Abstract
All Haemophilus ducreyi strains examined contain a lipooligosaccharide (LOS) consisting of a single but variable branch oligosaccharide that emanates off the first heptose (Hep-I) of a conserved Hep3-phosphorylated 3-deoxy-d-manno-octulosonic acid-lipid A core. In a previous report, identification of tandem genes, lbgA and lbgB, that are involved in LOS biosynthesis was described (Stevens et al., Infect. Immun. 65:651-660, 1997). In a separate study, the same gene cluster was identified and the lbgB (losB) gene was found to be required for transfer of the second sugar, d-glycero-d-manno-heptose (dd-Hep), of the major branch structure (Gibson et al., J. Bacteriol. 179:5062-5071, 1997). In this study, we identified the function of the neighboring upstream gene, lbgA, and found that it is necessary for addition of the third sugar in the dominant oligosaccharide branch, a galactose-linked β1→4, to the dd-Hep. LOS from an lbgA mutant and an lbgAB double mutant were isolated and were characterized by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, carbohydrate analysis, mass spectrometry, and nuclear magnetic resonance spectroscopy. The results showed that the mutant strains synthesize truncated LOS glycoforms that terminate after addition of the first glucose (lbgAB) or the disaccharide dd-Hepα1→6Glcβ1 (lbgA) that is attached to the heptose core. Both mutants show a significant reduction in the ability to adhere to human keratinocytes. Although minor differences were observed after two-dimensional gel electrophoresis of total proteins from the wild-type and mutant strains, the expression levels of the vast majority of proteins were unchanged, suggesting that the differences in adherence and invasion are due to differences in LOS. These studies add to the mounting evidence for a role of full-length LOS structures in the pathophysiology of H. ducreyi infection.
Haemophilus ducreyi is the causative agent of the genital ulcer disease chancroid. This sexually transmitted disease is prevalent in many developing countries and has been linked to the transmission of the human immunodeficiency virus (24, 48). Although there are few reported cases each year in the United States, outbreaks occur occasionally in urban areas (32, 46). Furthermore, chancroid may be greatly underreported due to inadequate methods of detection (15, 39).
Although the virulence mechanisms of H. ducreyi are not well understood, several factors have been identified that may play a role in the organism's pathogenicity. Two different cytotoxins that are toxic to human foreskin fibroblasts and epithelial cells have been identified and characterized (3, 11, 33, 34). Also, several studies have shown that the lipooligosaccharide (LOS) of H. ducreyi causes ulcers in rabbits and mice (10, 25, 47). In addition, LOS plays a role in the adherence of H. ducreyi to host cells (2, 16). One interesting aspect of the LOS from H. ducreyi, as well as other gram-negative mucosal pathogens, such as Haemophilus influenzae, Neisseria meningitidis, and Neisseria gonorrhoeae, is that some of the LOS glycoforms produced by these organisms mimic human antigens (5, 9, 27, 28). Molecular mimicry may allow these organisms to evade the host immune system or enable adherence through host cell receptors.
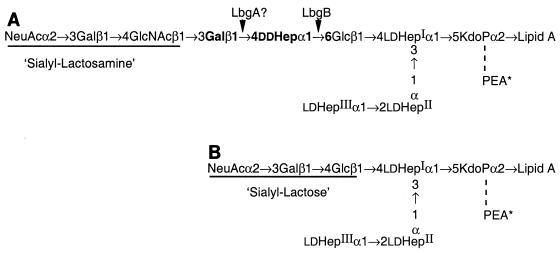
The structures of the LOS from several H. ducreyi strains have been reported by us and other workers (1, 8, 18, 29, 31, 40, 41). These structures have a common core structure consisting of three l-glycero-d-manno-heptose (ld-Hep) moieties linked to phosphorylated 3-deoxy-d-manno-octulosonic acid (Kdo) and substituted with a glucose on the first ld-Hep (Fig. 1). The glucose that is β1→4 linked to the first Hep residue in this core appears to be the common attachment point for an oligosaccharide branch extension that is assembled via two major, but apparently exclusive, biosynthetic pathways. In the vast majority of strains, four additional sugars are added to this branch glucose to form a pentasaccharide, Galβ1→4GlcNAcβ1→3Galβ1→4ddHepα1→6Glcβ1-, which is a lacto-N-neotetraose structure interrupted by a d-glycero-d-manno-heptose (dd-Hep). The second pathway bypasses this unusual dd-Hep and adds only a single galactose to form lactose, Galβ1→4Glc-, as typified by strains ITM 4747 and CCUG 4438 (41). LOS structures that terminate in N-acetyllactosamine or lactose are also partially substituted with sialic acid (1, 29). In one instance, African strain 33921, which was reported to be deficient in the ability to adhere to keratinocytes (7), was found to contain an oligosaccharide branch that extended this lactose disaccharide by the addition of N-acetylglucosamine (i.e., GlcNAcβ1→3Galβ1→4Glc-) that was not modified by sialic acid (29).
FIG. 1.
Structures of the two major sialylated LOS glycoforms from H. ducreyi. (A) LOS with pentasaccharide branch terminting in N-acetyllactosamine that is partially substituted with alpha-2,3-linked sialic acid. (B) LOS with disaccharide lactose branch that is partially substituted with alpha-2,3-linked sialic acid.
In a previous study Gibson et al. identified a gene that encodes the dd-heptosyltransferase, losB (lbgB), by using a mutant generated by transposon mutagenesis (16). This mutant was shown to be compromised in the ability to adhere to and invade human keratinocytes in vitro. It was also found to be unstable, with some reversion to wild-type LOS structures. In another study, Stevens et al. (44) identified a stable mutation in a neighboring upstream gene, lbgA (losA), that also resulted in an altered LOS phenotype. However, the function of this gene and the LOS structures from the stable, isogenic lbgA mutant were not determined. In this study, we determined the structures of the LOS from the stable lbgA mutant and a double mutant defective in both genes (lbgAB), and we identified the lbgA gene as a gene encoding a galactosyltransferase. The sequence of the lbgAB (losAB) gene cluster was deposited previously in the GenBank database under accession numbers U58147 and AF004712.
MATERIALS AND METHODS
Materials.
Anhydrous hydrazine, Salmonella enterica serovar Typhimurium TV 119 Ra lipopolysaccharide, 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS) (electrophoresis grade), thiourea, dithiothreitol (DTT) (electrophoresis grade), and iodoacetamide (electrophoresis grade) were purchased from Sigma (St. Louis, Mo.). 2,5-Dihydroxybenzoic acid was purchased from Aldrich (St. Louis, Mo.) and was recrystallized from water before use. An acrylamide/bis-acrylamide solution (40%, wt/vol; ratio of monomer to cross-linker, 37.5:1), electrophoresis quality Tris, glycine, and sodium dodecyl sulfate (SDS) were purchased from Bio-Rad (Hercules, Calif.). Trifluoroacetic acid was purchased from Pierce (Rockford, Ill.). IPG strips (width, 5 mm; length, 18 cm; pH 3 to 10 linear), urea, pharmalyte (pH 3 to 10), and glycerol (87%, wt/wt) were purchased from Pharmacia Biotech (Uppsala, Sweden).
H. ducreyi strains and growth conditions.
The H. ducreyi strains used in this study are wild-type strain 35000 isolated from the Winnipeg outbreak (19), the human-passaged variant 35000HP (42), and the two isogenic mutants of 35000, 35000.3 (lbgAB double mutant) and 35000.4 (lbgA mutant) (44). Bacteria were grown on chocolate agar or in brain heart infusion broth as previously described (33).
Assay of adherence to culture keratinocytes.
HaCaT cells were maintained in defined keratinocyte media (Life Technologies, Inc.) supplemented with 10% fetal bovine serum (Hy-Clone), 5% l-glutamine, and 5% nonessential amino acids (Life Technologies, Inc.). H. ducreyi adherence (percent association) to the HaCaT cells was evaluated by using a modification of the method described by Brentjens et al. (7). Briefly, the HaCaT cells were seeded into 24-well culture plates and allowed to reach confluency at 35°C in 5% CO2. The cells were washed four times with media devoid of serum and antibiotics. H. ducreyi strains to be tested were grown to the early log phase (optical density at 600 nm, 0.2), pelleted, and resuspended in serum-free keratinocyte media. The bacteria were added to the monolayers in triplicate at a multiplicity of infection of 100:1 and incubated for 1 or 2 h at 35°C in 5% CO2. The original inocula of H. ducreyi were verified by serial dilution and colony counting at time zero. After the desired time interval, the monolayers were extensively washed with serum-free media to remove nonadherent organisms, and the monolayers were released by using 0.25% trypsin. Each suspension was serially diluted, and the percent adherence was determined as described previously (7).
Preparation and SDS-polyacrylamide gel electrophoresis (PAGE) analysis of LOS.
LOS was prepared from H. ducreyi cells that were grown overnight in 1 liter of liquid medium (yielding 600 to 700 mg [dry weight] of cells) and extracted by using a modified version of the hot phenol-water procedure (4). The yields of LOS were 0.15 to 0.2% (wt/wt) of the bacterial dry weight. LOS was analyzed by using a 16% polyacrylamide resolving gel with a 4% polyacrylamide stacking gel (14 cm by 16 cm by 0.75 mm) and silver stained as previously described (6). LOS from H. ducreyi strain 35000HP was used as a standard.
Preparation of O-deacylated LOS and free oligosaccharides.
To prepare a water-soluble LOS species amenable to mass spectrometry (MS) analysis, O-acyl groups were removed by treatment with hydrazine (22). Anhydrous hydrazine (100 μl) was added to lyophilized LOS (290 to 370 μg), and O-deacylated LOS was prepared essentially as previously described (6). Oligosaccharides were prepared from LOS (∼600 to 900 μg) after hydrolysis in 1 ml of 1% (vol/vol) acetic acid at 100°C for 2 h. The resulting oligosaccharides were purified by size exclusion chromatography (31). Dephosphorylated oligosaccharides were prepared by dissolving the dried oligosaccharide in 20 μl of 48% HF and leaving it at 4°C for 24 h. The HF was removed under a vacuum by using an NaOH trap. For composition analysis this material was used directly, but for linkage and nuclear magnetic resonance (NMR) analyses, the dephosphorylated oligosaccharides were repurified by size exclusion chromatography.
Composition and linkage analyses.
To determine the monosaccharide composition of the oligosaccharide, dephosphorylated oligosaccharides were hydrolyzed with 100 μl of 2 M trifluoroacetic acid at 100°C for 3 h and analyzed by high-pH anion-exchange chromatography with pulsed amperometric detection by using a Dionex high-performance liquid chromatography system with a CarboPac PA1 column (4 by 250 mm; Dionex) (20). Quantitation was performed by using the response factors from a hydrolysate of an S. enterica serovar Typhimurium Ra dephosphorylated oligosaccharide standard (prepared from commercially available lipopolysaccharide which contained glucose, galactose, N-acetylglucosamine, and ld-Hep at a 2:2:1:3 ratio). The molar ratios obtained were expressed relative to glucose.
Monosaccharide linkages were determined by using a modification of the Levry-Hakomori microscale methylation procedure (26, 35). The partially methylated alditol acetates (PMAA) were analyzed by gas chromatography-MS in both the electron impact and chemical ionization modes as previously described (30). Quantitation was performed by measuring the peak area in the electron impact mode.
NMR spectroscopy.
Dephosphorylated oligosaccharides (<100 μg) were D2O exchanged several times in 99.96% D2O (Sigma) and then dissolved in 260 μl of 99.996% D2O (Cambridge Isotope Laboratories, Andover, Mass.) for NMR studies. The samples were run in Shigemi symmetrical NMR microtubes that were matched with D2O (Shigemi, Inc., Allison Park, Pa.). All 1H NMR spectra were recorded at 500 MHz with a GE Omega spectrometer and referenced to acetone (δ 2.225). Spectra were recorded at 15, 25, and 45°C for periods of time ranging from ∼5 h to overnight.
MS.
O-deacylated LOS was desalted with Dowex 50W-X8 100- to 200-mesh beads (NH4+ form) and analyzed with a Voyager-DE time of flight (TOF) mass spectrometer by using 2,5-dihydroxybenzoic acid as the matrix as previously described (6). Samples were desorbed with a nitrogen laser (337 nm), and the instrument was operated in the negative-ion mode by using an accelerating voltage of 25 kV, a grid voltage of 95%, a guide wire voltage of 0.1%, and a delay time of 150 ns. The instrument was calibrated externally by using bovine insulin β-chain (oxidized) (average [M-H]− = 3,494.9 Da) and angiotensin II (average [M-H]− = 1,045.2 Da).
Dephosphorylated oligosaccharides were analyzed in the positive-ion mode with a QSTAR hybrid quadrupole TOF mass spectrometer (PE Sciex Instruments) equipped with a Protana nanospray ion source. Samples were prepared in a solution containing 43 mM ammonium acetate (pH 4.5), 50% acetonitrile, and H2O, and ∼5 μl of each analyte was deposited into a Protana nanospray tip (medium). MS/MS spectra were acquired with an ion spray voltage of 1,200 V and a quadrupole mass analyzer mass window of 1 m/z unit. Mass spectra were corrected by using a two-point calibration with the singly charged fragment ions (m/z = 187.0719 and 1,285.5449) derived from the doubly charged parent ion (m/z = 785.8427) of the human [Glu1]-fibrinopeptide B.
Two-dimensional gel electrophoresis analysis of H. ducreyi proteins.
Bacteria were harvested from plates, washed in phosphate-buffered saline, lyophilized, and then stored at −20°C until they were used. Lyophilized bacteria were resuspended in 50 μl of water, and sufficient rehydration buffer (7 M urea, 2 M thiourea, 4% CHAPS, 65 mM DTT, Pharmalyte [pH 3 to 10] [1:50]) (37) was added to give a final concentration of 10 mg of dried bacteria per ml. Tubes were shaken for 2 h and centrifuged to remove any precipitate, and the supernatant was stored at −80°C until it was used. Protein concentration was determined with a Bio-Rad Protein Assay Kit II.
The lysate was diluted with rehydration buffer to obtain a solution containing 0.8 mg of protein/400 μl and was added to 18-cm linear pI 3 to 10 IPG strips (Pharmacia). The strips were rehydrated for 16 h, which was followed by isoelectric focusing with a Multiphor II (Pharmacia) at 20°C for 24 h using the following gradient program: step 1, 0.01 h, 500 V, 1 mA, and 7 W; step 2, 5 h, 500 V, 1 mA, and 7 W; step 3, 5 h, 3,500 V, 1 mA, and 7 W; and step 4, 14 h, 3,500 V, 1 mA, and 7 W. The strips were then equilibrated with gentle shaking in two subsequent steps (20 min each) in 5 ml of equilibration buffer (0.05 M Tris-Cl [pH 6.0], 35% glycerol, 1% SDS, trace of bromophenol blue) containing in addition 0.1 mg of DTT in the first step and 0.14 mg of iodoacetamide in the second step. The strips were placed on top of a 1-mm-thick 20- by 20-cm 12% acrylamide nongradient gel (vertical system, Bio-Rad Protean II cell with a two-dimensional conversion kit), and proteins were separated in the second dimension at 10°C until the bromophenol blue dye front reached the bottom of the gel (15 mA per gel, ∼16 h). The gels were stained with colloidal Coomassie blue Fast stain (Zoion, Newton, Mass.) and were scanned and analyzed by using an ImageMaster 2D gel scanner (Pharmacia).
RESULTS
SDS-PAGE analysis of the LOS from the lbgA and lbgAB mutants.
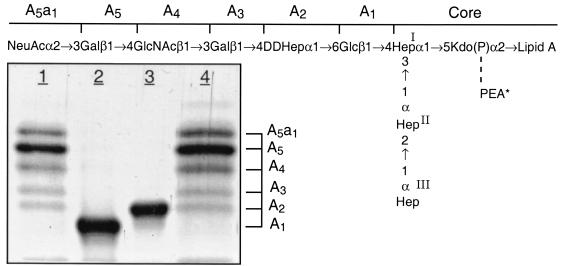
LOS isolated from the lbgA mutant (strain 35000.4), the lbgAB double mutant (strain 35000.3), and parental wild-type strain 35000HP were first analyzed by SDS-PAGE (Fig. 2). We previously assigned identities to the LOS bands obtained by SDS-PAGE for strain 35000HP on the basis of their mobilities and their relative intensities compared to those of O-deacylated LOS glycoforms observed by matrix-assisted laser desorption ionization (MALDI)-MS (6). For example, the major glycoform in strain 35000HP terminates in Gal and is designated A5 because it is the fifth monosaccharide in the branch extending from Hep-I. Both mutants produce LOS that appears to consist of only a single glycoform as determined by SDS-PAGE (Fig. 2, lanes 2 and 3). The LOS from the lbgA mutant has the same mobility as the fastest moving component (A2 glycoform) of the wild-type strain (Fig. 2, lane 3). The LOS from the lbgAB mutant (Fig. 2, lane 2) clearly migrates even faster than the LOS from the lbgA mutant. The distance separating the two bands suggests that the LOS from the lbgAB mutant has one less monosaccharide than the LOS from the lbgA mutant.
FIG. 2.
Silver-stained SDS-polyacrylamide gel of LOS from the lbgAB (lane 2) and lbgA (lane 3) mutants of H. ducreyi strain 35000HP (lanes 1 and 4). The designations on the right (A5a1, A5, etc.) refer to the proposed LOS glycoforms to which the bands are believed to correspond. Only the bottom portion of the gel where the LOS migrated is shown. The phosphate of Kdo can be partially substituted with PEA (asterisk). The structure corresponding to the LOS glycoform designated A1 was determined previously (16).
MS analysis of the O-deacylated LOS and oligosaccharide.
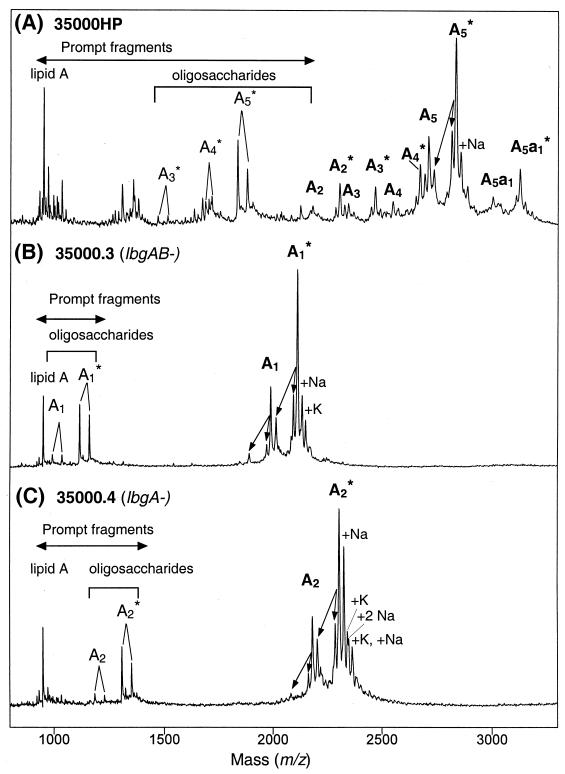
To determine the structure of the LOS from the lbgA and lbgAB mutants, MS and chemical analyses were performed. The LOS from the mutants were analyzed by MALDI-MS after O-deacylation and were compared to the wild-type strain 35000HP O-deacylated LOS (Fig. 3). The spectra of the two mutants are much simpler than the spectrum of the wild-type strain; however, there is clearly more heterogeneity than is evident from SDS-PAGE analysis. This can be explained by partial substitution of the LOS with phosphoethanolamine (PEA) that is not detected by SDS-PAGE, the presence of salt adducts, and fragmentation processes that occur in MALDI-MS experiments (6, 17). Overall, the results are consistent with the LOS from the lbgA mutant containing the A2 glycoform and the lbgAB mutant containing the A1 glycoform (one Hep less than A2); both of these glycoforms exist with no PEA moiety or one PEA moiety (Table 1).
FIG. 3.
Negative-ion MALDI-TOF spectra of O-deacylated LOS from the lbgAB (B) and lbgA (C) mutant strains, as well as parental strain 35000HP (A). Strain 35000HP is a human-passaged isolate of parent strain 35000 and produces an LOS profile similar to that of strain 35000 (6). There were prompt fragments corresponding to cleavage of the oligosaccharide from the O-deacylated lipid A, as well as fragments corresponding to loss of H2O and H3PO4 (indicated by arrows). Two additional fragments arose from each oligosaccharide via loss of CO2 (44 Da) from the terminal Kdo (17). Sodium and potassium adducts, primarily of PEA-containing LOS species, are also present. The singly deprotonated molecular ions (M-H)− are designated (in boldface type) according to the nomenclature shown in Fig. 1. An asterisk indicates addition of PEA. Oligosaccharide prompt fragments arising from the O-deacylated LOS glycoforms are indicated by lightface type. The lipid A prompt fragments observed are diphosphoryl N-diacyl lipid A (O-deacylated lipid A).
TABLE 1.
Masses of O-deacylated LOS from the lbgA and lbgAB mutants
| H. ducreyi strain | Form | Mol wt
|
Relative abundance (%)b | Proposed compositionc | |
|---|---|---|---|---|---|
| Observeda | Calculated | ||||
| 35000.3 (lbgAB) | A1 | 1,990.8 | 1,991.8 | 41 | Hex1 Hep3 Kdo(P)1 lipid A |
| A1∗ | 2,113.7 | 2,114.8 | 100 | Hex1 Hep3 PEA1 Kdo(P)1 lipid A | |
| 35000.4 (lbgA) | A2 | 2,184.3 | 2,184.0 | 45 | Hex1 Hep4 Kdo(P)1 lipid A |
| A2∗ | 2,307.4 | 2,307.0 | 100 | Hex1 Hep4 PEA1 Kdo(P)1 lipid A | |
All molecular weights for O-deacylated LOS were determined from the MALDI-MS data (Fig. 3) as average values based on singly deprotonated charged molecular ions, (M-H)−.
Relative abundance was determined from peak heights of the singly charged molecular ions.
After O-deacylation, the lipid A moiety is converted into diphosphoryl diacyl lipid A containing two N-linked β-hydroxymyristic acid chains with an average Mr of 953.0. Kdo(P), phosphorylated Kdo.
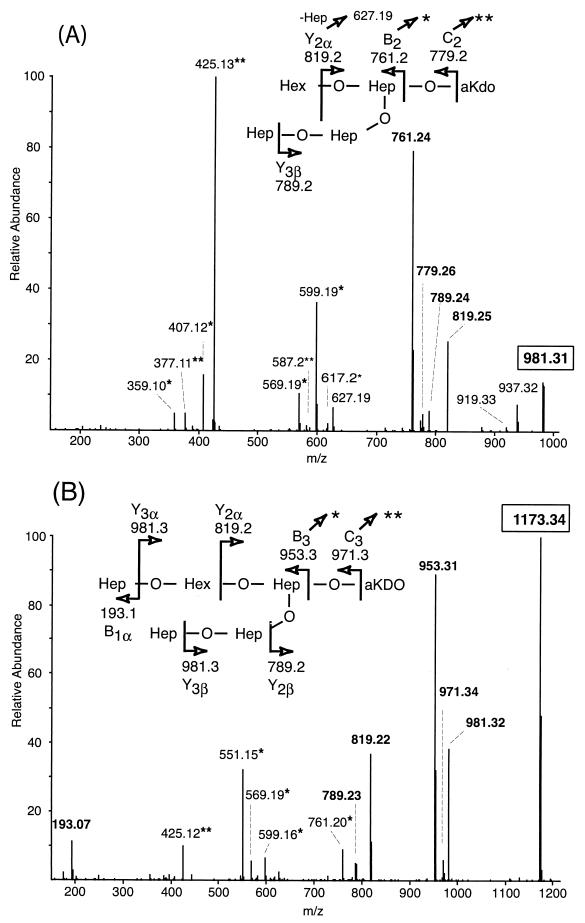
The sequential orders of sugars in the LOS from the mutant strains were determined by examining the corresponding dephosphorylated oligosaccharides by tandem MS (Fig. 4). Overall, collision-induced dissociation of the singly charged sodiated parent ions yielded a collection of B, C, and Y type product ions characteristic of branched structures (12). Although the MS/MS spectra of oligosaccharides from the mutants share several product ions, the ions unique to each structure were most useful for deducing the sequences of sugars added to lipid A. Consistent with the lbgAB mutant LOS being the A1 glycoform, product ions lacking a Hep (m/z 789.2) or hexose (Hex) (m/z 819.2) in one of two distinct nonreducing termini were observed (Fig. 4A). Likewise, consistent with the lbgA mutant LOS being the A2 glycoform, only a loss of Hep (m/z 981.3) was observed, indicating that both nonreducing termini are Hep residues (Fig. 4B). The observation of the B1 ion (m/z 193.1) arising from the oligosaccharide of the lbgA mutant provided further evidence of a nonreducing terminal Hep that is absent in the oligosaccharide of the lbgAB mutant. Sequential Y ions and internal fragmentation from the B3 (m/z 953.3) and B2 (m/z 761.2) ions provided further evidence that the lbgA mutant produces the A2 glycoform and the lbgAB mutant produces the A1 glycoform. Together, the two mutants produce truncated LOS structures in which the monosaccharides are sequentially connected to lipid A in a manner analogous to that in the core oligosaccharide from the wild-type strain (31).
FIG. 4.
Positive-ion MS/MS spectra of the dephosphorylated oligosaccharides from the LOS of lbgAB (A) and lbgA (B) H. ducreyi mutants. The singly charged parent ions are enclosed by boxes, and their structures are shown at the top of each panel. Anhydro-Kdo (aKDO) is formed by β-elimination of phosphate from phosphorylated Kdo during acetic acid hydrolysis. Peaks marked with one or two asterisks are internal ions that appear to originate from the highly abundant B3/B2 and C3/C2 ions that have lost the terminal anhydro-Kdo and subsequently undergo losses of Hex and/or Hep sugars from the nonreducing termini. For example, in spectrum A fragmentation of m/z 779.3 (C2) yields the internal ions at m/z 617.2 (-Hex), 587.2 (-Hep), and 425.1 (-Hex, Hep), and fragmentation of m/z 761.2 (B2) yields m/z 599.2 (-Hex), 569.2 (-Hep), 407.1 (-Hep, Hex), and 377.1 (-HepHep). In spectrum B fragmentation of m/z 953.3 (B3) yields the internal ions at m/z 761.2 (-Hep), 599.2 (-Hep, Hex), 569.2 (-HepHep), and 551.2 (-HepHep, H2O), and fragmentation of m/z 971.3 (C3) yields m/z 425.1 (-Hep, HepHex).
Composition and linkage analyses.
Composition analysis of the purified oligosaccharide fraction of the LOS from the two mutants revealed only glucose and heptose monosaccharides (Table 2). Galactose and glucosamine, present at the nonreducing terminus of the LOS from parent strain 35000 (30), were not detected. In addition, only ld-Hep was found in the oligosaccharide from the lbgAB mutant. Both ld-Hep and dd-Hep were detected as components of the oligosaccharide from the lbgA mutant. Kdo is not observed under the analysis conditions used (30).
TABLE 2.
Oligosaccharide composition and linkage analyses
| Analysis | Monosaccharide or derivative | Molar ratio
|
Relative peak area
|
||
|---|---|---|---|---|---|
| H. ducreyi 35000.3 (lbgAB) | H. ducreyi 35000.4 (lbgA) | H. ducreyi 35000.3 (lbgAB) | H. ducreyi 35000.4 (lbgA) | ||
| Compositiona | GlcNb | 0.0 | 0.0 | ||
| Gal | 0.0 | 0.0 | |||
| Glc | 1.0 | 1.0 | |||
| ld-Hep | 2.9 | 2.4 | |||
| dd-Hep | 0.0 | 0.3 | |||
| Linkagec | 1-Glc | 1.0 | NDd | ||
| 1-dd-Hep | ND | 0.65 | |||
| 1,6-Glce | 0.07 | 1.0 | |||
| 1,3,4-ld-Hepe | 0.26 | 0.09 | |||
| 1,2-ld-Hepe | 0.52 | 0.15 | |||
| 1-ld-Hepe | 0.42 | 0.19 | |||
The molar ratios are the ratios relative to Glc determined by using dephosphorylated S. enterica serovar Typhimurium Ra oligosaccharide as a standard. The dd-Hep ratio was estimated by using the response factor determined for ld-Hep.
No Glucosamine (GlcN) was observed since no GlcNAc was present on the branch structures of the mutants and lipid A was removed prior to analysis.
Abbreviations: 1-Glc, 1,5-di-O-acetyl-2,3,4,6-tetra-O-methylglucitol; 1-dd-Hep, 1,5-di-O-acetyl-2,3,4,6,7-penta-O-methylheptitol; 1,6-Glc, 1,5,6-tri-O-acetyl-2,3,4-tri-O-methylglucitol; 1,3,4-ld-Hep, 1,3,4,5-tetra-O-acetyl-2,6,7-tri-O-methylheptitol; 1,2-ld-Hep, 1,2,5-tri-O-acetyl-3,4,6,7-tetra-O-methylheptitol; 1-ld-Hep, 1,5-di-O-acetyl-2,3,4,6,7-penta-O-methylheptitol. The values are relative peak areas normalized to 1-Glc for strain 35000.3 and to 1,6-Glc for strain 35000.4.
ND, not detected.
PMAA observed in parent strain 35000 (30).
Methylation analysis of the oligosaccharides confirmed the results of the composition analysis (i.e., only Glc and Hep monosaccharides were detected) and provided the linkages of the sugars (Table 2). The three core ld-Hep residues (terminal, 2-linked, and 3,4-linked residues) were found in both mutants, but no branch dd-Hep was found in the lbgAB mutant. Furthermore, only a trace of 6-linked Glc was observed, and in its place a terminal Glc was detected. This provides strong evidence that the oligosaccharide terminates at Glc, forming the A1 structure, before addition of dd-Hep. Consistent with the lbgA mutant adding only a dd-Hep sugar to Glc, the normal 6-linked Glc was detected along with a terminal dd-Hep, and the 4-linked dd-Hep observed in the parent strain (30) was absent. As in the composition analysis, Kdo was not detected under the conditions used for the analysis (30, 35).
NMR spectroscopy of the oligosaccharides.
To confirm the anomeric configurations of the oligosaccharides from the lbgA and lbgAB mutants, the 500-MHz 1H NMR spectra of the dephosphorylated oligosaccharides were recorded at three temperatures (15, 25, and 45°C). The spectra were compared to the 1H NMR spectra of the oligosaccharides from H. ducreyi strain 35000 (16) and the TN 916 lbgB mutant (16). Consistent with the published spectra, the spectra for the lbgA and lbgAB mutants contained the characteristic downfield-shifted anomeric signals arising from the three alpha-linked Hep residues (residues I, II, and III, J < 1 Hz) and the beta-linked glucose (residue IV, J ≈ 7 to 8 Hz) of the inner core (Table 3). The glucose in the pentasaccharide from the lbgAB mutant was shown by methylation analysis to be a nonreducing terminal residue. Taken together, these data indicate that the oligosaccharide from the lbgAB mutant is identical to the pentasaccharide from the H. ducreyi Tn 916 lbgB mutant described previously (16). In addition to these resonances, the spectrum of the oligosaccharide from the lbgA mutant contained an additional signal at δ 4.92 (J < 1 Hz), which was assigned to the branch alpha-linked d-glycero-d-manno-heptose V residue in H. ducreyi 35000 (31). In this case, the dd-Hep is present on a nonreducing terminus. Thus, 1H NMR analysis and methylation linkage analysis confirmed that the pentasaccharide of the lbgAB mutant and the hexasaccharide of the lbgA mutant are simple truncations of the wild-type H. ducreyi 35000 oligosaccharide.
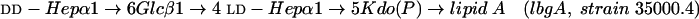 |
 |
 |
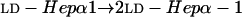 |
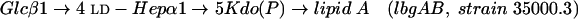 |
 |
 |
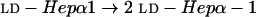 |
TABLE 3.
Anomeric proton NMR assignments of the major oligosaccharides from the H. ducreyi lbgA and lbgAB mutantsa
| H. ducreyi mutant | Anomeric protons, δ (ppm)
|
||||
|---|---|---|---|---|---|
| αHep (V) | βGlc (IV) | αHep (III) | αHep (II)c | αHep (I)c | |
| 35000.3 (lbgAB) | ≈4.52b | 5.111 | 5.705 | 5.097 | |
| 5.683 | 5.077 | ||||
| 5.670 | 5.027 | ||||
| 35000.4 (lbgA) | 4.921 | ≈4.50b | 5.119 | 5.777 | 5.105 |
| 5.755 | 5.084 | ||||
| 5.742 | 5.035 | ||||
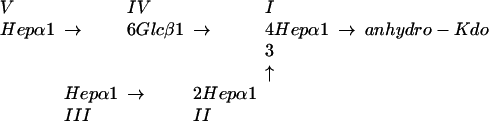 |
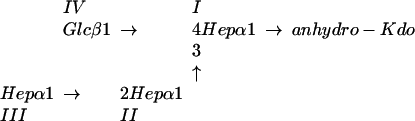 |
bAverage of overlapping signals arising from reducing terminal microheterogeneity in the anhydro-Kdo residue.
Two-dimensional SDS-PAGE of whole-cell lysates.
Cell lysates from the parent and two mutant strains were analyzed by two-dimensional SDS-PAGE (data not shown) to determine if there were any inadvertent changes in protein expression resulting from deletion of the lbgA and lbgAB genes. Comparison of the two-dimensional gels obtained for the wild-type strain and the two mutant strains showed that there were no major differences, although a few minor differences were seen in the basic region of the gel that were highly reproducible and characteristic for each strain.
Adherence to human keratinocytes.
The adherence of the LOS-defective 35000.3 (lbgAB) and 35000.4 (lbgA) mutant organisms to cultured human keratinocytes showed significant changes. After 1, 2, and 4 h of incubation, the mutant organisms showed <3% total adherence (1.2, 2.3, and 2% for strain 35000.3 and 1.6, 2.1, and 1.7% for 35000.4, respectively). In contrast, the parental wild-type strain 35000 showed the expected normal adherence levels between 22 and 28%, (22, 25, and 28% for the 1-, 2-, and 4-h time points, respectively), consistent with previous analyses (7, 16).
DISCUSSION
In previous work, Gibson et al. (16) and Stevens et al. (44) identified gene cluster lbgAB (also termed losAB) involved in H. ducreyi LOS biosynthesis. In both studies, mutants were selected based upon their inability to bind monoclonal antibodies that recognize the nonreducing terminus of the LOS. A Tn 916 lbgB mutant was found to have a greatly reduced ability to adhere to and invade human keratinocytes in tissue culture. However, the mutant strain was not stable and showed some reversion to wild-type LOS structures. In this study, we structurally characterized the LOS from stable, isogenic lbgA and lbgAB mutants to determine the function of lbgA and analyzed these mutants for the ability to adhere to cultured human keratinocytes.
Analysis of the LOS by SDS-PAGE showed that both mutants had very simple profiles compared to the wild-type LOS, with each mutant appearing to produce a single, truncated LOS species. Furthermore, the LOS from the lbgAB mutant migrated faster than that from the lbgA mutant and all of the species present in the wild-type LOS. Chemical, MS, and NMR spectroscopic analyses confirmed that the lbgAB mutant produces an LOS species that is truncated at the first Glc of the Hep-I branch (A1 glycoform) and is identical to the previously reported major LOS species produced by the Tn 916 lbgB mutant but without any reversion to wild-type LOS structures (16). Likewise, the lbgA mutant LOS is truncated at the dd-Hep of the branch (A2 glycoform), making the LOS one Hep larger than the LOS from the lbgAB mutant. The LOS from the mutants are simple truncations of the major LOS species from the parent strain. The lbgA mutant is blocked in the ability to add Galβ1→4 to dd-Hep to elongate the Hep-I branch of the LOS. This could occur due to defects either in synthesis of the activated nucleotide sugar, UDP-Gal, or in transfer of Gal to the LOS acceptor.
As noted previously (44), the lbgA gene product is similar (23 to 25% identity) to proteins involved in LOS biosynthesis from H. influenzae, but the exact functions of these proteins are unknown. Recently, two β1→4-specific galactosyltransferases from Helicobacter pylori and Escherichia coli with some similarity to LbgA (23 and 26% identity, respectively) and no significant similarity to enzymes involved in UDP-Gal synthesis (GalK, GalT, GalU, and GalE) have been identified (13, 21). Low sequence identities among glycosyltransferase of bacteria are common and may be further reduced due to differences in the acceptor molecules that they recognize (e.g., the H. ducreyi enzyme transfers Gal to dd-Hep, the H. pylori enzyme transfers Gal to GlcNAc, and the E. coli enzyme transfers Gal to Glc). However, LbgA is much more highly conserved in Actinobacillus pleuropneumoniae (64% identity) and Pasteurella multocida (56% identity); in both of these organisms a second neighboring gene of lbgA is present downstream that is similar to lbgB and in the same order (i.e., lbgAB). In these cases, the product in A. pleuropneumoniae is reported to be a putative d-glycero-d-manno-heptosyl transferase, in keeping with our prior identification of this enzyme in H. ducreyi (16), although its function is reported as unknown in P. multocida.
Previously, we showed that the Tn 916 lbgB mutant had a greatly reduced ability to adhere to and invade human keratinocytes in tissue culture (16). Studies with the stable isogenic lbgA and lbgAB mutants, the latter of which produces the same LOS structure as the TN 916 lbgB mutant, showed similar reductions in the ability to adhere to cultured human keratinocytes. No significant differences in protein expression between these two mutants and the parental wild-type strain were observed by two-dimensional SDS-PAGE separation. In addition, previously published data for one-dimensional gels of outer membrane proteins from similar isogenic strains of 35000 deficient in LOS branch structures also yielded patterns with no discernible differences (8, 14). Although the similarity in protein gel patterns supports the hypothesis that the biological differences are due to changes in LOS structure, the data do not preclude the possibility of a minor alteration in one or more proteins critical for adhesion.
The vast majority of H. ducreyi strains produce an LOS structure that is immunochemically similar to paragloboside that is partially sialylated. What function(s) these structures have in H. ducreyi virulence is still unknown. In other gram-negative human pathogens, however, evidence exists that LOS containing paragloboside and its sialylated analog are important in virulence, acting through molecular mimicry to inhibit key human defenses, such as opsonophagocytosis, neutrophil killing, and/or resistance to complement and serum factors (for a review, see reference 36). In support of such possible roles for H. ducreyi LOS, it has recently been reported that H. ducreyi is exquisitely sensitive to the exogenous concentration of sialic acid and can efficiently take up and incorporate this sugar into its LOS (38). However, expression of full-length LOS glycoforms with or without sialic acid is not necessary for the serum resistance of H. ducreyi (6, 45), nor is it needed to cause pustule formation in the experimental model of human infection or dermal lesions in the rabbit model (44, 49). Indeed, the lbgB isogenic mutant has been shown to be both serum resistant (23) and virulent in the human model (49). However, the human model (and rabbit model) of infection may be insensitive to virulence factors that are necessary during the early and late stages of establishing infection. For instance, in the early stage of infection, H. ducreyi is likely to bypass altogether the keratinocyte layer in both the human and rabbit models due to the mode of inoculation. In the late stages of ulcer formation, the human model is terminated at the pustule stage due to human safety concerns and therefore may miss key processes of tissue necrosis and subsequent pathogen transmission (43, 49). It is also worth noting that in late-stage conditions of H. ducreyi infection, extensive tissue damage is observed clinically and exogenous (cell-free) levels of sialic acid would be expected to be at their highest values. It is under these conditions that one would expect conversion of LOS containing terminal N-acetyllactosamine to its sialylated glycoforms.
In conclusion, structural characterization of the LOS from isogenic lbgA and lbgAB mutants has shown that these mutants produce LOS structures that are truncated versions of the major wild-type LOS structure due to mutations in the α-1,6-dd-heptosyltransferase gene (lbgB) or β-1,4-galactosyltransferase gene (lbgA). LbgB and LbgA act consecutively to add α-1,6-dd-Hep followed by β-1,4-Gal to the glucose emanating off Hep-I of the heptose core. These mutations prevent further elongation of the oligosaccharide, thereby blocking synthesis of sialylated and paragloboside-like LOS structures.
Acknowledgments
This study was supported by Public Health Service grants AI 31254 (to B.W.G), AI 30006 (to A.A.C), AI 38444 (to R.S.M.), and AI 32011 (to E.J.H.). We acknowledge Applied Biosystems (Framingham, Mass.) for its generous support of the MALDI-TOF instrumentation in our laboratory (B.W.G.) and the UCSF Mass Spectrometry Facility that is partially funded by the National Center for Research Resources (grant RR01614).
Editor: B. B. Finlay
REFERENCES
- 1.Ahmed, H. J., A. Frisk, J. E. Mansson, E. K. Schweda, and T. Lagergard. 1997. Structurally defined epitopes of Haemophilus ducreyi lipooligosaccharides recognized by monoclonal antibodies. Infect. Immun. 65:3151-3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alfa, M. J., and P. DeGagne. 1997. Attachment of Haemophilus ducreyi to human foreskin fibroblasts involves LOS and fibronectin. Microb. Pathog. 22:39-46. [DOI] [PubMed] [Google Scholar]
- 3.Alfa, M. J., P. DeGagne, and P. A. Totten. 1996. Haemophilus ducreyi hemolysin acts as a contact cytotoxin and damages human foreskin fibroblasts in cell culture. Infect. Immun. 64:2349-2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Apicella, M. A., J. M. Griffiss, and H. Schneider. 1994. Isolation and characterization of lipopolysaccharides, lipooligosaccharides, and lipid A. Methods Enzymol. 235:242-252. [DOI] [PubMed] [Google Scholar]
- 5.Apicella, M. A., R. E. Mandrell, M. Shero, M. E. Wilson, J. M. Griffiss, G. F. Brooks, C. Lammel, J. F. Breen, and P. A. Rice. 1990. Modification by sialic acid of Neisseria gonorrhoeae lipooligosaccharide epitope expression in human urethral exudates: an immunoelectron microscopic analysis. J. Infect. Dis. 162:506-512. [DOI] [PubMed] [Google Scholar]
- 6.Bozue, J. A., M. V. Tullius, J. Wang, B. W. Gibson, and R. S. Munson, Jr. 1999. Haemophilus ducreyi produces a novel sialyltransferase. Identification of the sialyltransferase gene and construction of mutants deficient in the production of the sialic acid-containing glycoform of the lipooligosaccharide. J. Biol. Chem. 274:4106-4114. [DOI] [PubMed] [Google Scholar]
- 7.Brentjens, R. J., S. M. Spinola, and A. A. Campagnari. 1994. Haemophilus ducreyi adheres to human keratinocytes. Microb. Pathog. 16:243-247. [DOI] [PubMed] [Google Scholar]
- 8.Campagnari, A. A., R. Karalus, M. Apicella, W. Melaugh, A. J. Lesse, and B. W. Gibson. 1994. Use of pyocin to select a Haemophilus ducreyi variant defective in lipooligosaccharide biosynthesis. Infect. Immun. 62:2379-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campagnari, A. A., S. M. Spinola, A. J. Lesse, Y. A. Kwaik, R. E. Mandrell, and M. A. Apicella. 1990. Lipooligosaccharide epitopes shared among gram-negative non-enteric mucosal pathogens. Microb. Pathog. 8:353-362. [DOI] [PubMed] [Google Scholar]
- 10.Campagnari, A. A., L. M. Wild, G. E. Griffiths, R. J. Karalus, M. A. Wirth, and S. M. Spinola. 1991. Role of lipooligosaccharides in experimental dermal lesions caused by Haemophilus ducreyi. Infect. Immun. 59:2601-2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cope, L. D., S. Lumbley, J. L. Latimer, J. Klesney-Tait, M. K. Stevens, L. S. Johnson, M. Purven, R. S. Munson, Jr., T. Lagergard, J. D. Radolf, and E. J. Hansen. 1997. A diffusible cytotoxin of Haemophilus ducreyi. Proc. Natl. Acad. Sci. USA 94:4056-4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Domon, B., and C. E. Costello. 1988. A systematic nomenclature for carbohydrate fragmentations in FAB-MS/MS spectra of glycoconjugates. Glycoconjugate J. 5:397-409. [Google Scholar]
- 13.Endo, T., S. Koizumi, K. Tabata, and A. Ozaki. 2000. Cloning and expression of beta1,4-galactosyltransferase gene from Helicobacter pylori. Glycobiology 10:809-813. [DOI] [PubMed] [Google Scholar]
- 14.Filiatrault, M. J., B. W. Gibson, B. Schilling, S. Sun, R. S. Munson, Jr., and A. A. Campagnari. 2000. Construction and characterization of Haemophilus ducreyi lipooligosaccharide (LOS) mutants defective in expression of heptosyltransferase III and β1,4-glucosyltransferase: identification of LOS glycoforms containing lactosamine repeats. Infect. Immun. 68:3352-3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flood, J. M., S. K. Sarafian, G. A. Bolan, C. Lammel, J. Engelman, R. M. Greenblatt, G. F. Brooks, A. Back, and S. A. Morse. 1993. Multistrain outbreak of chancroid in San Francisco, 1989-1991. J. Infect. Dis. 167:1106-1111. [DOI] [PubMed] [Google Scholar]
- 16.Gibson, B. W., A. A. Campagnari, W. Melaugh, N. J. Phillips, M. A. Apicella, S. Grass, J. Wang, K. L. Palmer, and R. S. Munson, Jr. 1997. Characterization of a transposon Tn 916-generated mutant of Haemophilus ducreyi 35000 defective in lipooligosaccharide biosynthesis. J. Bacteriol. 179:5062-5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gibson, B. W., J. J. Engstrom, C. M. John, W. Hines, and A. M. Falick. 1997. Characterization of bacterial lipooligosaccharides by delayed extraction matrix-assisted laser desorption ionization time-of-flight mass spectrometry. J. Am. Soc. Mass Spectrom. 8:645-658. [Google Scholar]
- 18.Gibson, B. W., W. Melaugh, N. J. Phillips, M. A. Apicella, A. A. Campagnari, and J. M. Griffiss. 1993. Investigation of the structural heterogeneity of lipooligosaccharides from pathogenic Haemophilus and Neisseria species and of R-type lipopolysaccharides from Salmonella typhimurium by electrospray mass spectrometry. J. Bacteriol. 175:2702-2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hammond, G. W., C. J. Lian, J. C. Wilt, and A. R. Ronald. 1978. Antimicrobial susceptibility of Haemophilus ducreyi. Antimicrob. Agents Chemother. 13:608-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hardy, M. R., R. R. Townsend, and Y. C. Lee. 1988. Monosaccharide analysis of glycoconjugates by anion exchange chromatography with pulsed amperometric detection. Anal. Biochem. 170:54-62. [DOI] [PubMed] [Google Scholar]
- 21.Heinrichs, D. E., J. A. Yethon, and C. Whitfield. 1998. Molecular basis for structural diversity in the core regions of the lipopolysaccharides of Escherichia coli and Salmonella enterica. Mol. Microbiol. 30:221-232. [DOI] [PubMed] [Google Scholar]
- 22.Helander, I. M., B. Lindner, H. Brade, K. Altmann, A. A. Lindberg, E. T. Rietschel, and U. Zahringer. 1988. Chemical structure of the lipopolysaccharide of Haemophilus influenzae strain I-69 Rd-/b+. Description of a novel deep-rough chemotype. Eur. J. Biochem. 177:483-492. [DOI] [PubMed] [Google Scholar]
- 23.Hiltke, T. J., M. E. Bauer, J. Klesney-Tait, E. J. Hansen, R. S. Munson, Jr., and S. M. Spinola. 1999. Effect of normal and immune sera on Haemophilus ducreyi 35000HP and its isogenic MOMP and LOS mutants. Microb. Pathog. 26:93-102. [DOI] [PubMed] [Google Scholar]
- 24.Jessamine, P. G., and A. R. Ronald. 1990. Chancroid and the role of genital ulcer disease in the spread of human retroviruses. Med. Clin. N. Am. 74:1417-1431. [DOI] [PubMed] [Google Scholar]
- 25.Lagergard, T. 1992. The role of Haemophilus ducreyi bacteria, cytotoxin, endotoxin and antibodies in animal models for study of chancroid. Microb. Pathog. 13:203-217. [DOI] [PubMed] [Google Scholar]
- 26.Levery, S. B., and S. Hakomori. 1987. Microscale methylation analysis of glycolipids using capillary gas chromatography-chemical ionization mass fragmentography with selected ion monitoring. Methods Enzymol. 138:13-25. [DOI] [PubMed] [Google Scholar]
- 27.Mandrell, R. E., and M. A. Apicella. 1993. Lipo-oligosaccharides (LOS) of mucosal pathogens: molecular mimicry and host-modification of LOS. Immunobiology 187:382-402. [DOI] [PubMed] [Google Scholar]
- 28.Mandrell, R. E., J. M. Griffiss, and B. A. Macher. 1988. Lipooligosaccharides (LOS) of Neisseria gonorrhoeae and Neisseria meningitidis have components that are immunochemically similar to precursors of human blood group antigens. Carbohydrate sequence specificity of the mouse monoclonal antibodies that recognize crossreacting antigens on LOS and human erythrocytes. J. Exp. Med. 168:107-126. (Erratum, 168:1517.) [DOI] [PMC free article] [PubMed]
- 29.Melaugh, W., A. A. Campagnari, and B. W. Gibson. 1996. The lipooligosaccharides of Haemophilus ducreyi are highly sialylated. J. Bacteriol. 178:564-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Melaugh, W., N. J. Phillips, A. A. Campagnari, R. Karalus, and B. W. Gibson. 1992. Partial characterization of the major lipooligosaccharide from a strain of Haemophilus ducreyi, the causative agent of chancroid, a genital ulcer disease. J. Biol. Chem. 267:13434-13439. [PubMed] [Google Scholar]
- 31.Melaugh, W., N. J. Phillips, A. A. Campagnari, M. V. Tullius, and B. W. Gibson. 1994. Structure of the major oligosaccharide from the lipooligosaccharide of Haemophilus ducreyi strain 35000 and evidence for additional glycoforms. Biochemistry 33:13070-13078. [DOI] [PubMed] [Google Scholar]
- 32.Morse, S. A. 1989. Chancroid and Haemophilus ducreyi. Clin. Microbiol. Rev. 2:137-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palmer, K. L., W. E. Goldman, and R. S. Munson, Jr. 1996. An isogenic haemolysin-deficient mutant of Haemophilus ducreyi lacks the ability to produce cytopathic effects on human foreskin fibroblasts. Mol. Microbiol. 21:13-19. [DOI] [PubMed] [Google Scholar]
- 34.Palmer, K. L., and R. S. Munson, Jr. 1995. Cloning and characterization of the genes encoding the hemolysin of Haemophilus ducreyi. Mol. Microbiol. 18:821-830. [DOI] [PubMed] [Google Scholar]
- 35.Phillips, N. J., C. M. John, L. G. Reinders, B. W. Gibson, M. A. Apicella, and J. M. Griffiss. 1990. Structural models for the cell surface lipooligosaccharides of Neisseria gonorrhoeae and Haemophilus influenzae. Biomed. Environ. Mass Spectrom. 19:731-745. [DOI] [PubMed] [Google Scholar]
- 36.Preston, A., R. E. Mandrell, B. W. Gibson, and M. A. Apicella. 1996. The lipooligosaccharides of pathogenic gram-negative bacteria. Crit. Rev. Microbiol. 22:139-180. [DOI] [PubMed] [Google Scholar]
- 37.Rabilloud, T. 1998. Use of thiourea to increase the solubility of membrane proteins in two-dimensional electrophoresis. Electrophoresis 19:758-760. [DOI] [PubMed] [Google Scholar]
- 38.Schilling, B., S. Goon, N. Samules, C. Bertozzi, and B. W. Gibson. 2001. Biosynthesis of sialylated lipooligosaccharides in Haemophilus ducreyi is dependent on exogenous sialic acid and not mannosamine. Incorporation studies using N-acylmannosamine analogs, and N-glycolyl and 13C-labeled N-acetyl neuraminic acid. Biochemistry 40:12666-12677. [DOI] [PubMed] [Google Scholar]
- 39.Schulte, J. M., F. A. Martich, and G. P. Schmid. 1992. Chancroid in the United States, 1981-1990: evidence for underreporting of cases. Morb. Mortal. Wkly. Rep. 41:57-61. [PubMed]
- 40.Schweda, E. K., J. A. Jonasson, and P. E. Jansson. 1995. Structural studies of lipooligosaccharides from Haemophilus ducreyi ITM 5535, ITM 3147, and a fresh clinical isolate, ACY1: evidence for intrastrain heterogeneity with the production of mutually exclusive sialylated or elongated glycoforms. J. Bacteriol. 177:5316-5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schweda, E. K., A. C. Sundstrom, L. M. Eriksson, J. A. Jonasson, and A. A. Lindberg. 1994. Structural studies of the cell envelope lipopolysaccharides from Haemophilus ducreyi strains ITM 2665 and ITM 4747. J. Biol. Chem. 269:12040-12048. [PubMed] [Google Scholar]
- 42.Spinola, S. M., A. Orazi, J. N. Arno, K. Fortney, P. Kotylo, C. Y. Chen, A. A. Campagnari, and A. F. Hood. 1996. Haemophilus ducreyi elicits a cutaneous infiltrate of CD4 cells during experimental human infection. J. Infect. Dis. 173:394-402. [DOI] [PubMed] [Google Scholar]
- 43.Spinola, S. M., L. M. Wild, M. A. Apicella, A. A. Gaspari, and A. A. Campagnari. 1994. Experimental human infection with Haemophilus ducreyi. J. Infect. Dis. 169:1146-1150. [DOI] [PubMed] [Google Scholar]
- 44.Stevens, M. K., J. Klesney-Tait, S. Lumbley, K. A. Walters, A. M. Joffe, J. D. Radolf, and E. J. Hansen. 1997. Identification of tandem genes involved in lipooligosaccharide expression by Haemophilus ducreyi. Infect. Immun. 65:651-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun, S., B. Schilling, L. Tarantino, M. V. Tullius, B. W. Gibson, and R. S. Munson, Jr. 2000. Cloning and characterization of the lipooligosaccharide galactosyltransferase II gene of Haemophilus ducreyi. J. Bacteriol. 182:2292-2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trees, D. L., and S. A. Morse. 1995. Chancroid and Haemophilus ducreyi: an update. Clin. Microbiol. Rev. 8:357-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tuffrey, M., F. Alexander, R. C. Ballard, and R. D. Taylor. 1990. Characterization of skin lesions in mice following intradermal inoculation of Haemophilus ducreyi. J. Exp. Pathol. (Oxford) 71:233-244. [PMC free article] [PubMed] [Google Scholar]
- 48.Wasserheit, J. N. 1992. Epidemiological synergy. Interrelationships between human immunodeficiency virus infection and other sexually transmitted diseases. Sex. Transm. Dis. 19:61-77. [PubMed] [Google Scholar]
- 49.Young, R. S., K. Fortney, J. C. Haley, A. F. Hood, A. A. Campagnari, J. Wang, J. A. Bozue, R. S. Munson, Jr., and S. M. Spinola. 1999. Expression of sialylated or paragloboside-like lipooligosaccharides are not required for pustule formation by Haemophilus ducreyi in human volunteers. Infect. Immun. 67:6335-6340. [DOI] [PMC free article] [PubMed] [Google Scholar]