ABSTRACT
Background/Purpose
Cumulative sunlight exposure and cataract surgery are reported risk factors for advanced age-related macular degeneration (AMD). Laboratory studies suggest that accumulation and photochemical reactions of A2E (N-retinylidene-N-retinylethanolamine) and its epoxides, components of lipofuscin, are important in AMD. To relate this data to the clinical setting, we modeled the effects of macular irradiance and spectral filtering on production of A2E and reactive oxygen intermediates (ROIs) in pseudophakic eyes with a clear or “yellow” intraocular lens (IOL) and in phakic eyes.
Methods
We calculated relative changes of macular irradiance as a function of light (390 to 700 nm) intensity, pupil size, age, and lens status, and modeled resulting all-trans-retinal concentration and rates of production of A2E-related photochemicals and photon-induced ROIs in rods and retinal pigment epithelium (RPE). We compared these photoproducts following cataract surgery and IOL implantation with and without spectral sunglasses to normal age-related nuclear sclerotic lens changes.
Results
Following cataract and IOL surgery, all-trans-retinal and lipofuscin photochemistry would theoretically increase average generation of 1) A2E-related photochemicals, 2) ROI in rods and 3) ROI in RPE, respectively, 2.6-, 15- and 6.6-fold with a clear IOL, and 2.1-, 4.1- and 2.6 fold with a yellow IOL, but decrease approximately 30-, approximately 20-and 4-fold with a vermillion filter sunglass and clear IOL compared to an average 70 year old phakic eye.
Conclusion
Sunglasses that strongly decrease both deep blue light and rod photobleaching, while preserving photopic sensitivity and color perception, would provide upstream protection from potential photochemical damage in subjects at risk for AMD progression after cataract surgery.
INTRODUCTION
The late stages of age-related macular degeneration (AMD), neovascularization and geographic atrophy, are important causes of severe visual loss and legal blindness in persons over 60 years of age in the United States.1–3 Clinical and epidemiological studies that report a significant association between both prior cataract surgery and cumulative exposure to sunlight and late-stage AMD lend support to the hypothetical role of photochemical reactions in the pathogenesis of AMD.4–10
In 1920, van der Hoeve11 observed that AMD was less common in eyes with cataracts and proposed that opacity of the lens diminished the severity of AMD; in 1925, Gjessing12 reported an inverse relation between lens opacity and AMD. Recent epidemiological studies have reported conflicting data on AMD and lens opacities but consistently suggest that prior cataract surgery, aphakia, and pseudophakia are risk factors for late-stage AMD.5,6,13,14
The Chesapeake Bay Waterman Study and the Beaver Dam Eye Study reported a significant association between AMD and cumulative sunlight exposure, 400 to 700 nm, the former with late-stage AMD and the latter with early AMD.7,8 Neither of these studies reported an association between AMD and exposure to ultraviolet A (320 to 400 nm) or B (290 to 320 nm) light.
Laboratory studies on acute photochemical injury to mammalian rod photoreceptors and retinal pigment epithelium (RPE) cells document two well-defined action spectra: (1) Ham-type photochemical injury to the RPE caused by intense blue and ultraviolet light with peak sensitivity at approximately 350 nm in aphakic monkeys and (2) rod damage associated with the rhodopsin absorption spectrum and enhanced by blue light.15,16 Recent in vitro studies have demonstrated that RPE lipofuscin granules generate oxygen free radicals, singlet oxygen, and other reactive oxygen intermediates (ROIs), with an action spectrum very similar to that in the Ham-type in vivo study.17–19 Additionally, lipofuscin granules contain a number of different fluorescent species that (1) originate largely from photochemical reactions involving all-trans-retinal and include A2E (N-retinylidene-N-retinylethanolamine) and its epoxides and (2) are capable of acting as photosensitizers of singlet oxygen.18,19 A2E-derived fluorophores in lipofuscin appear to be produced during periods of photopic vision associated with significant rhodopsin bleaching and high levels of all-trans-retinal in the rod outer segment (ROS) disks. All-trans-retinal in the ROS disks can absorb short-wavelength light with a 380-nm peak and known wavelength dependence (or action spectrum) leading to a long-lived triplet state that acts as a photosensitizer of oxidative damage.20 A2E-derived fluorophores that accumulate in the RPE lipofuscin are potent photosensitizers of oxidative damage with a known aggregate action spectrum.21
To relate the epidemiological and laboratory data to the clinical setting, we modeled the effects of retinal irradiance and spectral filtering on the relative rates of all-trans-retinal photosensitization, production of A2E and A2E-derived lipofuscin fluorophores, and 1O2 generation due to RPE lipofuscin photosensitization in phakic eyes and in eyes with a clear or “yellow” intraocular lens (IOL) with and without external spectrally selective filters. We discuss this model in relation to hypothetical pathogenic pathways in AMD and describe a possible preventive strategy for decreasing the potential risk of photochemical damage in AMD.
METHODS
We have developed a model relating retinal spectral irradiance as a function of age and lens status to the relative rates of rod bleaching, steady-state concentration of all-trans-retinal, its photosensitization of oxidative damage in the rod outer segments, or alternatively its reactions to form A2E-related species. In RPE cell culture experiments, A2E is rapidly ingested and concentrated through lysosomal processing into prototypical nascent lipofuscin granules.19 On irradiation, these granules form a complex mixture of oxidized A2E-related fluorophores that are potent photosensitizers of singlet oxygen generation and spectroscopically comparable to mature lipofuscin granules harvested from aged human retinas (R. Bonner, unpublished data, 2004). In our model, we assume that averaged over long time periods (1 year), the relative rate of “mature” lipofuscin accumulation within the RPE is proportional to the rate of production of A2E precursors in the rods. From the literature, we applied a variety of values describing the normal age dependence of critical ocular parameters to standard formulas in order to determine average retinal spectral irradiance as a function of age. We then used our model and published action spectra to estimate the normal average age dependence of these potential causes of chronic light injury at 7 to 11 degrees from the center of the fovea, a region of high lipofuscin accumulation and rod loss.22 Finally, we compared the estimates for the normally aging phakic subject following replacement of the “yellowed” aged lens with two different commercial IOLs (Alcon clear AcrySoft MA60BM UV-absorbing and light yellow AcrySoft Natural) with or without rod-sparing or blue-blocking spectrally selective sunglasses.
We calculated relative changes of macular irradiance as a function of light spectral (390 to 700 nm) intensity, pupil size, age, and lens status. The solar radiance has direct, diffuse (scattered light from the atmosphere), and ground-reflected components and varies with the day of year, time of day, altitude, and latitude. For our calculations we used the following formula and daylight radiances at the cornea between 9 and 4,400 candelas[cd]/m2 for the solar spectrum through air mass 1.2:
| (1) |
where HR is the retinal irradiance where the specific photochemical reaction occurs; Hpupil = Hp≅is the “effective” solar radiance at anterior corneal surface, Hcornea ; A is the area of pupil; f is the distance from pupil to macula; n is the index of refraction of ocular media; t is the transmission of the ocular media; and λ is the wavelength of light.
For diffuse sources, the solid angle of the retinal image determined by the pupil area (A n2/f2) greatly diminishes retinal irradiance. For diffusely reflected sunlight, the surface reflectance or albedo of viewed objects further diminishes corneal irradiance so that at 500 nm the spectral irradiance of the sun hitting the ground might be approximately 1 mW/cm2/10 nm, but the retinal irradiance only a few microwatts per cm2/10 nm. Acute phototoxicity experiments in animals or cell cultures capable of inducing apoptosis generally use light intensities hundreds or thousands times greater than normal retinal irradiance in daylight. We assumed n was 1.33 and f was 21.5 mm. This formula and the assumptions we made are similar to those used in studies to calculate retinal irradiance at the surface of the retina from indirect ophthalmoscopes, slit lamps, and surgical lamps.23
In the visible spectrum, ocular spectral transmission t(λ) is determined mainly by absorption of light by the lens(tL), the macular pigment(tMP), the photoreceptor visual pigments(tPH), and melanin in the RPE (tM) in the macula. Each of these components is a function of wavelength:
| (2) |
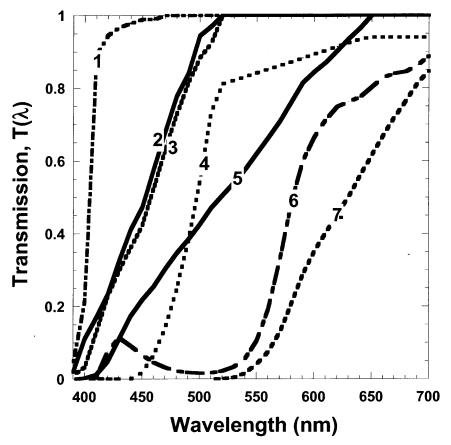
Spectral irradiance at the surface of the macula is determined by (1) the source spectral irradiance at the cornea, (2) the pupil diameter, and (3) the transmission of the lens (and cornea). The cornea absorbs virtually all light below 300 nm. The lens transmits virtually 100% of light over 660 nm. Because the lens and UV-absorbing IOLs transmit less than 1% light below 390 nm, we considered light below 390 nm to have an insignificant effect on our calculations. We are principally interested in the role of photo-protection of the lens as it ages (“yellows”) compared to IOLs used to replace the lens following cataract surgery. We used published data on lens transmittance as a function of age in normals (due to nuclear sclerosis) and data on the transmittance of the Alcon clear AcrySoft UV-absorbing and light yellow AcrySoft Natural IOLs and a similar yellow Spectrum IOL developed and used in Russia beginning in the mid-1980s.24–26 We also examined the effects of external spectrally selective sunglasses after cataract surgery (a vermilion, a yellow or 480-nm long-pass, and a deep red or 570-nm long-pass; see Figure 1). The selected vermilion filter allows (1) little attenuation of long wavelengths and photopic sensitivity, (2) sufficient midrange blue transmission to provide good color perception, and (3) blockage of wavelengths efficiently absorbed by rod rhodopsin and short-wavelength blue light.
Figure 1.
Spectral transmission of ocular filters analyzed with our model. Numbered from left to right (1) Acrysoft clear UV-absorbing IOL, (2) Russian Spectrum (light yellow) IOL, (3) Acrysoft light yellow IOL, (4) 480-nm long-pass filter, (5) 70-year-old lens, (6) vermilion filter, and (7) 570-nm long-pass filter.
Retinal irradiance is a strong function of pupil diameter and corneal irradiance. We used published average normal data on pupil size as a function of age and after cataract removal and IOL implantation.27,28 The light-filtering effects of macular and photoreceptor pigments and RPE melanin are complicated functions of their radial and depth (z) distributions.29,30 Although we have used literature values for the radial (r) and depth (z) dependence, we present here only our model predictions for the photochemistry and spectral irradiance at 7 to 11 degrees from the center of the fovea.
The effect of the macular pigment on the spectral irradiance of the underlying macular photoreceptors and RPE is highest in the central 1 degree of the fovea, rapidly decreases from 1 to 5 degrees from the center of the fovea, and is considered negligible beyond approximately 7 degrees.29 Rod loss and lipofuscin accumulation occur predominantly outside the region of significant macular pigment absorption, and therefore its possible photoprotective role does not affect our calculations for 7 to 11 degrees. Our calculations of the “internal filter” photoprotection of rhodopsin and melanin absorption have been estimated assuming age-independent concentrations of rhodopsin and melanin at 7 to 11 degrees from the fovea.30 We modeled only the average age-dependent effects of macular spectral irradiance in a typical subject before and after cataract surgery. In eyes with early AMD, pigmentary and drusenoid changes may critically affect the potential for local photodamage and not be reflected in the reported average values (such as rod dark-adaptation time constant) that we used. Such complex interactions with spectral irradiance could not be reliably predicted and are beyond the scope of our model.
In order to estimate the average age-dependent rates of all-trans-retinal photosensitization of ROI production in the rods or of its reactions to form A2E and related molecules, we related the macular irradiance to the corresponding steady-state concentration of all-trans-retinal, [all-trans-retinal]ss, produced in the rods. We used the formula of Thomas and Lamb to calculate the steady-state bleaching of the rods, B(I,age), using the age-dependent average values of the rod visual cycle time constant trh (age) in seconds reported for a normal population31,32:
| (3) |
Where Is is the retinal irradiance in scotopic trolands, Lrh is an empirical constant reported to be 107, [all-trans-retinal]ROS is the concentration of all-trans-retinal in the rod disks, and a is an age-independent constant.31 For different ambient corneal light levels, the scotopic trolands were calculated using the reported age-dependence of average pupil diameters as a function of corneal irradiance and the altered scotopic sensitivity of the solar spectrum transmitted through the aging lens (or IOLs). The addition of external filters (eg, spectrally selective sunglasses) merely adds another factor tSSG(γ) in equation 2.
Following rod bleaching of the normal mammalian retina, all-trans-retinal in the rod outer segments is the predominant transiently increased species in the retina. Therefore, we assume in our model that [all-trans-retinal]ss varies linearly with the steady-state rod bleaching (equation 3). All-trans-retinal, which reaches high concentrations in partially bleached rods (ie, for daylight ≥200 cd/m2), is a potent photosensitizer of oxidative damage. After absorbing a short-wavelength photon, the excited singlet-state all-trans-retinal undergoes intersystem crossing to a long-lived (approximately 10 msec) triplet state that can efficiently transfer its energy to ground state 3O2, creating highly reactive singlet oxygen 1O2.20 The action spectrum AS(λ)atr of this process is the absorption spectrum of all-trans-retinal with a maximum at 380 nm and rapidly diminishing with increasing wavelength. We assumed that the photochemical creation of reactive oxygen intermediates in the rods ROIrod is predominantly driven by the creation of excited states of all-trans-retinal and is given by:
| (4) |
where [all-trans-retinal] is obtained from equation 3, Hrod(λ) from equation 1, and b is a constant (affected by Po2 and quantum efficiency of the photosensitizer reaction but not age). In this and all the subsequent calculations, we were interested only in long-time averages over many days in which daily or even seasonal variations in light exposure can be neglected. Since equation 3 is nonlinear with normal photopic corneal irradiances, the integral over time allows calculation of the average ROI production over any standardized temporal distribution of ambient corneal irradiances that reflect the range of typical daily light exposures. We used 5% at 4,400 cd/m2, 20% at 1,100 cd/m2, 30% at 220 cd/m2, 25% at 44 cd/m2, and 20% at 9 cd/m2; even lower values would not contribute significantly to modeled photodamage. We modeled the effects due to spectral transmission changes in the lens assuming environmental light exposures do not vary significantly with age in order to evaluate the specific effects of aging on lens yellowing and pupil diameter changes. Epidemiological literature suggests that increased environmental light exposure is a risk factor for AMD, and higher or lower average ambient light distributions than the one we used would have roughly proportional changes in the computed rates of photochemistry at a given age.
Periods of rod bleaching and high [all-trans-retinal]ROS also lead to the formation of A2E and related fluorophores. After disk shedding, these fluorescent molecules are concentrated by lysosomal processing within lipofuscin granules in the RPE. A2E and related molecules avidly partition into cellular membranes and appear to be potent cytotoxic agents capable of inducing DNA damage and apoptosis in the dark, which is enhanced by blue light.18 Since A2E and its phosphorylated precursor require the reaction of two all-trans-retinal molecules, its reaction rate is second order in [all-trans-retinal] and therefore should be proportional to [all-trans-retinal]2 in the rods. An estimate of the average rate of A2E production over long periods of different ambient light levels is given by the time average of [all-trans-retinal]2 determined by retinal scotopic irradiance (Is) during environmental light exposure:
| (5) |
where x is the age in years and k is a rate constant assumed to be independent of age, or at least in the case of IOL implantation, independent of whether the aged lens has been replaced with an IOL.
A2E does not appear to be substantially broken down by lysosomal enzymes but rather accumulates in RPE lipofuscin granules where its photo-oxidization on irradiation with short-wavelength light results in a complex mixture of fluorophores.33 A2E avidly reacts with 1O2 to form epoxides of increasingly higher order, and this oxidation within the lipofuscin granules appears to be the principal means by which A2E concentration (as a distinct molecular species) is limited to approximately 1 pg per RPE cell (approximately 200 μM) in the human retina.18,19 We have modeled the accumulated A2E-derived lipofuscin fluorophores in the RPE (LFRPE[age]) over many years to be proportional to the time integral of the rate of A2E precursor formation in the rods during periods of significant bleaching.
| (6) |
where x is the age. The action spectrum of RPE lipofuscin AS(λ)lf has been determined by direct detection of 1O2 phosphorescence and falls exponentially with wavelength (approximately 20-fold from 360 to 460 nm).21 This action spectrum is very similar to that observed for macular RPE injury induced by 1,000-sec focal monochromatic irradiations in rhesus monkeys.15,16 In our model, we assumed that the potent photosensitizers in lipofuscin granules are derived from A2E by oxidation (predominantly photo-oxidation). We can describe the age dependence of generation of 1O2 by lipofuscin granule photochemistry by:
| (7) |
where ROIRPE (age) is the age dependence of lipofuscin 1O2 generation within a typical RPE cell in the macula at 7 to 11 degrees from center of the fovea.
To predict all of the above processes after cataract surgery, we substituted the spectral transmission of the IOL, tIOL(λ), for tL(λ) in equation 2 at the specified time of IOL implantation and recalculated equations 1 and 3 through 7 for the years after implantation. Similarly, spectral transmission of spectrally selective external sunglasses was added to equation 2, and the results recalculated.
RESULTS
We are primarily interested in estimating the effects of lens aging and lens replacement with a clear or yellow IOL on the retinal spectral irradiance and the rate of modeled macular photochemistries. Variations among aging individuals in degree of lens yellowing, pupil diameter, rod dark adaptation rate trh(age), and environmental light exposures might result in substantial differences in the amount of A2E-related compounds produced and modeled photo-oxidative damage in both the rods and RPE cells. In modeling macular photosensitization of ROI and production of related photochemicals, we did not evaluate the effects of antioxidant and molecular repair mechanisms. These ameliorating mechanisms may decrease with age but would not be expected to change due to cataract and IOL surgery.
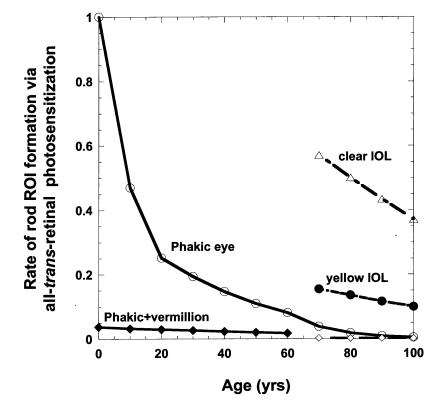
Under our modeled age-independent mixed light exposures, described above, the time average rate of rod bleaching and average [all-trans-retinal]ROS decreases only slightly from 0 to 60 years owing to a balance of the normal aging trend to smaller pupils (ie, less light) and slower trh(age), whereas the scotopic transmission of the lens is reduced only slightly. During this period, the modeled average relative rate of rod oxidative damage via all-trans-retinal photosensitization (Figure 2) is affected largely by changes in the fraction of short wavelength (<450 nm) reaching the rods, which decreases most rapidly between the ages of 0 and 20. Beyond age 60, the progressive yellowing of the lens further reduces macular irradiance in scotopic trolands and consequently the average rod bleaching and [all-trans-retinal]ROS. By age 70, the modeled average rate for rod ROI generation is reduced 20-fold and fourfold compared to a 1-year-old and 20-year-old, respectively. Removing the 70-year lens and replacing it with a clear UV-absorbing IOL (roughly equivalent to a 1-year-old lens) increases the average rate of rod ROI photochemical formation by a factor of 15 (Figure 2 and Table 1). Implanting an Alcon yellow IOL, roughly equivalent to a 20-year-old lens, limits the increase in rod ROI after cataract surgery approximately fourfold. Addition of spectrally selective sunglasses such as rod-sparing vermilion lens or long-wavelength-pass filters (yellow 480-nm long-pass or red 570-nm long-pass) would be expected to reduce dramatically rod ROI formation to levels well below those of the presurgery phakic eye as long as they are reliably worn during periods of moderate to high environmental lighting (≥200 cd/m2; Table 1). A 480-nm long-pass yellow sunglass following clear IOL implantation is roughly equivalent to a 60-year lens.
Figure 2.
Modeled average rate of all-trans-retinal photosensitization of rod reactive oxygen intermediates (ROIs) at 7 to 11 degrees from the center of the fovea in the phakic eye (○), for the addition of vermilion sunglasses for ambient conditions ≥200 cd/m2 in phakic eye before 70 (♦) and after implantation of clear UV-absorbing IOL at 70 (⋄), for clear IOL implanted at age 70 (▵), and for the yellow IOL implanted at age 70 (•). Environmental light exposures are assumed to be age-invariant.
Table 1.
Fractional changes in photochemistry values 7 to 11 degrees from the center of the fovea after cataract surgery at age 70 relative to values for typical phakic subject at age 70*
| Lens status | <Rod bleach> | <Rod roi> | <A2E production> | <RPE1O2> | <[A2ESS]RPE> |
|---|---|---|---|---|---|
| Native lens at age 70 | 1 | 1 | 1 | 1 | 1 |
| Clear IOL | 1.8 | 15 | 2.6 | 6.6 | 0.44 |
| Natural yellow IOL | 1.6 | 4.1 | 2.1 | 2.6 | 0.9 |
| Spectrum yellow IOL | 1.6 | 4.9 | 2.2 | 2.9 | 0.8 |
| Clear IOL + 480-nm LP | 1.1 | 0.20 | 1.2 | 0.7 | 1.8 |
| Clear IOL + vermilion | 0.14 | 0.05 | 0.03 | 0.24 | 0.09 |
| Clear IOL + 570-nm LP | 0.05 | <0.01† | <0.01† | <0.01† | No estimate† |
All quantities in angular brackets (< >) denote time averages over 1 year and our standardized ambient lighting changes. <Rod bleach>, cumulative rod bleaching; <Rod ROI>, cumulative reactive oxygen intermediates produced in rods via all-trans-retinal photosensitization; <A2E production>, cumulative A2E produced by rod bleaching; <RPE1O2> , cumulative production of singlet oxygen via lipofuscin photosensitization in the retinal pigment epithelium; < [A2Ess]RPE >, average steady-state concentration of A2E within the RPE.
The small amount of light capable of bleaching rods or driving photosensitizer production of ROI when wearing the 570-nm long-pass filter suggests that these photochemical products will be determined entirely by periods during which the external filters are not worn, and consequently the [A2Ess]RPE could not be reliably estimated.
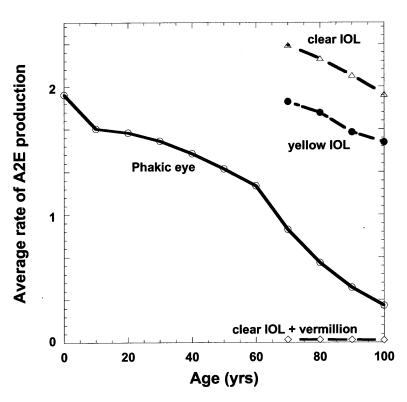
Small monotonic decreases in the time average rate of rod bleaching and average [all-trans-retinal] with increasing age induce larger age-dependent decreases in the annual average rate of A2E formation and build-up within the RPE, according to our model parameters (Figure 3). The modeled average relative rate of A2E formation in RPE cells 7 to 11 degrees from the center of the fovea (Figure 3) is affected by age-dependent decreases in scotopic retinal irradiance balanced against increases in trh with age. Lens yellowing more significantly reduces scotopic transmission for ages greater than 60. By age 70, the modeled average rate for A2E formation is reduced 2.2-fold and 1.9-fold compared to an infant and 20-year-old, respectively (Figure 3). Removing the 70-year lens and replacing it with a clear UV-absorbing IOL increases the average rate of A2E formation by a factor of 2.6 versus 2.1 for an AcrySoft Natural IOL (Figure 3 and Table 1). Addition of spectrally selective sunglasses such as rod-sparing vermilion lens or long-wave-pass filters (570-nm long-pass) would be expected to reduce dramatically average rod bleaching and A2E production to levels less than 1/30th of those for the presurgery phakic eye as long as they are reliably worn during periods of moderate to high environmental lighting (≥200 cd/m2).
Figure 3.
Modeled average annual rate of formation of A2E as a function of age and lens status: for the phakic eye (○), for the addition of vermilion sunglasses after implantation of clear IOL at 70 (⋄), for clear IOL implanted at age 70 (▵), and for the yellow IOL (•) implanted at age 70.
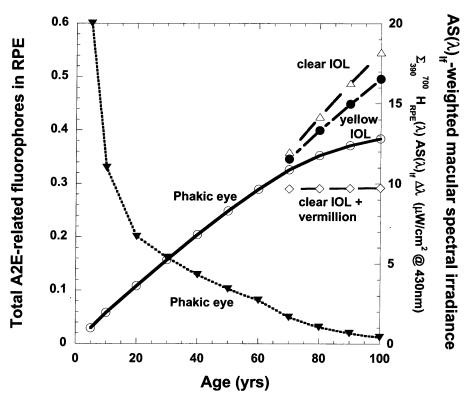
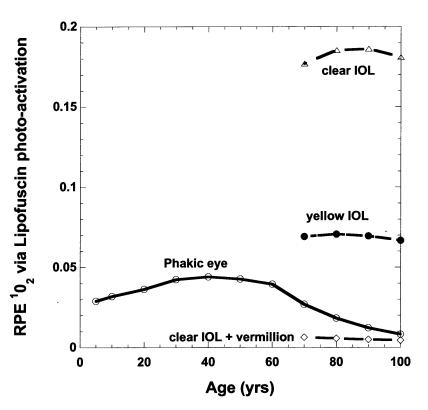
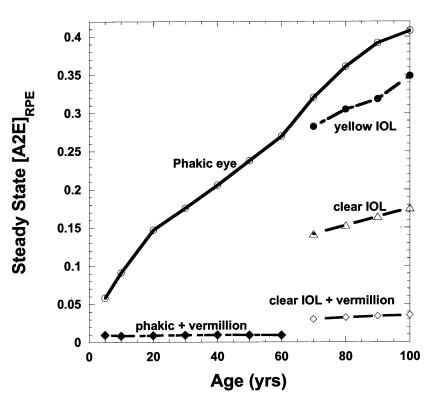
Assuming A2E and related photochemicals are largely concentrated by RPE lysosomal processing into lipofuscin granules rather than being enzymatically degraded or removed by transport to the choroidal micro-circulation, the model predicts that the accumulation rate of lipofuscin fluorophores would be nearly linear for the first 60 years with a noticeable rate of slowing of accumulation beyond 60 years (Figure 4). After cataract and IOL surgery, the modeled rate of A2E-related fluorophore accumulation increases approximately linearly at about the same rate as in early childhood (with its clear lens). The vermilion and 570-nm long-pass sunglasses prevent significant rod bleaching and A2E formation and thus prevent accumulation of further A2E-related fluorophores in the RPE. Although A2E-derived lipofuscin photosensitizer concentration increases consistently with age, the aging lens filters out increasing fractions of the short-wavelength photons capable of allowing lipofuscin to photosensitize 1O2 formation (macular-weighted spectral irradiance in Figure 4). This balance of aging effects leads to a predicted increase in RPE 1O2 generation between age 10 and age 60 followed by a progressive decrease beyond age 60 (Figure 5). By dramatically increasing the transmission of short-wavelength photons to the retina and increasing the rate of A2E-related lipofuscin fluorophore accumulation, cataract and clear IOL surgery at 70 years would be expected to increase RPE 1O2 generation approximately sevenfold relative to the phakic eye (Table 1). The Alcon yellow IOL (or Russian Spectrum yellow IOL) would result in approximately a threefold increase. The use of the vermilion sunglass with a clear IOL reduces RPE 1O2 generation greater than 20-fold compared to the clear IOL alone and fourfold with respect to the phakic eye. A 570-nm long-pass filter almost completely prevents RPE 1O2 generation during its use. A 480-nm long-pass filter combined with a clear IOL is roughly comparable to the aging phakic eye at 70 years (see Figure 1).
Figure 4.
Modeled average accumulation of A2E-related fluorophores in RPE lipofuscin (lefthand axis) as a function of age and lens status: for the phakic eye (○), for the addition of vermilion sunglasses after implantation of clear UV-absorbing IOL at 70 (⋄), for clear IOL implanted at age 70 (▵), and for the yellow IOL implanted at age 70 (•). Concomitant with increases in lipofuscin fluorophore accumulation with age, the average macular AS(λ lf –weighted spectral irradiance in μW/cm2 @430-nm ) equivalents (▾ righthand axis) falls monotonically in the phakic eye.
Figure 5.
Average retinal pigment epithelium 1O2 generation via lipofuscin photoactivation as a function of age and lens status. Curves labeled as in Figure 2: phakic eye (○), clear IOL plus vermilion sunglass (⋄), clear IOL (▵), and natural or yellow IOL (•). IOLs implanted at age 70 in these modeled results.
At ≥ 10 μM, A2E has been shown to be cytotoxic to RPE cells in culture even though its average extractable concentration found in the aged human RPE is approximately 1 pg/cell or approximately 200 μM. Therefore, RPE viability appears to require A2E segregation within the lipofuscin granules and some means to alter chemically A2E as it accumulates within the RPE with age. We modeled the removal of accumulated A2E through its oxidation by 1O2 generated in turn by short-wavelength light absorption by oxidized A2E molecules within the same lipofuscin granule. This oxidation process within a lipofuscin granule requires a cumulative exposure equivalent to 80 J/cm2 at 488 nm (less at shorter wavelengths), which for our modeled ambient exposures would be expected to require approximately 1 month in a subject under 20 years of age. We calculated our estimate of the relative steady-state A2E concentration in the RPE, [A2Ess]RPE, as the average A2E production rate divided by its photo-oxidation rate within a lipofuscin granule as a function of age and ocular status (Figure 6). This calculation predicts that [A2Ess]RPE should increase almost linearly with age in a normal phakic individual, even though the rate of modeled A2E production falls significantly with age, particularly after 60 (Figure 3). Following lens removal and IOL implantation, both the modeled production of A2E and the rate of its photo-oxidation increase. As shown in Figure 4, the total accumulated A2E-related fluorophores (largely oxidized A2E) in lipofuscin increase after IOL implantation more for the clear IOL than for the yellow IOL. In contrast, [A2Ess]RPE (Figure 6) falls 2.3-fold following clear IOL implantation at 70 years but only 10% for yellow IOL implantation (Table 1).
Figure 6.
Modeled steady-state A2E concentration in the perifoveal retinal pigment epithelium cell, [A2Ess]RPE, as a function of age assuming that each A2E molecule is largely photo-oxidized by 1O2 generated within the lipofuscin granule. Phakic eye (○); addition of vermilion sunglasses for ambient conditions ≥200 cd/m2 in phakic eye before 70 (♦) and after implantation of clear UV-absorbing IOL at 70 (⋄); clear IOL implanted at age 70 (▵); and the yellow IOL implanted at age 70 (•).
DISCUSSION
The model described in this study is based on the long-held hypothesis that cumulative photochemical damage (eg, generation of toxic photochemicals or ROIs that result in oxidative damage) is an important factor in the pathogenesis of AMD. It has been long proposed that the reduced short-wavelength (<500 nm) transmission of the aged lens should photoprotect from macular photochemical damage and this protection would be substantially removed following cataract surgery. In the early IOLs, UV transmission was a significant factor, shortly avoided by introduction of UV absorbents cross-linked to the IOL polymers. Subsequently (first in Russia in the mid-1980s and then more recently in the West), in response to early criticisms of the removal of natural photoprotection of the aged lens, deep blue (420-nm) absorbents have been similarly formulated to create a light yellow IOL approximately as photoprotective against blue light photodamage as a 20-year-old lens.26 In the model, we calculated the relative effects of macular irradiance, lens aging (nuclear sclerosis), a clear or yellow IOL, and additional selective spectral glasses on the production of possibly cytotoxic photoproducts. The modeled retinal area, 7 to 11 degrees from the center of the fovea, is a region of high rod loss and lipofuscin accumulation with age.22,30 Consequently, possible changes in photochemical photosensitization of rod and RPE ROIs within this region are likely to show the greatest changes on aging and following cataract and IOL surgery.
Shortly after cataract and IOL surgery, our model predicts that the average production of A2E-related photochemicals and ROIs in rods and RPE due to all-trans-retinal and lipofuscin photochemistry would theoretically increase, respectively, 2.6-, 15-, and 6.6-fold with a clear UV-absorbing AcrySoft IOL and 2.1-, 4.1-, and 2.6-fold with a “yellow” IOL (Table 1). An external vermilion filter sunglass in ambient lighting of ≥200 cd/m2 would decrease the above values for a clear IOL approximately 30-, approximately 20-, and 4-fold compared to an average 70-year-old phakic eye. We would expect an increase in these photochemistries in eyes with larger pupil diameters, in phakic eyes with a higher lens transmittance, and in subjects with greater environmental light exposure compared to the age-dependent average values and light exposures we used in our calculations.
Our model is based in part on selected laboratory studies that have identified specific photo-oxidative damage mechanisms in rods and RPE cells and their action spectra.15–21 However, it is important to note that the short-wavelength thresholds for acute injury found in these studies (eg, >1 mW/cm2 and >1 J/cm2 at 430 nm) are greater than 100-fold higher than macular irradiances achieved under normal daylight conditions (Figure 4: inverted triangles, righthand axis). Although the empirical damage thresholds in animal experiments demonstrate an expected reciprocal relation between exposure intensity and duration for exposures up to a few thousand seconds, for much lower rates of ROI production over days or months, antioxidant protection and the rate of repair of oxidative damage can markedly increase damage threshold fluences (eg, J/cm2 at 430 nm) and determine whether significant photo-oxidative stress or cellular damage occurs.
Direct photochemical injury to the rods mediated by photo-activation of rhodopsin has been hypothesized to result from excited-state photochemistry of all-trans-retinal in the rod outer segments. On absorbing short-wavelength light (380-nm peak absorbance), an all-trans-retinal molecule can be driven into a long-lived triplet excited state that can initiate photo-oxidative damage to ROS membrane lipids and rim proteins.34 The reported photo-aggregation of ABCA4, a photoreceptor transporter enzyme of the visual cycle, would theoretically decrease the rate of transport of all-trans-retinal from the ROS disks as they age.34 Under moderate daylight conditions, the older, more distal disks would increase local all-trans-retinal concentration under steady-state illumination, which in turn would increase the production of A2PE, A2E, and related species requiring the reaction of two all-trans-retinal molecules and one phosphatidylethanolamine. These reactions would be driven by significant rod photobleaching and associated high steady-state [all-trans-retinal]ROS in moderate to bright daylight. Changes in our modeled rates of rod ROI production with increasing age are largely determined by the lower lens transmission of blue light, smaller pupil diameter, and slower rod dark adaptation time constant tRh. The resulting modeled rate of rod ROI production via all-trans-retinal photochemistry monotonically decreases, on average, approximately 40% per decade throughout life (Figure 2). All-trans-retinal photo-induced ROI created within the rods should create much more damage when macular short-wavelength irradiances are high. Consequently, the model predicts approximately 20-fold higher rod ROI production in a young child than in a 70-year-old phakic person. An immediate 15-fold increase in ROIrod production is predicted on cataract removal and clear UV-absorbing IOL implantation in a 70-year-old; this suggests a possible mechanism for accelerated rod loss following cataract and IOL surgery. The yellow IOL limits the postoperative increase to fourfold. In contrast, wearing the vermilion glasses (during ambient conditions ≥ 200 cd/m2) following clear IOL implantation reduces modeled rod ROI production approximately 20-fold compared to the preoperative 70-year-old phakic eye (Table 1). In the cones, the more rapid visual cycle kinetics would markedly reduce steady-state all-trans-retinal concentrations under intense light conditions compared to rods. Consequently, the cones may be less vulnerable to all-trans-retinal–mediated photo-oxidative injury and generate less A2E and related species. This may be due in part to the recently described alternative pathway for recycling all-trans-retinal in cones involving Mueller cells independent of the RPE.35
The accumulated A2E precursors in the shed distal disks are phagocytosed by the RPE cells and concentrated as A2E by lysosomal processing into the lipofuscin granules.18 Retinal pigment epithelial lipofuscin granules accumulate with age and contain a heterogeneous mixture containing at least 12 distinct extractable fluorophores, three of which exhibit fluorescence spectra similar to A2E.33 A2E epoxides formed by the reaction of 1O2 with A2E are much more potent photosensitizers than A2E with a blue-shifted spectra and are likely to correspond to the major green-emitting and yellow-emitting fluorophores that Eldred isolated from lipofuscin.33 Recently, Rozanowska and associates36 have reported that the amount and total activity of chloroform-insoluble photosensitizers within each lipofuscin granule appear to increase with age, possibly through the cumulative action of increasing ROI photo-oxidation within the aging lipofuscin granule. In our model, we assumed that lipofuscin photosensitization within the RPE results in A2E becoming oxidized within the lipofuscin granule and converted into a more potent photosensitizer of ROI. As shown in Figure 3, the modeled annual average of A2E production gradually decreases with age up to age 60, but falls rapidly after age 60 because of increased attenuation of macular scotopic irradiance. With a nearly constant input of A2E, the model (Figure 4) predicts a nearly linear increase within the RPE of A2E-related fluorophores and concentration of potent photosensitizers up to age 60, but this increase slows significantly thereafter. The removal of the aged lens at age 70 and insertion of either a clear or a yellow IOL almost doubles the macular irradiance in scotopic trolands, leading to a greater rate of incorporation of A2E and A2E-related fluorophores into the RPE lipofuscin. In contrast, individuals wearing the vermilion filter after IOL implantation would be expected to experience much reduced rod bleaching and no significant increases in A2E-related photosensitizers (Table 1).
In our model, the total rate of ROI generation by lipofuscin photosensitizers is proportional to the product of (1) the accumulated A2E-related fluorophore photosensitizer concentration in a RPE cell and (2) the action-spectrum-weighted macular irradiance (the integral product of HRPE(λ) and AS(λ)lf – both plotted▾ in Figure 4). This spectrally weighted macular irradiance falls monotonically with age, while lipofuscin photosensitizer concentration rises in the phakic eye. The product of these two factors results in the complex curve of RPE ROI (age) via lipofuscin photoactivation plotted in Figure 5. The modeled ROI increases with age over the first 60 years. However, due to the reduction in short-wavelength macular irradiance, it falls progressively (32% per decade) after 60. The large increase in short-wavelength macular irradiance on removal of the lens and replacement with an IOL leads to modeled increases in RPE ROI photosensitization (6.6-fold for clear IOL and 2.6-fold for the yellow IOL at 70 years), which continue to increase in successive years as the rate of lipofuscin photosensitizer accumulation increases. Wearing the vermilion spectral glasses following clear IOL implantation should reduce the RPE ROI photosensitization to modeled levels roughly one fourth of those for preoperative phakic eye.
Cytoplasmic A2E concentrations of ≥10 μM have been shown to damage mitochondrial membranes in vitro (in the dark), and approximately 1 pg of A2E per RPE cell (which is approximately 200 μM A2E on average in situ) has been extracted from human eyes.18 A2E molecules are avidly taken up by RPE cell cultures, concentrated within phagosomes, and within a few days appear as highly fluorescent lipofuscin-like granules. This efficient mechanism appears necessary to limit the cytotoxic (dark reaction) damaging effects of the detergent-like A2E molecules. The amount of chloroform-insoluble photosensitizers within each lipofuscin granule appears to increase with age.36 The high content of unsaturated carbon bonds in lipofuscin granules and the granule size (much greater than the distance 1O2 diffusion distances in its 4-μsec lifetime) suggest that a large fraction of cellular oxidation induced by lipofuscin photogenerated 1O2 may be confined to the granule in which it was generated. This process might have two positive attributes: (1) limit the concentration of 1O2 escaping from the lipofuscin granules and thereby limit cell oxidative damage and (2) photodegrade A2E into A2E-related insoluble fluorophores that are incapable of redistributing into critical cellular membranes (thereby limiting A2E cytotoxic damage in the dark). We suggest this is reflected in a balance between the rate of A2E production and the rate at which A2E molecules would be photo-oxidized within a lipofuscin granule, both of which are light-driven processes. The relative steady-state [A2Ess(age)]RPE in Figure 6 is predicted from the ratio of the modeled rate of A2E production (Figure 3) and the modeled rate of its photodegradation within a lipofuscin granule (equation 7 and plotted as ▾ in Figure 4). Interestingly, the modeled[A2Ess(age)]RPE shows a continual increase beyond the age of 60 even though the A2E production rate falls. The age-related changes in lens transmission decrease short-wavelength photodegradation of A2E by lipofuscin generated 1O2 more than they reduce rod bleaching and A2E production. Delori and associates30 have made in vivo fluorescence measurements in the human macula that are specific and proportional to [A2E] and show a similar age dependence in normals to our modeled [A2Ess(age)]RPE (Figure 6). If A2E is cytotoxic at the levels found in the aging RPE, then its increasing average steady-state levels in the RPE with advancing age might be a factor in the pathogenesis of AMD. Unlike our analysis of increases in ROI generation following cataract surgery at 70, the modeled [A2Ess(age)]RPE shows a 2.3-fold reduction within a few months following lens removal and clear IOL implantation (Table 1). The yellow IOL increases both the modeled light-driven A2E production and its photodegradation by roughly the same factor so that the resulting [A2Ess(age)]RPE is within 20% of the phakic preoperative eye. The vermilion sunglasses, worn after clear IOL implantation, reduce modeled A2E production much more than its rate of photodegradation and further reduce the [A2Ess(age)]RPE to approximately 10% of the preoperative levels. In contrast, a bright yellow 480-nm long-pass filter worn after clear IOL implantation increases the modeled [A2Ess(age)]RPE 1.8-fold over the preoperative levels. The technique of Delori and associates30 may be suitable to measure some of the larger predicted changes following cataract and IOL surgery. Comparisons with and without external filters that might decrease macular photo-oxidation but exhibit diverging effects on modeled [A2Ess]RPE in patients following cataract and IOL surgery might test the assumptions and predictions of our model.
Overall after cataract removal and clear IOL implantation, the model predicts a marked increase in ROI photoproduction in both the rods and RPE but an associated decrease in the [A2Ess]RPE cytotoxicity compared with the preoperative phakic eye. The total lipofuscin fluorophores, which are largely oxidized forms of A2E, are predicted to increase at a greater rate following lens removal and IOL implantation, but the yearly fractional rate of change is small given the lifetime accumulation of lipofuscin prior to cataract surgery. These modeled effects were for a typical aging eye without preoperative early AMD changes that may critically affect the potential for photo-oxidative damage or stress. The interaction between photo-oxidation and antioxidants or macromolecular repair mechanisms is presently not well understood in relation to macular aging changes or to AMD and thus is beyond the scope of our simplified model. Vermilion filters are widely used in commercial glasses and are well suited to acceptance by patients after cataract surgery. In a pilot study, patients who had late AMD in one eye and early AMD changes in the other eye adapted well subjectively to wearing the red 570-nm long-pass filters outside during bright daylight (S. Meyers and R. Bonner, unpublished data, 2003).
The pooled data of the Beaver Dam and Blue Mountain Eye Studies indicate a substantially higher risk for developing late-stage AMD in nonphakic compared with phakic eyes during a 5-year period (odds ratio equals 5.7 after adjustment for confounding variables).6 Short-wavelength macular irradiance is expected to have larger fractional increases on removal of nuclear cataracts than for the normally aged lens that we modeled. The epidemiological data coupled with our model predictions suggest that patients immediately after cataract removal and IOL implantation appear to be a particularly good population to evaluate the possible role of chronic photochemical injury in AMD. A randomized clinical trial to test the potential protective effects of an optimized “rod-sparing” external filter (eg, vermilion) would be a timely and important study in AMD. Furthermore, it would be of interest to evaluate such a filter in young patients with Stargardt’s disease and ABCA4 mutations, who would be of greater theoretical risk for photochemical injury associated with rod bleaching. Such studies might also discriminate among possible mechanisms for potential chronic photochemical injury.
DISCUSSION
Dr Robert N. Frank
Meyers and his colleagues have presented a very elegant, theoretical model of irradiance of the macula over the visible spectrum as a function of age, age-related changes in the lens, and following cataract surgery in the presence of several types of ultraviolet-absorbing intraocular lenses (IOLs). The hypothesis underlying this analysis is that age-related maculopathy (ARM) and in particular its most severe forms (geographic atrophy and choroidal neovascularization, which we term age-related macular degeneration [AMD]) are in large part due to a lifetime of irradiance of the retinal rods with light in the short wavelength portion of the visible spectrum. Following the bleaching of the rod visual pigment, rhodopsin (cone visual pigments are less susceptible to damage), the photoisomerized rhodopsin chromophore, all-trans-retinal, can both act as a photosensitizer of ROI and can undergo dark reactions leading to creation of the fluorescent molecule known as A2E, which accumulates in the retinal pigment epithelium (RPE) where it is incorporated into lipofuscin. Short-wavelength photo-excitation of A2E and its oxidized products in lipofuscin granules generate reactive oxygen intermediates (ROIs). Either the increasing concentrations of A2E and related molecules or the ROI’s produced might damage the RPE, ultimately leading to the lesions of ARM/AMD. The model of Meyers, Ostrovsky, and Bonner predicts that though A2E production rates generally decrease with advanced age, the steady-state levels of A2E in the RPE rise as a result of larger decreases in the rate of its photo-oxidation within lipofuscin granules due to increasing short-wavelength light absorption as the lens yellows. Cataract extraction and implantation of a UV-absorbing IOL increases production of A2E and even more markedly of ROIs, but these processes can both be profoundly inhibited by the wearing of specific red or vermilion sunglasses.
I won’t go into the details of this model in my discussion, since much of the photochemistry and the calculations are beyond my limited expertise. But it is important to note that this model is entirely a theoretical one. Its validity is based on the hypothesis I have stated above and, because it is a generalized model, it does not consider biological variability that might influence whether a particular individual is more or less susceptible to the development of AMD, even given the same lifetime history of short wavelength visible light exposure as another individual of differing genetic background or other characteristics. Evidence that sunlight exposure itself is a risk factor has been somewhat controversial, with different studies reaching different conclusions.1 However, there is very strong evidence from the combined Beaver Dam and Blue Mountains Eye Study results2 that cataract surgery substantially increases the five-year incidence of AMD (odds ratio 5.7, with 95 percent confidence interval 2.4–13.6 after multiple adjustments).
With this result in mind, and given the predictive and, above all, testable hypothesis of Meyers, et al., it seems to be time to initiate a controlled clinical trial of protective sunglass wearing for individuals who undergo cataract surgery. The major problem of such a trial, as it is with many clinical trials, is the duration (at least five years) and the sample size. The combined Beaver Dam/Blue Mountains study populations included 6,019 participants from whom 11,391 eyes were evaluated over a five-year follow-up. Of these, only 315 (fewer than 3 percent) were non-phakic, i.e. would qualify for the proposed clinical trial (and because of the requirement to wear sunglasses for the proposed study, it is subjects and not eyes that must be counted). Of these eyes, fewer than 7 percent (a total of 21) developed advanced AMD over the five-year follow-up. With these figures-and using a five-year relative risk (sunglass wearers vs. controls) of 0.8, approximately equal to the results of the Age-Related Eye Disease Study,3 with an alpha of 0.05, a power of 0.8, and approximately equal numbers of sunglass wearers and controls-using the uncorrected chi-square test to calculate results, I calculate a sample size of 4,700 in the treated group and an equal number of controls. If the relative risk is decreased to 0.5, the sample size decreases to 640 subjects in each group. Of course, this calculation will differ if, as in the AREDS, the entry criteria are modified to include only subjects who have early ARM at the outset. Given the frequency of cataract surgery and of ARM/AMD and the detailed hypothesis that Meyers and his colleagues have presented, I urge that such a trial be given strong consideration.
REFERENCES
- 1.Tomany SC, Cruickshanks KJ, Klein R, et al. Sunlight and the 10-year incidence of age-related maculopathy. Arch Ophthalmol. 2004;122:750–757. doi: 10.1001/archopht.122.5.750. [DOI] [PubMed] [Google Scholar]
- 2.Wang JJ, Klein R, Smith W, et al. Cataract surgery and the 5-year incidence of late-stage age-related maculopathy: pooled findings from the Beaver Dam and Blue Mountains eye studies. Ophthalmology. 2003;110:1960–1967. doi: 10.1016/s0161-6420(03)00816-9. [DOI] [PubMed] [Google Scholar]
- 3.Age-Related Eye Disease Study Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta-carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119:1417–1436. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dr Douglas D. Koch
I wonder if some of the experimental work could be done in vitro. Janet Sparrow has done a lot of work with various filters protecting against generation of A2E. You mentioned that you need some blue light coming in. If you could model that experimentally and then in an in vitro model, you might be able to confirm your hypothesis.
Dr John T. Flynn
I might suggest another experimental approach. Take patients that need a cataract extraction but also have a very strong family history of macular degeneration. Consider entering those patients in a study. They receive a clear lens in one eye and they receive a vermilion lens in the other eye, or they receive two clear lenses but they wear a vermilion contact lens. It seems to me the sample size to get to a P value of <0.05 is six, and you may not have to study a large number of patients to prove your hypothesis. You have to have cooperative patients who are willing to subject their eyes to be their own control, so to speak, to answer this question.
Dr Rick Ferris
I applaud the authors for trying to add some science to the understanding of what’s accumulating in the RPE and how it accumulates. With regard to light toxicity, I spent many years in various studies looking at this question with a number of sunlight questionnaires and other approaches. If it is a risk factor, it is hard to demonstrate it, and it appears not to be an overwhelmingly strong risk factor. However, it does point out that this may be a complex issue with a number of other important factors leading to AMD.
It is important to discuss the issue of post-cataract surgery and the development of AMD. Unfortunately, the two epidemiologic studies that have recently been reported suffer from a major flaw. The flaw is that patients with early AMD suffer slight decreased vision from the AMD, and wind up getting a cataract removed, not because the cataract caused the decreased vision, but because the AMD caused the decreased vision.
So, in this case, the AMD causes the cataract surgery. When you do the epidemiologic and statistical studies, it could appear that the cataract surgery caused the AMD, but it is actually the other way around. Both sets of authors of those studies suggest that the ideal way of evaluating this would be to carefully examine these patients’ retinas before cataract surgery. We have been doing that in the age-related eye disease study for the last eight years or 10 years, and we have been looking at the development of AMD after cataract surgery in our study. At present, we cannot show an increased risk of the development of late AMD or advanced AMD following cataract surgery. The weakness of our study is that we have modest follow-up of five to six years after cataract surgery. We are doing additional analyses because it may take you many years of increased exposure before you are likely to see the increased risk develop.
Dr Sanford M. Meyers
Our model probably has its greatest relevance in very early AMD or in patients without overt disease but who have a strong family history or hereditary risk. A trial of spectral filters comparing one eye with the other in patients after bilateral cataract and IOL implant surgery, as suggested by Dr Flynn, would avoid the genetic and non-genetic variables of interindividual comparisons. In such a study, compliance would be an important issue. Over the past few years, we studied about 25 unilateral late-stage AMD patients, phakic and pseudophakic, who wore reddish glasses with a 570-nm-long pass filter outside during bright daylight. We did not observe a dramatic effect. However, one of the patients, a physician who was bilaterally pseudophakic, fell in love with the reddish glasses and was 100 percent compliant. He had one photodynamic laser treatment after which he began wearing the reddish glasses. In the subsequent three years, he has not had a recurrent choroidal neovascular membrane in that eye (vision 20/60) and has not developed late AMD in the other eye. Overall, the compliance was good, but some of the patients were more compliant than others on subjective questioning. The vermilion filter may increase compliance (due to improved color perception and photopic sensitivity) and provides similar protection as the red 570 nm filter. A vermilion contact lens is a possibility but would create logistical issues on its use in subjects over the age of 70. A vermilion IOL would create problems for night vision unless suitable photochromic materials were developed.
Dr Ferris raises critical questions about some of the epidemiological studies. Patient responses on the history of sunlight exposure 30 years in the past are very subjective, especially in relation to our model with sophisticated mathematical analysis. Additionally, differences between individuals in regard to genetic factors, levels of antioxidants, and cellular damage repair mechanisms are confounding variables in epidemiological studies.
To address these issues and the large size of a clinical trial needed to test the efficacy of spectral filters, a small pilot study could be done in patients after cataract and IOL implant surgery to determine the feasibility of using in vivo techniques for retinal spectral fluorescent measurements that quantify levels of A2E and its epoxides and the effects of different ocular filters. If this pilot study verifies the model’s predictions, it would support a randomized clinical trial. We also suggest that the vermilion filter be considered in a study of Stargardt’s patients with mutations in the ABCA4 enzyme, which is critical in the processing of all-trans-retinal as stated in our manuscript. Dr Koch’s comments are addressed in the manuscript.
REFERENCES
- 1.Leibowitz HM, Kruger DE, Maunder LR, et al. The Framingham eye study monograph: an ophthalmological and epidemiological study of cataract, glaucoma, diabetic retinopathy, macular degeneration, and visual acuity in a general population of 2631 adults, 1973–1975. Surv Ophthalmol. 1980;24((Suppl)):335–610. [PubMed] [Google Scholar]
- 2.Klein R, Klein BEK, Linto KLP. Prevalence of age-related maculopathy. Ophthalmology. 1992;99:933–943. doi: 10.1016/s0161-6420(92)31871-8. [DOI] [PubMed] [Google Scholar]
- 3.Ferris FL, III, Fine SL, Hyman LG. Age-related macular degeneration and blindness due to neovascular maculopathy. Arch Ophthalmol. 1984;102:1640–1642. doi: 10.1001/archopht.1984.01040031330019. [DOI] [PubMed] [Google Scholar]
- 4.Pollack A, Marcovich A, Bukelman A, et al. Age-related macular degeneration after extracapsular cataract extraction with intraocular lens implantation. Ophthalmology. 1996;103:1546–1554. doi: 10.1016/s0161-6420(96)30464-8. [DOI] [PubMed] [Google Scholar]
- 5.Klein R, Klein BEK, Jensen SC, et al. The relationship of ocular factors to the incidence and progression of age-related maculopathy. Arch Ophthalmol. 1998;116:506–513. doi: 10.1001/archopht.116.4.506. [DOI] [PubMed] [Google Scholar]
- 6.Wang JJ, Klein R, Smith W, et al. Cataract surgery and the 5-year incidence of late-stage age-related maculopathy: pooled findings from the Beaver Dam and Blue Mountains Eye Studies. Ophthalmology. 2003;110:1960–1967. doi: 10.1016/s0161-6420(03)00816-9. [DOI] [PubMed] [Google Scholar]
- 7.Taylor HR, Munoz B, West S, et al. The long-term effects of visible light on the eye. Arch Ophthalmol. 1992;110:99–104. doi: 10.1001/archopht.1992.01080130101035. [DOI] [PubMed] [Google Scholar]
- 8.Tomany SC, Cruickshanks KJ, Klein R, et al. Sunlight and the 10-year incidence of age-related maculopathy: the Beaver Dam Eye Study. Arch Ophthalmol. 2004;122:750–757. doi: 10.1001/archopht.122.5.750. [DOI] [PubMed] [Google Scholar]
- 9.Mainster MA. Light and macular degeneration: a biophysical and clinical perspective. Eye. 1987;1:304–310. doi: 10.1038/eye.1987.49. [DOI] [PubMed] [Google Scholar]
- 10.Young RW. Solar radiation and age-related macular degeneration. Surv Ophthalmol. 1988;32:252–269. doi: 10.1016/0039-6257(88)90174-9. [DOI] [PubMed] [Google Scholar]
- 11.van der Hoeve J. Eye lesions produced by light rich in ultraviolet rays: senile cataract senile degeneration of the macula. Am J Ophthalmol. 1920;3:178–194. [Google Scholar]
- 12.Gjessing HGA. Gibt es einen Antagonismus zwischen cataracta senilis und habscher seniler Makulaveranderungen? Z Augenheilkd. 1925;56:79–90. [PubMed] [Google Scholar]
- 13.Sperduto RD, Hiller R, Seigel D. Lens opacities and senile maculopathy. Arch Ophthalmol. 1981;99:1004–1008. doi: 10.1001/archopht.1981.03930011004003. [DOI] [PubMed] [Google Scholar]
- 14.Liu IY, White L, LaCroix AZ. The association of age-related macular degeneration and lens opacities in the aged. Am J Public Health. 1989;79:765–769. doi: 10.2105/ajph.79.6.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ham WT, Jr, Mueller HA, Sliney DH. Retinal sensitivity to damage from short wavelength light. Nature. 1976;260:153–155. doi: 10.1038/260153a0. [DOI] [PubMed] [Google Scholar]
- 16.Ham WT, Jr, Mueller HA, Ruffolo JJ, et al. Action spectrum for retinal injury from near-ultraviolet radiation in the aphakic monkey. Am J Ophthalmol. 1982;93:299–306. doi: 10.1016/0002-9394(82)90529-3. [DOI] [PubMed] [Google Scholar]
- 17.Boulton M, Dontsov A, Jarvis-Evans J, et al. Lipofuscin is a photoinducible free radical generator. J Photochem Photobiol B. 1993;19:201–204. doi: 10.1016/1011-1344(93)87085-2. [DOI] [PubMed] [Google Scholar]
- 18.Sparrow JR, Nakanishi K, Parish CA. The lipofuscin fluorophore A2E mediates blue light induced damage to retinal pigmented epithelial cells. Invest Ophthalmol Vis Sci. 2000;41:1981–1989. [PubMed] [Google Scholar]
- 19.Sparrow JR, Zhou J, Ben-Shabat S, et al. Involvement of oxidative mechanisms in blue-light induced damage to A2E-laden RPE. Invest Ophthalmol Vis Sci. 2002;43:1222–1227. [PubMed] [Google Scholar]
- 20.Pawlak A, Wrona M, Rozanowska M, et al. Comparison of the aerobic photoreactivity of A2E with its precursor retinal. Photochem Photobiol. 2003;77:253–258. doi: 10.1562/0031-8655(2003)077<0253:cotapo>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 21.Pawlak A, Rozanowska M, Zareba M, et al. Action spectra for the photoconsumption of oxygen by human ocular liofuscin and lipofuscin extracts. Arch Biochem Biophys. 2002;403:59–62. doi: 10.1016/S0003-9861(02)00260-6. [DOI] [PubMed] [Google Scholar]
- 22.Curcio CA, Owsley C, Jackson GR. Spare the rods, save the cones in aging and age-related maculopathy. Invest Ophthalmol Vis Sci. 2000;41:2015–2018. [PubMed] [Google Scholar]
- 23.Calkins JL, Hochheimer BF, D’Anna SA. Potential hazards from specific ophthalmic devices. Vision Res. 1980;20:1039–1053. doi: 10.1016/0042-6989(80)90042-5. [DOI] [PubMed] [Google Scholar]
- 24.Pokorny J, Smith VC. How much light reaches the retina? The Verriest Lecture. In: Cavonius CR, ed. Colour Vision Deficiencies XIII. Dordrecht, Netherlands: Kluver Academic Publishers; 1997:491–511.
- 25.Mainster MA, Sparrow JR. How much blue light should an IOL transmit? Br J Ophthalmol. 2003;87:1523–1529. doi: 10.1136/bjo.87.12.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Linnik LF, Ostrovsky MA, Zak PP, et al. Analysis of long-term clinical and functional results of implantation of IOL spectrum. Ophthalmochirurgia. 1992;1:40–44. [Google Scholar]
- 27.Winn B, Whitaker D, Elliot DB, et al. Factors affecting light adapted pupil size in normal human subjects. Invest Ophthal Vis Sci. 1994;35:1132–1137. [PubMed] [Google Scholar]
- 28.Koch DD, Samuelson SW, Villarreal R, et al. Changes in pupil size induced by phacoemulsification and posterior chamber lens implantation: consequences for multifocal lenses. J Cataract Refract Surg. 1996;22:579–584. doi: 10.1016/s0886-3350(96)80013-7. [DOI] [PubMed] [Google Scholar]
- 29.Bone RA, Landrum JT, Fernandez L, et al. Analysis of the macular pigment by HPLC: retinal distribution and age study. Invest Ophthalmol Vis Sci. 1988;29:843–849. [PubMed] [Google Scholar]
- 30.Delori FC, Goger DG, Dorey CK. Age-related accumulation and distribution of lipofuscin in RPE of normal subjects. Invest Ophthalmol Vis Sci. 2001;42:1855–1866. [PubMed] [Google Scholar]
- 31.Thomas MM, Lamb TR. Light adaptation and dark adaptation of human rod photoreceptors measured from the a-wave of the electroretinogram. J Physiol. 1999;518:479–496. doi: 10.1111/j.1469-7793.1999.0479p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jackson GR, Owsley C, McGwin G. Aging and dark adaptation. Vision Res. 1999;39:3975–3982. doi: 10.1016/s0042-6989(99)00092-9. [DOI] [PubMed] [Google Scholar]
- 33.Eldred GE. Lipofusccin fluorescence—chromatographic and spectral characterization of yellow-emitting components. Age. 1983;6:135–136. [Google Scholar]
- 34.Sun H, Nathans J. ABCR, the ATP-binding cassette transporter responsible for Stargardt macular dystrophy, is an efficient target of all trans-retinal-mediated photooxidative damage in vitro. J Biol Chem. 2001;276:11766–11774. doi: 10.1074/jbc.M010152200. [DOI] [PubMed] [Google Scholar]
- 35.Mata NL, Radu RA, Clemmons RS, et al. Isomerization and oxidation of vitamin A in cone-dominant retinas: a novel pathway for visual-pigment regeneration in daylight. Neuron. 2002;36:69–80. doi: 10.1016/s0896-6273(02)00912-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rozanowska M, Pawlak A, Rozanowski B, et al. Age-related changes in the photoreactivity of retinal lipofuscin granules: role of chloroform-insoluble components. Invest Ophthalmol Vis Sci. 2004;45:1052–1060. doi: 10.1167/iovs.03-0277. [DOI] [PubMed] [Google Scholar]