not plateau, as does endothelial cell density loss. It appears that the rate of increase in pachymetry from 10 to 15 years, if not accelerating, is at least remaining constant.
Thus in five years, if enough patients survive and are available for follow up, Dr Bourne could present us with another important paper on the natural history of grafts with marginal endothelial cell counts and increasing endothelial dysfunction. I am curious as to what Dr Bourne thinks is likely to happen over the next five years as he further studies these important corneal transplants for endothelial cell densities and pachymetry.
Dr Dan B. Jones
The Australian Graft Registry determined that the greatest predictor of corneal graft failure is prior grafting. Have you looked at that subset of patients who have prior grafting and can you tell us about their pattern of cell loss? In following the patients with graft failure, did you see any pattern of more accelerated cell loss prior to the event? Was contact lens wear a risk factor for endothelial cell loss and failure?
Dr Jules L. Baum
In the early ‘70s, there was an anecdotal paper published looking at a few patients who had corneal transplant in keratoconus and then developed an acute corneal graft rejection. Over six months, in the absence of another transplant, there was spontaneous clearing of the graft in some. This observation suggested that, if you a had healthy recipient endothelium as you might expect in keratoconus, as opposed to someone with Fuchs’ dystrophy, the endothelial cells of the recipient would grow in and then deturgess the cornea. Your data, if you dissect out the keratoconus patients, suggests that’s not the case.
Dr Ivan R. Schwab
Over 25 percent of the patients died. That is to be expected in an elderly cohort, but I would wonder if, like cataract extraction, there is a more rapid rate of death for those patients who have corneal transplantation as compared to an age- and sex-matched control group.
Dr Woody S. Van Meter
Since specular microscopy was done presumably before preservation on the donor cornea, was there any change in your technique for doing specular microscopy that might account for any shift in numbers over the course of 15 years? There was a natural skew in the data if you look at the number of patients that are represented by diagnosis in your final cohort that made it through for 15 years. The majority cohort had keratoconus, and the second most common cohort had Fuchs’ dystrophy, and the third most common cohort had aphakic and pseudophakic corneal edema. This of course would be expected because keratoconus patients tend to be younger and so that data may reflect the age difference in your patients at the time of graft. Do you think we need to do anything different for younger patients versus older patients based on your data?
Dr John T. Flynn
When you compare the endothelial cell counts of normal control subjects with the endothelial cell counts of your graft at 10–15 years post graft, did the normal controls also have an increase in corneal thickness accompanying whatever changes they had in their corneal cell count? If you can’t find it in the numbers of endothelial cells, is there anything about the morphology of the endothelial cells that might be giving you a clue as to what’s happening to cause that thickening of the cornea?
Dr Jacob T. Wilensky
It’s been a classic teaching that high pressure is inimical to the health of corneal grafts. Was there anything in your data that suggests that the 28 percent of patients who developed glaucoma might have a higher rate of failure than the non-glaucomatous eyes?
Dr Sanjay V. Patel
I’d like to thank Dr Gottsch for reviewing the paper and discussing it. There are several questions to answer. Over the next five years, I think we will see stabilization of the endothelial cell count, although individual grafts vary quite widely from the mean of all the grafts. The overall rate of cell loss over a long period is now approaching that of normal corneas but will be different for individual grafts. Some will develop late endothelial failure.
We have not performed a subanalysis to determine whether prior grafting affected cell loss. The effects of contact lenses were not analyzed for this study. Concerning the question about keratoconus and acute prolonged rejection, a European study suggests there is greater cell loss in Fuchs’ dystrophy corneas than in keratoconus corneas. It’s proposed that there is a migration hypothesis of endothelial cells from the healthy donor onto the abnormal host rim in Fuchs’ dystrophy. Our data do not support that hypothesis. Rejection in the keratoconus group is actually low in our group, so we don’t think that rejection is why keratoconus grafts lose cells at a similar rate to Fuchs’ dystrophy grafts. It’s possible that there are some subclinical innate processes going on which we have not identified, possibly because these recipients in keratoconus are younger and actually have healthier tissues.
Twenty-five percent of our patients died during the 15-year period. Our study did not assess a control group for a comparison of risks of death. There have been some changes in the laboratory techniques of specular microscopy, but we have made the appropriate adjustments to the calculations. Grafts for younger recipients would be expected to have a longer survival than grafts for older recipients. Our data suggest that this could be achieved by using graft tissue from younger donors.
Why does corneal thickness continue to increase despite no evident decrease in cell density? I wonder if there are cellular or extracellular matrix changes in the stroma, such as changes in glycosaminoglycan composition, that may account for an increase in corneal thickness. We have not looked at endothelial cell or stromal morphology to determine a reason for increasing cell thickness at this time.
Footnotes
Supported in part by grant EY 02037 from the National Institutes of Health (W.M.B.) and by Research to Prevent Blindness, Inc, New York, New York.
REFERENCES
- 1.Olson RJ. Corneal transplantation techniques. In: Kaufman HE, Barron BA, McDonald MB, et al, eds. The Cornea. New York: Churchill Livingston; 1988:743–784.
- 2.Bourne WM, Hodge DO, Nelson LR. Corneal endothelium five years after transplantation. Am J Ophthalmol. 1994;118:185–196. doi: 10.1016/s0002-9394(14)72898-3. [DOI] [PubMed] [Google Scholar]
- 3.Ing JJ, Ing HH, Nelson LR, et al. Ten-year postoperative results of penetrating keratoplasty. Ophthalmology. 1998;105:1855–1865. doi: 10.1016/S0161-6420(98)91030-2. [DOI] [PubMed] [Google Scholar]
- 4.Bourne WM. Morphologic and functional evaluation of the endothelium of transplanted human corneas. Trans Am Ophthalmol Soc. 1983;81:403–450. [PMC free article] [PubMed] [Google Scholar]
- 5.Armitage WJ, Dick AD, Bourne WM. Predicting endothelial cell loss and long-term corneal graft survival. Invest Ophthalmol Vis Sci. 2003;44:3326–3331. doi: 10.1167/iovs.02-1255. [DOI] [PubMed] [Google Scholar]
- 6.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1985;53:457–481. [Google Scholar]
- 7.Bourne WM. Functional measurements on the enlarged endothelial cells of corneal transplants. Trans Am Ophthalmol Soc 1995;93:65–79; discussion 79–82. [PMC free article] [PubMed]
- 8.Bourne WM. Clinical estimation of corneal endothelial pump function. Trans Am Ophthalmol Soc 1998;96:229–239; discussion 239–242. [PMC free article] [PubMed]
- 9.Bourne WM, Nelson LR, Hodge DO. Central corneal endothelial cell changes over a ten-year period. Invest Ophthalmol Vis Sci. 1997;38:779–782. [PubMed] [Google Scholar]
- 10.Zacks CM, Abbott RL, Fine M. Long-term changes in corneal endothelium after keratoplasty. A follow-up study. Cornea. 1990;9:92–97. [PubMed] [Google Scholar]
- 11.Bourne WM, Nelson LR, Hodge DO. Continued endothelial cell loss ten years after lens implantation. Ophthalmology 1994;101:1014–1022; discussion 1022–1023. [DOI] [PubMed]
- 12.Reinhard T, Bohringer D, Huschen D, et al. Chronic endothelial cell loss of the graft after penetrating keratoplasty: influence of endothelial cell migration from graft to host. Klin Monatsbl Augenheilkd. 2002;219:410–416. doi: 10.1055/s-2002-32876. [DOI] [PubMed] [Google Scholar]
- 13.Langenbucher A, Seitz B, Nguyen NX, et al. Corneal endothelial cell loss after nonmechanical penetrating keratoplasty depends on diagnosis: a regression analysis. Graefes Arch Clin Exp Ophthalmol. 2002;240:387–392. doi: 10.1007/s00417-002-0470-2. [DOI] [PubMed] [Google Scholar]
- 14.Foulks GN. Glaucoma associated with penetrating keratoplasty. Ophthalmology. 1987;94:871–874. doi: 10.1016/s0161-6420(87)33542-0. [DOI] [PubMed] [Google Scholar]
- 15.Simmons RB, Stern RA, Teekhasaenee C, et al. Elevated intraocular pressure following penetrating keratoplasty. Trans Am Ophthalmol Soc 1989;87:79–91; discussion 91–93. [PMC free article] [PubMed]
- 16.Alldredge OC, Krachmer JH. Clinical types of corneal transplant rejection. Their manifestations, frequency, preoperative correlates, and treatment. Arch Ophthalmol. 1981;99:599–604. doi: 10.1001/archopht.1981.03930010599002. [DOI] [PubMed] [Google Scholar]
- 17.Pleyer U, Steuhl KP, Weidle EG, et al. Corneal graft rejection: incidence, manifestation, and interaction of clinical subtypes. Transplant Proc. 1992;24:2034–2037. [PubMed] [Google Scholar]
- 18.Nishimura JK, Hodge DO, Bourne WM. Initial endothelial cell density and chronic endothelial cell loss rate in corneal transplants with late endothelial failure. Ophthalmology. 1999;106:1962–1965. doi: 10.1016/S0161-6420(99)90409-8. [DOI] [PubMed] [Google Scholar]